Manuscript accepted on :19-03-2022
Published online on: 25-03-2022
Plagiarism Check: Yes
Reviewed by: Dr. Heera Ram
Second Review by: Dr. Salman Ahmed Pharmacognosy
Final Approval by: Dr. Ian James Martin
Nur Amalina Noralidin 1 , Vasantha Kumar Rajoodorai 1
, Vasantha Kumar Rajoodorai 1 , Kamarul Ariffin Hambali2
, Kamarul Ariffin Hambali2 , Mohd Farhan Hanif Reduan1
, Mohd Farhan Hanif Reduan1 , Nur Zul Izzati Mohd Rajdi1
, Nur Zul Izzati Mohd Rajdi1 , Nurshahirah Shaharulnizim1
, Nurshahirah Shaharulnizim1 , Fathin Faahimaah Abdul Hamid 1
, Fathin Faahimaah Abdul Hamid 1 , Jasni Sabri1, Imad Ibrahim Ali Al Sultan3, Rumaizi Shaari1
, Jasni Sabri1, Imad Ibrahim Ali Al Sultan3, Rumaizi Shaari1 and Muhammad Luqman Nordin1*
and Muhammad Luqman Nordin1*
1Faculty of Veterinary Medicine, Universiti Malaysia Kelantan, Pengkalan Chepa, 16100 Kota Bharu, Kelantan, Malaysia
2Faculty of Earth Science, Universiti Malaysia Kelantan, 17600, Jeli, Kelantan, Malaysia
3Faculty of Medicine, Lincoln University College, 47300 Petaling Jaya, Malaysia
Corresponding Author E-mail: luqman.n@umk.edu
DOI : https://dx.doi.org/10.13005/bpj/2348
Abstract
Parkia speciosa is frequently consumed as a raw salad due to the notion that the plant exhibits numerous pharmacological activities that could benefit health particularly among Asians. This study was aimed to investigate the cytotoxicity and acute oral toxicity consequences of Parkia speciosa seeds extract against 4T1 mouse mammary cancer cells on C57BL/6 female mice. The antiproliferative effect of aqueous and ethanolic extracts was studied using the in-vitro antiproliferative assay, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Meanwhile, for acute toxicity study, twenty female mice were categorized into 5 groups, each with three aqueous extract treatment groups: 50 mg/kg, 300 mg/kg, 2000 mg/kg, meanwhile one vehicle group (treated with sterile distilled water), and one control group (no treatment given). Changes in behavioural signs, mortality rate, relative body weight, haematology, serum biochemistry, and organ histological evaluation were observed. Based on the MTT assay, the aqueous extract of Parkia speciosa was weakly active (IC50 = 312.5 ± 1.20 ug/ml) against 4T1 mammary cancer cells. In the acute study, no mortality, behavioural and physical changes were observed in any of the mice groups throughout the 14-day experiment. The haematological and serum biochemistry results of the treated and control groups showed no alteration. The kidney and liver were histopathologically evaluated and found to have normal organ architectures. Analyzed results could conclude that aqueous extract of Parkia speciosa has weakly active against cancer cells but has no deleterious effects on C57BL/6 mice given at high doses up to 2000 mg/kg.
Keywords
Antiproliferative; Acute Toxicity Study; C57BL/6; Cytotoxicity; Parkia speciosa
Download this article as:| Copy the following to cite this article: Noralidin N. A, Rajoodorai V. K, Hambali K. A, Reduan M. F. H, Rajdi N. Z. I. M, Shaharulnizim N, Hamid F. F. A, Sabri J, Al Sultan I. I. A, Shaari R, Nordin M. L. Cytotoxicity and Acute Oral Toxicity Effects of Parkia Speciosa Seeds Extract in C57bl/6 Mice.Biomed Pharmacol J 2022;15(1). |
| Copy the following to cite this URL: Noralidin N. A, Rajoodorai V. K, Hambali K. A, Reduan M. F. H, Rajdi N. Z. I. M, Shaharulnizim N, Hamid F. F. A, Sabri J, Al Sultan I. I. A, Shaari R, Nordin M. L. Cytotoxicity and Acute Oral Toxicity Effects of Parkia Speciosa Seeds Extract in C57bl/6 Mice.Biomed Pharmacol J 2022;15(1). Available from: https://bit.ly/3qy1811 |
Introduction
Cancer is the second greatest cause of mortality worldwide, according to the World Health Organization (WHO), with a reported nine million six hundred thousand deaths in 2018. Although many cancer medicines are in the investigation and most are in clinical trials, there is still a pressing need to seek medications that are considerably more effective and have fewer adverse effects. Natural products are said to account for nearly half of all medicines in clinical trials, with many of them having the possibility to inflict programmed cell death in different cell lines1. Plants have been recognised as a source of safer and less expensive alternatives with beneficial qualities such as fewer side effects on the body and ecology2. The anticancer activities of some plant extracts and their bioactive components have been thoroughly investigated3. Some of the most clinically effective chemotherapeutic agents produced from natural substances include vincristine, podophyllotoxin, paclitaxel, and camptothecin4.
