Manuscript accepted on :20-01-2022
Published online on: 02-02-2022
Plagiarism Check: Yes
Reviewed by: Dr. Hany Akeel
Second Review by: Dr. Prathibha Nair
Final Approval by: Dr. Francesca Gorini
Sunil Mhatarba Vishwasrao1* , Sufala Sunil Vishwasrao2
, Sufala Sunil Vishwasrao2 and Amar Nagesh Kumar3
and Amar Nagesh Kumar3
1Department of Pharmacology, Karpaga Vinayaga Institute of Medical Sciences and Research Centre, GST Road, Chinakolambakkam, Madhuranthagam, Tamil Nadu, India, 603308.
2Department of Anesthesiology, Karpaga Vinayaga Institute of Medical Sciences and Research Centre, GST Road, Chinakolambakkam, Madhuranthagam, Tamil Nadu, India, 603308.
3Department of Biochemistry, Karpaga Vinayaga Institute of Medical Sciences and Research Centre, GST Road, Chinakolambakkam, Madhuranthagam, Tamil Nadu, India, 603308.
Corresponding Author E-mail: sunilpharmac@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2371
Abstract
Background Post operative nausea vomiting (PONV) is distressing for patient as well as clinician as it affects post-operative care and recovery substantially. Causes of PONV are multi factorial which are primarily categorized into patient related factors, pre- surgical factors and post-surgical factors. There are several classes of drugs that constitute basic of anti-emetic therapy. Primary objective of the study is to assess the efficacy and safety of intravenous (IV) Palonosetron in preventing post operative nausea vomiting (PONV) in comparison with IV ondansetron. Methodology This is a double blinded randomized controlled study conducted during the period of January 2015 to February 2016 in patients with ASA (American Society of Anesthesiologist) grade I category who underwent surgical intervention under general anaesthesia. Both male and female patients in the age range of 15-60 years with ASA grade I status and willing to give written informed consent were recruited for the study. 116 out of 129 patients were recruited for the study based upon inclusion and exclusion criteria. The patients were randomly assigned to two equal groups, Group A, who received palonosetron 0.075 mg intravenously and Group B, who received ondansetron 8 mg intravenously. The efficacy and safety of palonosetron was tested on the use of ondansetron. Statistical analysis was done by Chi-square test and Student t-test. P value less than 0.05 was considered statistically significant. Results Efficacy of palonosetron was assessed by complete response (CR), use of rescue medication, gratification score and severity of nausea. The P value of all efficacy parameters was <0.05 which was statistically significant. Safety parameters include adverse reactions related to palonosetron or other adverse drug events. Adverse drug reactions were less in group A compared to Group B. Conclusion Palonosetron was more efficacious than ondansetron in controlling PONV in a post-surgical patient undergoing general anaesthesia. Palonosetron was found equally safe as ondansetron.
Keywords
General Anesthesia; Ondansetron; PONV; Palonosetron; Postsurgical vomiting
Download this article as:| Copy the following to cite this article: Vishwasrao S. M, Vishwasrao S. S, Kumar A. N. Efficacy and Safety of Intravenous Palonosetron against Ondansetron in Preventing Postoperative Nausea Vomiting in Patients Undergoing General Anaesthesia: Double blind Randomized Control Study in Tertiary Care Hospital, Tamil Nadu, India. Biomed Pharmacol J 2022;15(1). |
| Copy the following to cite this URL: Vishwasrao S. M, Vishwasrao S. S, Kumar A. N. Efficacy and Safety of Intravenous Palonosetron against Ondansetron in Preventing Postoperative Nausea Vomiting in Patients Undergoing General Anaesthesia: Double blind Randomized Control Study in Tertiary Care Hospital, Tamil Nadu, India. Biomed Pharmacol J 2022;15(1). Available from: https://bit.ly/3Lfsemr |
Introduction
Post-operative nausea vomiting (PONV) is a displeasing sensation. The patient usually expresses it as worse than postoperative pain. Despite the development of new drugs & treatment strategies to reduce its incidence & severity to some extent, it continues to rank as the most undesirable surgical outcome1. Since the inception of general anaesthesia, PONV remains an important complication after surgery for which no complete solution is available till date. PONV had gained more attention in 1991 after Kapur described this problem as big “little problem”2. It is distressing for both the patient and the clinician as postoperative care and recovery are substantially affected. Causes of PONV are multi factorial which are primarily categorised into patient related factors, pre- surgical factors and post-surgical factors. Due to various factors that contribute to the development of PONV, quantification of the risk of PONV in the individual patient is difficult. Apfel and colleagues mentioned major predictors of PONV that include age, obesity, female patient, past history of PONV or motion sickness, use of opioids as an adjunct to anaesthesia and non-smoker group3-8. Other pre-surgical and intra-surgical factors that contribute to PONV are pre-operative anxiety, underlying medical condition, hydration status, use of volatile anaesthetics, type and duration of surgery and type of anaesthesia3, 5, 9.