Furthermore, Parkia speciosa is a native leguminous tree in tropical countries5. P. speciosa is recognised as petai in Southeast Asian countries such as Indonesia, Malaysia, and Singapore. Meanwhile, it is known as Sataw or Sator and u’pang in the Thailand and Philippines, respectively6. Malaysians have been using the plant to cure various illnesses and complications, including diabetes, kidney failure, and headaches6. P. speciosa was reported to have hypoglycemic7, anticancer8, and angiogenesis inhibitor properties9. All components of the Parkia speciosa plant contains various bioactive compounds such as flavonoids10, alkaloids, terpenoids11, and phenolics12 that have a wide range of nutritional benefits13. Despite numerous fundamental findings regarding the plant’s biological activities, in vivo toxicological effects should be studied prior to any in vivo test. This is since the chemical composition of each natural herb substance varies depending on several factors14,15 mentioned that variabilities in pharmacological activity could be resulting in considerable pharmacological activity discrepancies, impacting both pharmacodynamic and pharmacokinetic issues. Regardless of the plant’s naturalness, some phytochemical compounds could pose a lethal threat to humans and animals when consumed16. The efficacy and potential toxicity of commonly consumed plant preparations are unidentified16. Even though many plant-based products are natural, however, they are not necessarily always safe to consume. In this study, the acute toxicity of Parkia speciosa seed extract was determined in C57BL/6 mice using an aqueous solvent. Acute toxicity is important for determining the clinical signs that appear in the consumer as a result of high doses of the test substance or drug, the time of onset and cessation of the signs, the potential determination of a minimum lethal dose, and the sequence and timing of effects leading to death or recovery17.
Materials and Methods
Ethical approval
Faculty of Veterinary Medicine, Universiti Malaysia Kelantan Animal Ethics Committee, granted animal ethics approval (UMK/FPV/ACUE/FYP/17/2019) in January 2020. 20 female C57BL/6 mice aged 7 to 18 weeks were bought from Animal Research and Service Centre Universiti Sains Malaysia (ARASC USM), Health Campus, Kubang Kerian, Kelantan.
Housing and feeding condition
Groups of four C57BL/6 mice were housed in the polycarbonate cage measuring 28.5cm x 16.5cm x 13cm in an experimental room with a temperature of 22 °C (± 3°C), relative humidity of 60% (± 5%), and a cycle of 12-hour light and 12-hour dark. The mice were fed ad libitum with conventional rodent laboratory diets and distilled water. The bedding was made of wood shavings, and it was changed daily. Mice were chosen at random and kept in a cage as a group of four for six days prior to dosing to allow them to adapt to the laboratory settings.
Plant collection and Identification
Seeds of P. speciosa were purchased from a market in Pengkalan Chepa, Kota Bahru, Kelantan. The seeds were certified with a deposited voucher specimen from the Botany Department Herbarium, Universiti Kebangsaan Malaysia (UKM), Bangi, Malaysia. The seeds were cleaned, rinsed with distilled water, then followed with oven drying for seven days.
Speciosa seeds extraction
Clean seeds were pulverised in a heavy-duty grinding mill (High Power Smart Essence Blender, Model SSL – 766) to obtain the coarse particles. Afterwards, 100 g of tiny particles were mixed to 1000 ml of cleaned distilled water, in which the mixture was then boiled for 5 minutes. After the mixture was been cooled at room temperature, Filter paper (Whatman No.1) was used to separate the mixture from the blended P. speciosa seeds. The filtered solution was evaporated using a rotary evaporator (Heidolph, Germany) under reduced pressure (72 mbar) with a water bath at 50-60°C, then freeze-dried to gain the dried extract. The final product of dried P. speciosa extracts was weighed and until further use, it was stored at -20 °C in a tight and sterile container. Prior orally administered to mice, the final production of the extract was diluted with sterile distilled water, and doses of 50, 300 and 2000 mg/kg body weight (in sterile distilled water, 2 ml/kg body weight) was prepared.
In vitro antiproliferative assay
The 4T1 mouse mammary carcinoma cell was generously provided by Prof Dr. Nik Soriani Yaacob from the Department of Chemical Pathology, School of Medical Sciences, Universiti Sains Malaysia (USM). The cells were cultured in 10 % RPMI 1640 media with L-glutamine, supplemented with 10% foetal bovine serum (FBS), and 1% antibiotic-antimycotic. Hundred microlitres of media containing 1×104 cells (4T1 mammary cancer cells) were seeded into 96-well plates. This was followed by incubation of the plates at 37 °C with 5% CO2 overnight to allow cells attached to the bottom of the well plates. After overnight incubation, PSAE was dissolved in 1x PBS alone to obtain the stock solution for the extract with the concentration of 1mg/ml. Parkia speciosa of aqueous extract (PSAE) was serially diluted with concentrations ranging from 1000 μg/mL to 15.63 μg/mL and was added into the appropriate wells in three replicates for each concentration. For the untreated cells (0μg/mL), it was used as the negative control in every plate. To get a consistent result for each concentration, triplicate in one plate, and the experiment was repeated three times. The plates were later being incubated for 3 days with under control environment and temperature of 5% CO2, 37 °C, respectively. The old media were removed and replaced with 100 𝜇L of the fresh medium after the treatment duration for the incubated plated was completed (72 hours). In each well, 20 𝜇L of filtered MTT solution (5mg/mL PBS) was added. By tilting the plate, the medium was aspirated from each well carefully without interfering with the attached cells and, DMSO was added to each well at 100 𝜇L of and mixed thoroughly with pipette with up-and-down pipetting technique. The plates were re-incubated for another 10 minutes with the same environment control. Followed by optical density (OD) measuring by using a spectrophotometer (BioTek ELx 808) at 490 nm. The cell viability in percentage (%) was calculated using the formula:
Acute Toxicity Study
The body weight of each animal was taken followed by dose calculation before dosing. The animals were fasted for 3-4 hours from food but not the water and aqueous seeds of P. speciosa extract were given per os in a single dose. According to OECD guideline, only up to 2 ml/100 g of body weight was been given. Single-dose of P. speciosa aqueous extract (PSAE) at a low dose (50 mg/kg), medium dose (300 mg/kg), and high dose (2000 mg/kg) was orally gavage to the treatment groups of mice, whereas the control groups were received with sterile distilled water (vehicle) and no treatment for the negative control group. After administration of the extract, food was withheld for a further 3 to 4 hrs.
Observation Period
Each mouse was observed once within the first 30 minutes, 1 hour, 2 hours, 4 hours, and 8 hours. Afterwards, daily observations were conducted for the next 14 days. They were excluded from the sample in case they were discovered dead. They were humanely slaughter for animal welfare purposes if they did not meet the research criteria. Physical and behavioural observations, autonomic effects, sensory responses and reflexes, respiratory abnormalities, and somatosensory activities were monitored and recorded.
Necropsy
On the termination day, all of the mice were sacrificed by carbon dioxide inhalation and decapitation by guillotine. The vital organs, primarily the liver, kidneys, lungs, and other organs such as the spleen and ovaries, were removed, cleaned, and weighed during the post-mortem. Subsequently, all of the organs were preserved in 10% formalin for further histopathological assessment. The relative weight of the organ was calculated during the necropsy using the following formula:
Haematological and Biochemical Analyses
The decapitation method was performed to harvest the blood and then deposited it in a heparin tube. The blood was then subjected to a complete blood count test, followed by biochemistry analyses. Packed cell volume (PCV), haemoglobin (Hb) concentration, red blood cell count, mean cell volume, mean corpuscular haemoglobin and platelet count were calculated. Furthermore, the biochemical analyses included alanine aminotransferase (ALT), creatinine, urea and total protein parameter measurements.
Histopathology examination
Vital organs such as kidneys and liver were preserved in 10% formalin for approximately 24 hours before the processed tissues were examined for toxicity-related abnormalities. A few parameters, for instance, granular cast, cellular cast, protein cast, necrosis (pyknotic nucleus), inflammation, and hydropic degeneration in the kidney, were included in histopathological evaluations. In addition, histopathological lesions in the liver included activated Kupffer cells, necrosis (karyolysis and eosinophilic cytoplasm), necrosis (pyknotic nucleus), degeneration (hydropic degeneration), and inflammation were observed.
Results and Discussions
Qualitative phytochemical analysis of plant extract
Table 1 shows the finding of qualitative phytochemical screening of aqueous extract of Parkia speciosa. Among the phytochemical constituents that were screened for their presence in the extracts are phenolics, saponins, tannins, terpenoids, and flavonoids compounds. The result showed that Parkia speciosa seeds aqueous extract (PSAE) have all the phytochemical constituents except saponin and tannin.
Table 1: Phytochemical constituents of seeds derived from aqueous extract of Parkia speciosa.
| Phytochemical Constituent | Results | |
| PSAE | Colour | |
| Tannin | – | Absent |
| Phenolics | + | Weak |
| Terpenoids | ++ | Moderate |
| Flavonoids | +++ | Intense |
| PSAE | Froth | |
| Saponin | – | No froth for 10 minutes |
In vitro antiproliferative study
Table 2 represents the inhibitory concentration (IC50) of aqueous extract of Parkia speciosa on 4T1 mammary cancer cell lines.
Table 2: Minimum growth inhibitory concentration (IC50) of Parkia speciosa seeds extract on 4T1 and mammary carcinoma cell lines
| Type of extract | IC50 on 4T1 cells |
| Parkia speciosa seeds aqueous extract (PSAE) | 312.5 ± 1.20 ug/ml |
The inhibitory concentrations (IC50) of the extract against 4T1 mammary cancer cells were determined through the non-linear regression principle (Figure 1). The results demonstrated that aqueous extract of Parkia speciosa has a growth inhibitory effect on 4T1 mammary cancer cells with the IC50 value of 312.5 ± 1.20 ug/ml.
 |
Figure 1: The inhibitory concentrations (IC50) of the extract against 4T1 mammary cancer cells. |
Acute toxicity study results
Mortality
At 50, 300 nor 2000 mg/kg body weight of aqueous seed extract of Parkia speciosa demonstrated no fatality in female C57BL/6 mice for a single treatment in 14-days of study.