The incidence of PONV in the general population is approximately 30-40%, with a further increase in high-risk individuals of up to 80%6. In addition to this displeasing sensation, PONV may have adverse consequences such as pulmonary aspiration, hypovolemia, electrolyte imbalance, and wound dehiscence that prolongs postoperative and total hospital stay, leading to increased hospital cost10. The prevention of the above-mentioned complications improve quality of life, reduces unexpected hospital admissions and duration of hospital stay, and induces reduction in direct & indirect cost to the patient.
Several pharmacological agents have been tried, such as anti-histamines, butyrophenones, dopamine receptor antagonist and dexamethasone, for the prevention of PONV but none of them was found to be superior1. Despite extensive research and introduction of novel anti-emetic agents with better safety and efficacy profile, there seems to be little progress in reducing incidence of PONV. As a single agent has not been proven to be a complete solution to tackle this problem; recent research has advanced the use of combination anti-emetic therapy acting at more than one molecular site to control PONV. Use of more than two anti-emetic drugs offer its own disadvantages with added side effects and drug interactions. Therefore, research was strengthened on development of single molecule with prolonged action and lesser side effects. Ondansetron, a 5HT3 receptor antagonist is used as antiemetic in patients of malignancy along with chemotherapy and also approved in prevention of PONV11. Palonosetron is considered the latest 5HT3 receptor antagonist of the second generation with a unique action and a half-life much longer than other 5HT3 antagonists with a comfortable dose frequency option of once a day. It has higher receptor affinity compared to other 5HT3 antagonists and requires much smaller dose (0.075mg I.V) than ondansetron for the prophylaxis of PONV11,12.
Very minimal data is available on efficacy of palonosetron in all different types of surgeries under individual research. Hence palonosetron study was undertaken to compare its safety and efficacy with ondansetron in all adult patients planned for surgical procedures under general anaesthesia.
Aims and Objectives
The primary objective of the study includes the evaluation of the efficacy and safety of IV palonosetron in preventing post-operative nausea vomiting (PONV) compared to IV ondansetron. Secondary objective of the study is to find whether both drugs are comparable with demographic parameters like age, sex, height and weight.
There are few similar studies published on use of Palonosetron in PONV which includes specific group of population undergoing laparoscopic cholecystectomy, day care surgery, thyroidectomy, laparoscopic surgery, but our study aimed to be different from other published studies by selecting a broad group of patients undergoing various types of surgeries under general anesthesia rather than single specific type of surgery.
Materials and Methods
The study was initiated after getting approval from the Institutional Ethical committee dated 21.01.2015.
Study Design: Double blinded randomized controlled study.
Study period: January 2015 to February 2016.
Source of Data
All eligible patients of ASA grade I category undergoing surgical intervention under general anaesthesia in Karpaga Vinayaga Institute of Medical Sciences and Research Centre were enrolled.
Sample Size
Sample size was calculated with 5% (p<0.05) level of significance and a power of study at 80%. (β error 20%). Sample size required for our study was 50 in each group but 8 more samples in each group were added to improve accuracy of study results.
Inclusion Criteria
Both male and female patients in the age range of 15-60 years with ASA grade I status were recruited for the study.
Exclusion Criteria
Pregnant women, patients with a diagnosed case of acid peptic disease, a history of nausea and vomiting before surgery, a patient taking antiemetics or steroids, a patient with major organ involvement such as liver, kidney, heart, brain, and lungs, chronic alcoholics, a patient with hypersensitivity to any of the study trial drugs, a patient with a history of motion sickness, patients diagnosed with malignancy were excluded from the study.
Subject enrollment
A written informed consent was obtained from all participants in each group prior to surgery. Meticulous care was taken while obtaining demographic data, details of previous illness and retrieving details like past history of motion sickness or PONV.