Physical observations
The physical parameters such as skin and fur colour in all treated mice have no deviations during the study as shown in Table 3.
Table 3: Physical observations in C57BL/6 mice at all groups after a single administration of aqueous seed extract of Parkia speciosa.
| Observations | 0 – 30 minutes | 1 -2 hrs | 4 – 8 hrs | 24 hrs | 7th day | 14th day |
| Skin colour | N | N | N | N | N | N |
| Fur colour | N | N | N | N | N | N |
Note: N (Normal)
Behavioural observation
There was no abnormal behavioural characteristic such as the central nervous system (CNS) excitation and depression observed in controls and treatment groups, respectively throughout 14 days of study. All animals showed normal behaviours and without changes in mood during the study after the administration of the extract. The animals also did not show any CNS stimulatory side effects for example tremors, twitches, convulsion, and CNS depressant effects included sedation, catatonia, and ataxia.
Respiratory effect
Table 4 illustrates the respiratory system demonstrated no respiratory effects such as dyspnoea or apnoea. This indicating absents of respiratory distress in the mice by the single dose of PSAE.
Table 4: Respiratory effects of aqueous seed extract of P. speciosa in C57BL/6 mice in all groups.
| 0-30 minutes | 1-2 hrs | 4 – 8 hrs | 24 hrs | 7th day | 14th day | |
| Dyspnoea | Nil | Nil | Nil | Nil | Nil | Nil |
| Apnoea | Nil | Nil | Nil | Nil | Nil | Nil |
Relative body and organ weight
The relative body weights and organ weight gain comparison of mice throughout 14 days’ experiment in an acute oral toxicity study of aqueous seed extracts of P. speciosa are presented in Table 5 and Table 6, respectively. Mice in medium dose (300 mg), high dose (2000 mg), and control groups were increased in body weight after a week of the study. However, mice in the low dose (50 mg) group showed a slight decline in their body weight, but it was increasing until the end of the study. Nevertheless, the minimal weight change in the treated groups was revealed no significant difference between the control groups’ body weight changes patterns throughout the experiment. The relative organ weights of C57BL/6 female mice are shown in Table 6. There was an insignificant difference (p > 0.05) in organs relative weights of liver, kidney, lungs, heart, brain, ovaries, and spleen which indicate that aqueous extract of P. speciosa seeds did not produce any demonstrable toxic effects on the organs in the mice.
Table 5: The effect of P. speciosa seed extracts on the body weight (mean ± SEM) (n = 4) of C57BL/6 mice.
| Day | Groups | ||||
| Low Dose
(50 mg) |
Medium Dose
(300 mg) |
High Dose
(2000 mg) |
Vehicle | Control | |
| 0 | 19.70 ± 0.61a | 18.70 ± 0.81 a | 19.28 ± 0.77 a | 18.48 ± 0.88 a | 18.93 ± 0.41 a |
| 7 | 19.53 ± 0.54 a | 19.05 ± 0.56 a | 19.90 ± 0.61 a | 18.83 ± 0.50 a | 18.76 ± 0.63 a |
| 14 | 19.65 ± 0.60 a | 19.90 ± 0.38 a | 19.85 ± 0.47 a | 19.83 ± 0.50 a | 19.22 ± 0.37 a |
*Values in the same row with similar superscript letters do not significantly different from each other (p > 0.05).
Table 6: The relative organs weights (mean ± SEM) (n = 4) of C57BL/6 mice in acute oral toxicity of aqueous extract of Parkia speciosa seeds.
| Organs | Groups | ||||
| Low Dose
(50 mg) |
Medium Dose
(300 mg) |
High Dose
(2000 mg) |
Vehicle | Control | |
| Liver | 4.44 ± 0.03 a | 4.38 ± 0.36 a | 4.54 ± 0.10 a | 4.62 ± 0.22 a | 4.90 ± 0.15 a |
| Kidney | 1.19 ± 0.04 a | 1.23 ± 0.04 a | 1.25 ± 0.05 a | 1.26 ± 0.05 a | 1.30 ± 0.03 a |
| Lungs | 0.91 ± 0.03 a | 0.95 ± 0.07 a | 0.89 ± 0.06 a | 0.84 ± 0.10 a | 0.77 ± 0.05 a |
| Heart | 0.58 ± 0.03 a | 0.60 ± 0.02 a | 0.57 ± 0.06 a | 0.65 ± 0.08 a | 0.63 ± 0.05 a |
| Brain | 2.10 ± 0.08 a | 2.19 ± 0.07 a | 2.07 ± 0.07 a | 2.02 ± 0.07 a | 2.05 ± 0.09 a |
| Ovaries | 0.58 ± 0.11 a | 0.70 ± 0.04 a | 0.72 ± 0.12 a | 0.68 ± 0.11 a | 0.42 ± 0.03 a |
| Spleen | 0.33 ± 0.03 a | 0.38 ± 0.03 a | 0.33 ± 0.002 a | 0.36 ± 0.05 a | 0.37 ± 0.01 a |
*Values in the same row with similar superscript letters do not significantly different from each other (p > 0.05).
Haematological and serum biochemical analyses
The results showed insignificant differences (p > 0.05) in the haematological parameters of treatment groups between the control group (Table 7).
Table 7: The haematology values (mean ± SEM) (n = 4) of C57BL/6 mice in acute oral toxicity of aqueous extract of P. speciosa seeds.
|
Parameters |
Groups | |||||
| Low Dose
(50 mg) |
Medium Dose
(300 mg) |
High Dose
(2000 mg) |
Vehicle | Control | ||
| RBC | 8.30 ± 0.81 a | 7.73 ± 0.50 a | 8.25 ± 0.33 a | 7.93 ± 0.22 a |
7.80 ± 0.12 a |
|
| Hb | 13.33 ± 0.27 b | 11.05 ± 0.49 a | 13.48 ± 0.36 b | 13.55 ± 0.46 b | 12.73 ± 0.23 b | |
| PCV | 37.00 ± 0.82 a | 35.03 ± 2.89 a | 37.55 ± 1.16 a | 37.98 ± 1.28 a | 35.78 ± 0.84 a | |
| MCV | 44.65 ± 0.51 a | 45.35 ± 0.82 a | 45.68 ± 0.73 a | 47.83 ± 0.37 b | 45.45 ± 0.45 a | |
| MCHC | 36.50 ± 0.58 a | 29.63 ± 5.98 a | 35.93 ± 0.47 a | 35.70 ± 0.29 a | 35.60 ± 0.23 a | |
| Platelet | 45.75 ± 1.93 a | 82.00 ± 39.10 a | 80.00 ± 12.43 a | 166 ± 90.68 a | 65.50 ± 13.77 a | |
*Values in the same row with similar superscript letters do not significantly different from each other (p > 0.05).
Differential white blood cell count values showed an insignificant (p > 0.05) difference among treatment and control groups as shown in Table 8.
Table 8: The differential white blood cell (WBC) count (mean ± SEM) (n = 4) of C57BL/6 mice in acute oral toxicity of aqueous extract of Parkia speciosa seeds.
| Parameters | Groups | |||||
| Low Dose
(50 mg) |
Medium Dose
(300 mg) |
High Dose
(2000 mg) |
Vehicle | Control | ||
| Total White Blood Cells | 4.25 ± 0.58 a | 3.45 ± 0.42 a | 6.0 ± 0.65 a | 5.05 ± 1.12 a | 3.95 ± 0.58 a | |
| Neutrophils | 0.25 ± 0.10 a | 0.17 ± 0.06 a | 0.27 ± 0.11 a | 0.18 ± 0.05 a | 0.22 ± 0.06 a | |
| Lymphocytes | 3.27 ± 1.10 ab | 3.10 ± 0.34 a | 5.64 ± 0.11 b | 0.18 ± 0.05 ab | 0.22 ± 0.06 ab | |
| Monocytes | 0.09 ± 0.6 a | 0.05 ± 0.03 a | 0.07 ± 0.04 a | 0.19 ± 0.17 a | 0.03 ± 0.006 a | |
| Eosinophils | 0.00 ± 0.00 a | 0.03 ± 0.02 a | 0.04 ± 0.02 a | 0.03 ± 0.02 a | 0.00 ± 0.00 a | |
| Basophils | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | |
*Values in the same row with similar superscript letters don’t significantly different from each other (p > 0.05)
Vital parameter for serum biochemistry in acute toxicity study including both kidney and liver parameters (Table 9). For both parameters, there were insignificant differences (p > 0.05), suggest that both organs were unaffected by acute administration of P. speciosa seed extract.
| Parameters | Groups | ||||
| Low Dose
(50 mg) |
Medium Dose
(300 mg) |
High Dose
(2000 mg) |
Vehicle | Control | |
| Urea | 23.50 ± 0.29 ab | 22.50 ± 2.60 ab | 18.75 ± 3.33 a | 21.75 ± 1.31 ab | 22.10 ± 0.85ab |
| Creatinine | 0.23 ± 0.03 a | 0.20 ± 0.00 a | 0.25 ± 0.05 a | 0.23 ± 0.03 a | 0.33 ± 0.08 a |
| ALT | 134.75 ± 9.04 b | 98.00 ± 18.89 ab | 136.75 ± 13.10 b | 101.07 ± 10.91 a | 128.75 ± 5.12 b |
| TP | 8.25 ± 1.33 a | 7.13 ± 1.64 a | 5.63 ± 0.61 a | 6.90 ± 0.48 a | 6.40 ± 0.23 a |
| Albumin | 3.03 ± 0.03 ab | 2.70 ± 0.21 ab | 2.53 ± 0.45 a | 3.55 ± 0.44 b | 3.10 ± 0.13 ab |
| Globulin | 5.30 ± 1.35 a | 4.45 ± 1.46 a | 3.10 ± 0.18 a | 3.38 ± 0.10 a | 3.23 ± 0.13 a |
Table 9: The serum biochemistry analyses (mean ± SEM) (n = 4) of C57BL/6 mice in acute oral toxicity of aqueous extract of P. speciosa seeds.