116 out of 129 patients were recruited for the study based upon inclusion and exclusion criteria. Routine investigations like Hb%, Total Leukocyte Count (TLC), Fasting Blood Sugar Level (FBSL), Postprandial Blood Sugar Level (PPBSL), Blood Urea Level (BUL), Serum Creatinine, Chest X-ray and ECG were recorded for all the study participants.
Patients were randomly assigned into two equal groups.
Group A: received palonosetron 0.075 mg intravenously.
Group B: received ondansetron 8 mg intravenously.
Block randomization method was used for assigning equal groups. Four lettered 6 blocks were prepared as: AABB, ABAB, ABBA, BAAB, BABA, BBAA and patients were allocated accordingly. For example, if a randomly selected block would be BAAB then the first patient would go to group B, the second and third patient would go to group A, and the fourth patient would go to group B. In this way there was equal distribution of subjects in each group (Figure 1).
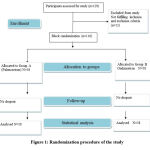 |
Figure 1: Randomization procedure of the study |
Before induction of anaesthesia vitals like pulse, respiratory rate (RR), systolic and diastolic blood pressure (BP), temperature and oxygen saturation (SPO2) were recorded. A covered envelope was provided to anaesthetist where name of drug group was mentioned. (Obtained from block randomization) Accordingly either palonosetron or ondansetron was administered 10 minutes before anaesthesia. After premedication with fentanyl 2µg/kg and glycopyrrolate 5µg/kg, patients were induced with IV propofol 2mg/kg and intubated with succinyl choline and muscle relaxation was achieved with vecuronium bromide 0.08mg/kg13. Patients were reversed back from general anaesthesia with neostigmine 0.05mg/kg and glycopyrrolate 0.2 mg. All vital parameters like pulse, BP, RR, Temperature, SPO2 and ECG were monitored intra operatively and post operatively at 0, 6,12,24,48 hrs.
The patients were questioned by trained staff or on duty doctors using a validated questionnaire to assess safety and efficacy. Efficacy was evaluated by complete response, (no episode of nausea or vomiting and no use of rescue medication) severity of nausea, use of rescue medication, and overall satisfaction score by 5-point Likert scale within 48 hrs of surgery14, 15. Nausea severity was measured by Verbal Rating Scale (VRS) and patients were graded into: no nausea 0, mild nausea 1-3, moderate nausea 4-6 and severe nausea 7-10. Those who had developed severe nausea or vomiting, rescue antiemetic IV metoclopramide (10mg) was administered. The presence of rash, itching, hypotension, or any serious adverse event during and after surgery after the administration of the drug was evaluated. Cardiovascular safety was assessed by comparing pre and post-operative ECG by assessing QTc interval.
Statistical analysis
Mean, standard deviations and proportions were calculated among the groups. Data was entered into excel spread sheet and analyzed by using SPSS software. Statistical analysis was done by Chi-square test and Student t-test. P-value less than 0.05 was considered statistically significant.
Results
It is evident from Table 1 that the mean age among Group A and Group B were 33.93± 10.32 and 34.86 ± 11.43 years respectively. This difference was not statistically significant (p>0.05). Large numbers of subjects observed in younger age group (18-28years) while small numbers of participants were present in elder age group (48-58years) (Table 1).
Table 1: Distribution of subjects according to age.
| Age
Group |
Group A
N (%) |
Group B
N (%) |
Total
N (%) |
| 18-28 | 20 | 23 | 43 (37.07) |
| 28-38 | 17 | 13 | 30 (25.86) |
| 38-48 | 16 | 14 | 30 (25.86) |
| 48-58 | 05 | 08 | 13 (11.21) |
| Total | 58 | 58 | 116 (100) |
| Mean age | 33.93 ±10.32 | 34.86±11.43 | * P> 0.05 |
*p>0.05 = not statistically significant. There is no statistically significant age difference between the study groups A and B.
Demographic data for both groups is mentioned in Table 2. Mean age observed in both groups were 33.93 and 34.86 years respectively. The average height and weight in Group A and Group B were 152.95, 153.02 cms, and 54.93 and 54.83 kg (Table 2).
Table 2: Demographic data of the study population.
| Parameters | Group A | Group B | P-value |
| Mean age | 33.93± 10.31 | 34.86 ± 11.43 | 0.46 |
| Mean Height | 152.95 ± 6.81 | 153.02± 6.38 | 0.06 |
| Mean weight | 54.93 ± 9.84 | 54.83 ± 8.72 | 0.06 |
| Mean BMI | 23.54±2.56 | 23.25±2.45 | 0.8 |
p>0.05 = not statistically significant. There is no statistically significant difference in height, weight, BMI between the study groups A and B.
About 56.90% were men and 43.10% were women. The distribution of men and women among both the groups were nearly similar and there was no statistically significant difference (Table 3).
Table 3: Distribution of subjects according to sex.
| Sex | Group A
N (%) |
Group B
N (%) |
Total
N (%) |
| Male | 27 (23.27) | 39 (33.62) | 66 (56.90) |
| Female | 31 (26.73) | 19 (16.38) | 50 (43.10) |
| Total | 58 (50) | 58 (50) | 116 (100) |
In group A, female patients were 20% more compared to group B. In both groups, non-smokers were having almost equal percentage. In group A, surgical time was prolonged for more than 2 hours in 88% of subjects which was higher than group B (65%) (Table 4). There was no difference in mean vital statistics in both groups during the preoperative, preinduction, intraoperative, and postoperative period.
Table 4: Risk factors among study groups
|
Risk factor
|
Group A | Group B | P-value |
| Female Gender | 31/58 (53%) | 19/58(33%) | 0.02 |
| Non smokers | 52/58 (89%) | 49/58 (84%) | 0.4 |
| Duration of surgery > 2 hrs | 7/8(88%) | 13/20 (65%) | 0.23 |
p>0.05= not statistically significant. There is no statistically significant difference in risk factors studied in Group A and Group B.
Mean Hb%, TLC values, Blood sugar values and Renal parameters did not show any significant difference among the groups.
Efficacy of palonosetron was assessed by complete response (CR), number of time rescue medication used, overall gratification and nausea severity score by VRS showed statistically significance (Table 5).
Table 5: Efficacy parameters tested
|
Efficacy parameters |
Group A (n=58) |
Group B (n=58) |
P-value |
| 1.Complete response | 50 | 38 | 0.009** |
| 2. Use of rescue medication | 8 | 20 | 0.009** |
| 3.Gratification score |
0.0001***
|
||
| Disgratified (DG) | 2 | 9 | |
| Not Gratified Not Disgratified (NGNDG) | 8 | 22 | |
| Gratified (GR) | 43 | 26 | |
| Highly Gratified (HGR) | 5 | 1 | |
| 4. Severity of nausea |
0.03* |
||
| Nil | 50 | 38 | |
| Mild | 4 | 12 | |
| Moderate | 4 | 08 | |
*p<0.05 = statistically significant, **p<0.01 =highly significant, ***p<0.001 =very highly significant.
The maximum incidence of PONV in group B was seen in laparoscopic surgeries followed by thyroid surgeries. The incidence of PONV in ENT surgeries in group B was 15% (Table 6). Least incidence was seen in percutaneous nephrolithotomy (PCNL). In group A, incidence of PONV was higher in females as compared to males (3:1) but was equal (1:1) in group B. In the early phase (0-24 hours) the incidence of PONV in group A was less (25%) compared to group B (95%) but in the late phase the incidence of PONV was high (Table 5 & Table 6).
Table 6: Distribution of various surgeries in study groups
| Type of surgery | Group A | Group B | Chi-square test
|
P-value |
| Oromaxillary | 08/58 (14%) | 07/58 (12%) | 0.07 | 0.7 |
| Laparoscopic abdominal | 14/58 (25%) | 17/58 (29%) | 0.39 | 0.5 |
| LSCS | Nil | 01/58 (2%) | – | – |
| Gynaecological Surgeries | 04/58 (7%) | 04/58 (7%) | – | – |
| Orthopaedic surgeries | 04/58 (7%) | 05/58 (9%) | 0.12 | 0.7 |
| ENT surgeries | 10/58 (17%) | 10/58 (17%) | – | – |
| Thyroid surgery | 02/58 (3%) | 04/58 (7%) | 0.7 | 0.4 |
| Spine Surgery | 03/58 (5%) | nil | – | - |
| Dental surgery | 02/58 (3%) | nil | – | – |
| General surgery excluding thyroid and laparoscopic procedures | 03/58 (5%) | 05/58 (9%) | 0.54 | 0.4 |
| Radical Neck Dissection | 07/58 (12%) | 02/58 (3%) | 3.01 | 0.08 |
| PCNL | 01/58 (2%) | 03/58 (5%) | 1.04 | 0.3 |
| Total | 58/58 | 58/58 | – | – |
Both groups did not show any serious adverse event. The most common side effect was headache in both groups and the least common side effect was rash or itching. QTc prolongation was observed in the ondansetron group in a single patient, while none in palonosetron receivers (Table 7).