*Values in the same row with similar superscript letters don’t significantly different from each other (p > 0.05).
Histopathological analysis
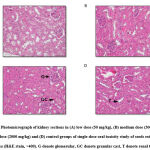
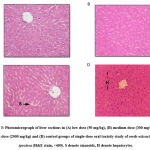
Histopathological evaluations of the kidney and liver of all the mice were conducted based on parameters such as granular cast, cellular cast, protein cast, necrosis (pyknotic nucleus), inflammation, and hydropic degeneration in the kidney. Meanwhile, in the liver, the histopathological lesions, for instance, activated Kupffer cells, necrosis (karyolysis and eosinophilic cytoplasm), necrosis (pyknotic nucleus), degeneration (hydropic degeneration) and inflammation were observed. There were no pathological changes observed in both kidney and liver tissues in the group of mice treated with extract of P. speciosa.
Discussion
Herbs or plants nowadays are used to cure various ailments and problems, including acute and chronic conditions, for instance, cardiovascular disease, prostate problems, depression, and inflammation. Unfortunately, certain phytochemical compounds in plants, when consumed, might pose a lethal threat to humans and animals16. Data on P. speciosa, like many other commonly consumed plants, are scant, especially on their efficacy and possible toxicity16. As a result, determining the toxicological effects of any plant extract intended for use in animals or human medicine is an important aspect of risk assessment, and the extract should not be harmful to the consumers. Consequently, assessing the toxicological effects of any plant extract intended for use in animals or human medicine is a crucial part of risk assessment, and it should have no toxic effects or severe interfering effects on consumers17. The cytotoxic effect of PSAE and was measured based on the minimum extract concentration that provides at least 50% of the cancer cell survivability (IC50). According to United State National Cancer Institute (USNCI) plant cytotoxic classification, there are four extract categories: very active (IC50 ≤ 20 μg/mL), moderately active (IC50 > 20–100 μg/mL), weakly active (IC50 > 100–1000 μg/mL) and inactive (IC50 > 1000 μg/mL). The MTT assay revealed that the aqueous extract of Parkia speciosa was weakly active (IC50 = 312.5 ± 1.20 ug/ml) against 4T1 mammary cancer cells. The presence of phenolic, flavonoid and terpenoid compounds was discovered in a qualitative phytochemical analysis of an aqueous extract of Parkia speciosa seeds. Terpenoids compounds such as β-sitosterol, stigmasterol, lupeol, campesterol, and squalene were detected in Parkia speciosa seeds using gas chromatography in a previous study11 . Breast cancer, ovarian cancer, lung cancer, stomach cancer, leukaemia, prostate cancer, and colon cancer are only a few cancers that these compounds have demonstrated anticancer properties against in both humans and animals18. The previous study has researched botanical compounds, such as β-sitosterol, for use in chemotherapy as potential future therapies, in which it expressed variable action mechanisms in cell culture, including cell cycle, apoptosis, proliferation, survival, invasion, angiogenesis, metastasis, and inflammation19. Besides, lupeol and stigmasterol are anti-angiogenic compounds that inhibit cancer formation by downregulating inflammatory cytokine production, macrophage recruitment, and tumour angiogenesis20. Lupeol is also a pharmacologically active triterpenoid that accounts for 0.71% of the total fatty acid content of Parkia speciosa. It has antinociceptive, anti-inflammatory, and anti-mutagenic properties and inhibits nitric oxide production, iNOS and COX-2 expression21. Furthermore, the squalene found in Parkia speciosa is known for its antioxidant, tumour reducing and anticancer properties22. Lastly, thiazolidine-4-carboxylic acid found in Parkia speciosa seed has been reported to have antiproliferative effects against cancer cells as it inhibits the endogenous formation of carcinogenic N-nitroso compounds23.
PSAE revealed the intense colour as per the flavonoid qualitative test. According to another study, kaemferol is the most common flavonol in the diet and has some therapeutic qualities as a breast cancer chemotherapy treatment21,24. 25mentioned that kaempferol not only had in vitro anti-breast cancer activity, but it also produced in vivo anti-cancer effects specifically by inhibiting cell proliferation, inhibit cell migration, and triggering apoptosis. According to 12, ethanol extract of empty Parkia speciosa pod had higher total flavonoid and total phenolic contents as compared to aqueous extract of Parkia speciosa. Gallic acid, catechin, ellagic acid, and quercetin were the most abundant phenolic constituents in Parkia speciosa extract. Flavonoids such as quercetin, myricetin, luteolin, kaempferol, and apigenin were also found in the ethanol extract of P. speciosa seeds13. Even though the aqueous extraction of Parkia speciosa seeds exhibited lesser bioactive compounds than other solvents, the extract nevertheless was mild-weakly active (IC50 = 312.5 ± 1.20 ug/ml) against 4T1 mammary cancer cells. Further in vivo research is needed to better understand the action mechanism of PSAE with the involvements of the animal immune system and microenvironment in breast cancer. Normal cell line cytotoxicity studies can be performed on the selectivity index (SI) to identify selective anti-cancer activity in the aqueous extract of Parkia speciosa seeds.