Table 7: Safety parameters tested in the study population
| Adverse effects | Group A (n=58) | Group B (n=58) |
| Headache | 2 | 4 |
| Constipation | 1 | 1 |
| Dizziness | 1 | 2 |
| Fatigue | 1 | 1 |
| Itching | 0 | 0 |
| Insomnia | 1 | 1 |
| QTC prolongation | 0 | 1 |
Discussion
The present study was carried out to assess the safety and efficacy of palonosetron versus ondansetron. Two groups with equal number of participants were chosen and total 116 participants were recruited in the study. Efficacy parameters were assessed by complete response (CR), number of rescue anti-emetics used, nausea severity and overall satisfaction score. Complete response was evaluated as no nausea, vomiting and no need of rescue anti-emetics.
Of the 116 patients, 88 were complete responders, of which 50 (86%) were in the palonosetron group and 38 (65%) were in the ondansetron group. The difference in numerical value of 12 between the groups was highly significant (p<0.01). Similar results were seen in a study published by Musso and colleagues which was prospective study conducted on different types of cancer patients16 showed 80% CR for chemotherapy induced nausea vomiting (CINV) in palonosetron group and 60% in ondansetron group. Mattiuzzi et al. also demonstrated higher CR in the palonosetron arm versus the ondansetron arm17. Further, it was concluded that patient receiving palonosetron had less severe nausea from day 1 to day 5 and less impact of CINV. The study conducted by Chattopadhyay and associates where PONV was assessed in post caesarean delivery CR was observed in 85% of subjects using palonosetron and 83% of subjects using ramosetron18. In another study for the prevention of CINV, Schwartzberg and colleagues stated overall CR of 51% in the palonosetron group and 40% in the ondansetron, dolasetron, or granisetron group19. Our study demonstrated higher CR rates compared to previous studies. This may be due to recruitment of subjects with a smaller number of high-risk populations.
In our study, there was statistically significance on use of rescue medication between palonosetron & ondansetron group (p<0.01). Sharma and colleagues study also showed higher (20%) use of rescue medication in ondansetron group as compared to palonosetron group (4%)20. Kim and associates found less use of rescue anti-emetics in palonosetron group than ondansetron or ramosetron group21.
A non-inferiority randomized controlled trial for prophylaxis of PONV conducted by Davolos FJC et al concluded high incidence of PONV in ondansetron (43.4%) group compared to palonosetron (36.8 %) group. The calculated risk difference between palonosetron and ondansetron was 0 for initial 2 hours and 6.6 at 2-6 hours. The statistically significant results were observed on use of rescue medication between palonosetron and ondansetron.22
While considering severity of nausea among the groups, results were statistically significant in our study. (p< 0.01) Similar results were observed in the prospective double-blind study by Bajwa et al. where 6.66% had nausea and 3.33% had vomiting in the palonosetron group, while 20% observed nausea and 13.33% observed vomiting in the ondansetron group and the difference was statistically significant14.
Schwartzberg and associates demonstrated no significant difference between palonosetron and other 5HT3 antagonists during early post-chemotherapy period but significant difference was observed in delayed chemotherapy period19.
PONV episodes during the first 48 hours were 8 (13.76%) in the palonosetron group and 20 (34.4%) in the ondansetron group, which was highly significant. Consistent results were also observed in a previous study conducted by Kim and associates, where the incidence of PONV was 22.2% and 77% in the palonosetron and ondansetron group, respectively23. The lower values observed in our study were due to factors related to the patient and surgery. The higher incidence was due to the recruitment of more high-risk predictors of PONV in another study. The study conducted by Choudhary A and Parashkar V concluded that palonosetron is more effective in treating long term PONV in patients undergoing laparoscopic surgery under general anesthesia24.
The total satisfaction score in palonosetron group was high (82.75 %) as compared to ondansetron group (46.55%) which was very highly significant (p< 0.001). An analogous results were observed in a double-blind active control study done by Emad E Mansour. In three different groups, palonosetron, saline, or metoclopramide along with dexamethasone had a total satisfaction score of 88%, 48%, and 62%, respectively25.