The aqueous extract of P. speciosa seed was relatively non-toxic at a single dose of 2000 mg/kg in the current study. Upon administering aqueous extract at a dose of 2000 mg/kg, all-female mice displayed neither mortality nor behavioural changes such as mood, central nervous system excitation, or depression throughout the entire 14-day observation timeframe. In line with the Globally Harmonised System of Classification and Labelling of Chemicals, PSAE belonged to Category 5, and its LD50 value was more than 2000mg/kg. This LD50 value was similar to other studies on aqueous seed extracts, for instance, Myrsine Africana26, Vigna Unguiculata27, Parkia biglobosa28, and Parkia speciosa ethanolic leaf extract29. In contrast to previous studies, certain herb plants such as Atropa belladonna, Hyoscyamus niger, and Cannabis indica demonstrated potential neurotoxic effects on the animals30.
The increase or decrease in body weight is another indicator of drug and chemical adverse effects31. Bodyweight changes are the result of drug and chemical adverse effects. It will be deemed a statistically significant animal if the loss of body weight exceeds 10% of the original body weight. The non-significant changes in relative body weights in all groups of mice in this study indicated that the administration of the extract had little effect on the animals’ growth. Since receiving an oral dose of aqueous extract of P. speciosa seeds, all mice gained weight normally, with no significant distinctions between the control and treated groups. Low-dose (50 mg) animals, on the other hand, lost weight within the first week, and although they began to gain weight after a week, their mean body weight remained lower than the control mean during the analysis. Despite this, the study concluded that losing weight had a statistically insignificant effect. This mild insignificant weight loss may be attributed to the body’s natural physiology adaptation responses to plant extract compounds. In a different experiment in Calycotome villosa (Poiret) Link (subsp. intermedia)32, an acute oral toxicity study revealed a substantial reduction in body weight gain in the treatment groups. Furthermore, based on the relative organ weights of the liver, kidney, lungs, heart, brain, ovaries, and spleen, the aqueous extract of P. speciosa seeds did not demonstrate toxic effects.
The toxic levels of chemical compounds in the blood can be observed by altering various haematological indices33. Any reduction or rise in blood cell counts and their elevation beyond the acceptable reference range could equally demonstrate haematoxicity34. The haematological component is one of the pathological parameters used in analysing pathogenic processes or pharmacological responses to therapeutic intervention33. In this study, all the treated groups’ haematology results, including RBC, haemoglobin concentration, MCV, MCHC, and platelet counts, showed insignificant differences (p > 0.05) compared to the control group. There were also insignificant differences (p > 0.05) in the WBC count and differential white blood cell count in leukocyte parameters. A study on aqueous extract of P. biglobosa seeds, on the other hand, found deviations in PCV, MCH, and MCHC, as well as a significant decrease in platelet and WBC levels28.
In plant toxicological studies, the use of blood serum or plasma enzymes as a marker to detect organ damage in the kidneys and liver, specifically cell destruction, enzyme induction, activation, or inhibition of enzymes. Numerous blood tests could be used to assess the level of tissue injury, identify potential target organs and evaluate organ function degradation. Besides, the kidneys and liver are major organs with a critical role in the body’s metabolic detoxification28.
Nephrotoxicity occurs if the kidneys cannot excrete urine and body waste materials, commonly due to any drugs or toxins with a nephrotoxic effect34. The total protein, albumin, globulin, urea, and creatinine levels were used to evaluate the kidney function, and elevation of these markers can indicate nephrotoxicity16. There were no significant differences (p > 0.05) in all kidney function parameters of the treated and control groups. In comparison, in plant-extract-induced impairment of renal function, for example, elevated creatinine level and presence of significant renal lesions in an acute toxicity study of Aquilaria malaccensis leaf extract16 and a significant decrease of urea level in the serum post administration of aqueous extract of Myrsine africana seeds26.
Hepatotoxicity is liver damage caused by chemicals, drugs, herbs, or dietary supplements35. The clinical symptoms of the liver injury include abdominal discomfort, nausea, vomiting, changes in urine and stool colour, jaundice, hive, recurrent weariness, weakness, exhaustion, and pyrexia are the clinical symptoms of liver damage35. There will be increased ALT, AST, ALP, and bilirubin levels in the case of liver injury, but a decrease of albumin level in the serum biochemistry analyses of the animal. Liver function testing in this study revealed no significant differences (p > 0.05) in alanine aminotransferase (ALT) and albumin between the control and P. speciosa treatment groups. With all of the justifications, it was possible to conclude that PSAE is non-hepatotoxic at a dosage of 2000 mg/kg.
In addition to behavioural and haematological analyses for toxicity research, histopathological analysis provides supporting data for biochemical and haematological assessments. Besides kidneys, the liver is the essential organ in detoxification and metabolism since it excretes most toxicants and metabolic wastes17. There was no evidence of toxicity in the kidney and liver tissues, supported by insignificant results from the haematological parameters of treated mice throughout the 14-day experiment. This implies that at a dose up to 2000 mg/kg, PSAE is likely to be non-toxic to the kidneys and liver. After oral administration of extracts at doses of 430 mg, 700 mg, and 1480 mg, groups treated with M. christia vespertillionis extract demonstrated a significant difference (p < 0.05) in hepatic lesion characterized by mild to moderate necrosis. Mild hepatic degeneration and necrosis were also significant (p < 0.05) despite no significant difference (p > 0.05) in liver function tests (total protein, albumin, globulin, and alanine aminotransferase).