In our study, patients from palonosetron group had higher CR, lesser nausea, lesser vomiting and higher satisfaction score as compared to ondansetron group. Even though both drugs belong to same structural group, palonosetron was much superior in controlling PONV. Few studies conducted26-29 among two groups have shown domination of palonosetron as antiemetic agent. palonosetron has ranked one in anti-emetic property than other 5HT3 antagonists like ramosetron and granisetron23. The study conducted by S H Kim and associates observed incidence of nausea, vomiting & retching lower in palonosetron as compared to ondansetron & ramosetron groups23. Even with combination chemotherapy palonosetron appears to be effective in controlling PONV. Sharma A N & associate concluded a study on PONV in palonosetron with dexamethasone and ondansetron with dexamethasone. In their study, combination of palonosetron and dexamethasone was more effective in controlling early & late phases of PONV in patients of laparoscopic hysterectomies20.
Although there was a higher number of women in our study group, the incidence of PONV was less (14%) compared to the ondansetron group (34.48%). Palonosetron proved its utility not only in normal patients but also in high risk individuals29-32 in controlling episodes of PONV. Superior efficacy of palonosetron could be due to its higher receptor affinity, due to allosteric site30,33 and longer half life11,26. Palonosetron was not only effective in reducing overall incidence of PONV but in controlling PONV episodes during early post-operative period (0-24hrs). This cardinal finding has more value when a previous study has demonstrated the efficacy of another 5HT3 antagonist to palonosetron in decreasing early episodes of PONV34. Study conducted by Elrashidy AA et al also showed that palonosetron group had less nausea, vomiting, retching as compared to ondansetron group in first 4-12 hours. Also, total episodes of nausea, vomiting and retching were significantly less in palonosetron group32. From the above-mentioned findings, we can conclude that palonosetron is also equally competent to the other 5 HT3 antagonists in controlling early phase PONV.
Various clinical trials had been supporting the safety of palonosetron36,37. In our study, palonosetron was well tolerated and was equally safe as ondansetron because both groups had mild and less side effects. The side effects in both groups were similar to those of previous studies. The common side effects observed were headache, constipation, fatigue, and insomnia. The most common side effect in each group was headache. Mattiuzzi et al, demonstrated most frequent adverse effect as headache and constipation17. A study by Sadaba et al, also listed headache, constipation, and diarrhoea as frequent adverse events38. No one in either group developed rash, itching, or diarrhoea. A single participant had QT prolongation in the ondansetron group but no one had it from the palonosetron group. Very few studies have demonstrated cardiac safety of palonosetron with increasing dose36. In our study, no effect was observed on the electrocardiogram measured by QT prolongation. Mean QTc for palonosetron group before and after surgery was 0.391 and 0.396 milli second while mean QTc for ondansetron group before and after surgery was 0.393 and 0.396 milli second respectively.
In our study, there was no loss of follow-up, as patients were monitored from 0 to 48 hours after surgery with regular intervals. Additionally, no deaths were observed in either group.
Conclusion
Ondansetron is most frequently used anti-emetic agent prescribed 8mg every 8 hourly. It has serious adverse effect of QTc interval prolongation. Palonosetron is having high receptor binding as compared to ondansetron is preferred with smaller dose (0.075mg) and once a day frequency. Also, QTc prolongation with use of palonosetron is associated with increase in dose. From the present study findings, it can be concluded that, palonosetron was more efficacious than ondansetron in controlling PONV in a post-surgical patient undergoing general anaesthesia. In addition, palonosetron was also effective in reducing PONV in first 24 hours of post-operative period. Overall satisfaction was higher in palonosetron recipients than in patients given ondansetron. Palonosetron was found equally safe as Ondansetron.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding source.
References
- Watcha M.F, White P.F. Postoperative nausea and vomiting. Its etiology, treatment and prevention. Anesthesiology 1992; 77:162-184.
CrossRef - Kapur PA. The big “little problem”. AnesthAnalg 1991; 73: 243-245.
CrossRef - Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology 1999; 91: 693-700.
CrossRef - Gan TJ, Meyer T, Apfel CC, Chung F, Davis PJ, Eubanks S, et al. Consensus guidelines for managing postoperative nausea and vomiting. AnesthAnalg. 2003; 97: 62–71.
CrossRef - Mckechnie K, Froese A. Ventricular tachycardia after ondansetron administration in a child with undiagnosed long QT syndrome. Can J Anaesth. 2010; 57:453-457.
CrossRef - Islam S, Jain PN. Postoperative nausea and vomiting (PONV): A review article. Indian J Anaesth. 2004; 48: 253-258.
CrossRef - Tramèr MR. Treatment of postoperative nausea and vomiting. BMJ. 2003; 327: 762–763.
CrossRef - Muchatuta NA, Paech MJ. Management of postoperative nausea vomiting: focus on palonosetron. Ther clin Risk Manag 2009; 5: 21-34.
CrossRef - Chatterjee S, Rudra A, Sengupta S. Current Concepts in the Management of Postoperative Nausea and Vomiting. Anesthesiology Research and Practice. 2011;2011:748031. doi:10.1155/2011/748031.
CrossRef - Golan David E, Tashjian Jr Armen H, Armstrong Ehrin J, Armstrong April W. Principles of Pharmacology. 3rd New Delhi: wolterskluwer India Pvt Ltd; 2012.
- Candiotti KA, Kovac AL, Melson TI. A randomized, double- blind study to evaluate the efficacy and safety of three different doses of palonosetron versus placebo for preventing postoperative nausea and vomiting. AnesthAnalg 2008;107: 445-451.
CrossRef - Rang H.P, Dale M.M, Ritter J.M, Flower R.J, Henderson G. Rang and Dale’s Pharmacology. 7th Edinburgh: Elsevier Churchill Livingstone; 2012.
CrossRef - Miller R.D, Cohen N, Eriksson Lars I, Fleisher Lee, Jeanine P, Wiener K et al. Miller’s Anaesthesia. 8th Philadelphia, Pennsylvania: Elsevier Churchill Livingstone; 2015.
- Bajwa SS, Bajwa SK, Kaur J, Sharma V, Singh A, Singh A, Goraya S, Parmar S, Singh K. Palonosetron: A novel approach to control postoperative nausea and vomiting in day care surgery. Saudi J Anaesth. 2011; 5(1): 19-24.
CrossRef - Gralla R,Lichinitser M, Van Der Vegt S, Sleeboom H, Mezger J, Peschel C, Tonini G, Labianca R, Macciocchi A, Aapro M. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann 2003; 14(10):1570-1577.
CrossRef - Musso M,Scalone R, Bonanno V, Crescimanno A, Polizzi V, Porretto F et al. Palonosetron (Aloxi) and dexamethasone for the prevention of acute and delayed nausea and vomiting in patients receiving multiple-day chemotherapy. Support Care Cancer. 2009; 17(2): 205-209.
CrossRef - Mattiuzzi GN, Cortes JE, Blamble DA, Bekele BN, Xiao L, Cabanillas M, Borthakur G, O’Brien S, Kantarjian H. Daily palonosetron is superior to ondansetron in the prevention of delayed chemotherapy-induced nausea and vomiting in patients with acute myelogenous leukemia.2010; 116(24): 5659-5666.
CrossRef - Chattopadhyay S, Goswami S. Palonosetron Versus Ramosetron Prophylaxis for Control of Postoperative Nausea and Vomiting after Cesarean Delivery under Spinal Anesthesia. J ObstetGynaecol India. 2015; 65(1): 28-33.
CrossRef - Schwartzberg L,Barbour SY, Morrow GR, Ballinari G, Thorn MD, Cox D. Pooled analysis of phase III clinical studies of palonosetron versus ondansetron, dolasetron, and granisetron in the prevention of chemotherapy-induced nausea and vomiting (CINV). Support Care Cancer. 2014; 22(2):469-477.
CrossRef - Sharma AN, Shankaranarayana P. Postoperative Nausea and Vomiting: Palonosetron with Dexamethasone vs. Ondansetron with Dexamethasone in Laparoscopic Hysterectomies. Oman Med J.2015; 30(4): 252-256.
CrossRef - Kim YY, Moon SY, Song DU, Lee KH, Song JW, Kwon YE. Comparison of palonosetron with ondansetron in prevention of postoperative nausea and vomiting in patients receiving intravenous patient-controlled analgesia after gynecological laparoscopic surgery. Korean J Anesthesiol. 2013; 64(2): 122-126.
CrossRef - Davolos FJC, Modolo NS, Braz LG, et al. Palonosetron versus ondansetron for prophylaxis of nausea and vomiting in a laparoscopic cholecystectomy: a non-inferiority randomized controlled trial. Brazilian Journal of Anesthesiology 2021 (Article in Press). Available online 16 July 2021. https://doi.org/10.1016/j.bjane.2021.06.020. Last accessed on 18 December 2021.
CrossRef - Kim SH, Hong JY, Kim WO, Kil HK, Karm MH, Hwang JH. Palonosetron has superior prophylactic antiemetic efficacy compared with ondansetron or ramosetron in high-risk patients undergoing laparoscopic surgery: a prospective, randomized, double-blinded study. Korean J Anesthesiol. 2013; 64(6): 517-523.
CrossRef - Choudhary A, Parashkar V. Comparison study of palonosentron and ondansetron in prevention of post-operative nausea vomiting after laparoscopic gynecological surgery. Indian Journal of Clinical Anesthesia 2020; 7(1): 59-63.
CrossRef - Emad E Mansour. Postoperative nausea and vomiting prophylaxis: The efficacy of a novel antiemetic drug (palonosetron) combined with dexamethasone. Egyptian J of Anaesthesia; 2013; 29(2): 117-123.
CrossRef - De Leon A. Palonosetron (Aloxi): a second-generation 5-HT3 receptor antagonist for chemotherapy-induced nausea and vomiting. Proc (BaylUniv Med Cent).2006; 19(4): 413-416.
CrossRef - Laha B, Hazra A, Mallick S. Evaluation of antiemetic effect of intravenous palonosetron versus intravenous ondansetron in laparoscopic cholecystectomy: a randomized controlled trial. Indian J Pharmacol. 2013; 45(1): 24-29.
CrossRef - Chun HR, Jeon IS, Park SY, Lee SJ, Kang SH, Kim SI. Efficacy of palonosetron for the prevention of postoperative nausea and vomiting: a randomized, double-blinded, placebo-controlled trial. Br J Anaesth. 2014; 112(3) :485-490.
CrossRef - Morrow GR, Schwartzberg L, Barbour SY, Ballinari G, Thorn MD, Cox D. Palonosetron versus older 5-HT3 receptor antagonists for nausea prevention in patients receiving chemotherapy: a multistudy analysis.J Community Support Oncol. 2014; 12(7): 250-258.
CrossRef - Del Cadia M,De Rienzo F, Weston DA, Thompson AJ, Menziani MC, Lummis SC. Exploring a potential palonosetron allosteric binding site in the 5-HT(3) receptor. Bioorg Med Chem. 2013; 21(23): 7523-7528.
CrossRef - Moon YE, Joo J, Kim JE, Lee Y. Anti-emetic effect of ondansetron and palonosetron in thyroidectomy: a prospective, randomized, double-blind study. Br J Anaesth. 2012; 108(3): 417-422.
CrossRef - Bhattacharjee DP, Dawn S, Nayak S, Roy PR, Acharya A, Dey R. A comparative study between palonosetron and granisetron to prevent postoperative nausea and vomiting after laparoscopic cholecystectomy. J Anaesthesiol Clin Pharmacol. 2010; 26: 480-483.
- Lummis SC,Thompson AJ. Agonists and antagonists induce different palonosetron dissociation rates in 5-HTA and 5-HTB Neuropharmacology. 2013; 73: 241-246.
CrossRef - Swaika S, Pal A, Chatterjee S, Saha D, Dawar N. Ondansetron, ramosetron or palonosetron: which is a better choice of antiemetic to prevent postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy? Anesthesia Essays Res 2011; 52: 182-186.
CrossRef - Elrashidy AA, Elsherif M, Elhag W., et al. Palonosetron versus ondansetron as prophylaxis against Post-operative Nausea and Vomiting After Laparoscopic Sleeve Gastrectomy: A Randomized Controlled Trial. Open Journal of Anaesthesiology 2020; 10(10): 349-360.
CrossRef - Morganroth J, Flaharty KK, Parisi S, Moresino C. Effect of single doses of IV palonosetron, up to 2.25 mg, on the QTc interval duration: a double-blind, randomized, parallel group study in healthy volunteers. Support Care Cancer. 2016; 24(2): 621-627.
CrossRef - Aapro MS,Grunberg SM, Manikhas GM, Olivares G, Suarez T, Tjulandin SA et al. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy.Ann Oncol. 2006; 17(9): 1441-1449.
CrossRef - Sadaba B, del Barrio A, Campanero MA, Azanza JR, Gomez-Guiu A, Lopez-Picazo JM et al. Randomized pharmacokinetic study comparing subcutaneous and intravenous palonosetron in cancer patients treated with platinum based chemotherapy.PLoS One. 2014; 9(2): e89747. doi: 10.1371/journal.pone.0089747.
CrossRef