Conclusion
In conclusion, the findings of in vitro and in vivo studies conveyed that an aqueous extract of Parkia speciosa seeds was safe; there were no deleterious effects on C57BL/6 mice even at doses up to 2000 mg/kg for 14 days after a single-dose treatment of PSAE. Additionally, PSAE is weakly active cytotoxic to the 4T1 mammary cancer cells, with a minimum inhibitory concentration (IC50) of 312.5 ± 1.20 ug/ml. Sub-acute and sub-chronic PSAE should be performed in more mouse models to determine the safety and possible toxicity effects of a higher dose and long-term intake.
Acknowledgment
This study was supported by the Ministry of Higher Education Malaysia via the Fundamental Research Grant Scheme for Research Acculturation of Early Career Researchers (FRGS-RACER) with grant number RACER/1/2019/SKK15/UMK/1. The authors are thanks Prof Dr. Nik Soriani Yaacob from the School of Medical Sciences, Universiti Sains Malaysia (USM) for support, especially in cell culture works.
Conflict of interest
The authors declare that there is no conflict of interest in this research.
Funding Sources
This study was funded by the Ministry of Higher Education Malaysia via Fundamental Research Grant Scheme for Research Acculturation of Early Career Researchers (FRGS-RACER) with grant number RACER/1/2019/SKK15/UMK/1.
References
- Kim, J., & Park, E. (2002). Cytotoxic anticancer candidates from natural resources. Current Medicinal Chemistry-Anti-Cancer Agents, 2(4), 485-537.
CrossRef - Nordin, S. F., Nordin, M. L., Osman, A. Y., Hamdan, R. H., Shaari, R., & Arshad, M. M. (2017). The effect of Matricaria Chamomilla L on the growth performance of red hybrid tilapia. Biomedical and Pharmacology Journal, 10(4), 1905-1915.
CrossRef - Nile, S. H., & Park, S. W. (2014). Edible berries: Bioactive components and their effect on human health. Nutrition, 30(2), 134-144.
CrossRef - Demain, A. L., & Vaishnav, P. (2011). Natural products for cancer chemotherapy. Microbial Biotechnology, 4(6), 687–699.
CrossRef - Samuel, A. J. S. J., Kalusalingam, A., Chellappan, D. K., Gopinath, R., Radhamani, S., Husain, H. A., Promwichit, P. (2010). Ethnomedical survey of plants used by the Orang Asli in Kampung Bawong, Perak, West Malaysia. Journal of Ethnobiology and Ethnomedicine, 6, 1–6.
CrossRef - Kamisah, Y., Othman, F., Qodriyah, H. M. S., & Jaarin, K. (2013). Parkia speciosa hassk.: A potential phytomedicine. Evidence-Based Complementary and Alternative Medicine, 2013.
CrossRef - Jin, C. B., & Noor, H. (2008). The hypoglycemic effect of aqueous seed extract of Parkia speciosa on rats. J Trop Med Plants, 9(1), 39-42.
- Suvachittanont, W., Kurashima, Y., Esumi, H., & Tsuda, M. (1996). Formation of thiazolidine-4-carboxylic acid (thioproline), an effective nitrite-trapping agent in human body, in Parkia speciosa seeds and other edible leguminous seeds in Thailand. Food Chemistry, 55(4), 359–363.
CrossRef - Aisha, A. F. A., Abu-Salah, K. M., Alrokayan, S. A., Ismail, Z., & Abdul Majid, A. M. S. (2012). Evaluation of antiangiogenic and antioxidant properties of Parkia speciosa Hassk extracts. Pakistan Journal of Pharmaceutical Sciences, 25(1), 7–14.
- Tapas, A., Sakarkar, D., & Kakde, R. (2008). Flavonoids as Nutraceuticals: A Review. Tropical Journal of Pharmaceutical Research, 7(3), 1089–1099.
CrossRef - Mohd Azizi, C. Y., Salman, Z., Nik Norulain, N., & Mohd Omar, A. (2008). Extraction and identification of compounds from Parkia Speciosa seeds by supercritical carbon dioxide. Journal of Chemical and Natural Resources Engineering, 2, 153–163.
- Ko, H. J., Ang, L. H., & Ng, L. T. (2014). Antioxidant activities and polyphenolic constituents of bitter bean Parkia speciosa. International Journal of Food Properties, 17(9), 1977-1986.
CrossRef - Chhikara, N., Devi, H. R., Jaglan, S., Sharma, P., Gupta, P., & Panghal, A. (2018). Bioactive compounds, food applications and health benefits of Parkia speciosa (stinky beans): A review. Agriculture and Food Security, 7(1), 1–9.
CrossRef - Firenzuoli, F., & Gori, L. (2007). Herbal medicine today: Clinical and research issues. Evidence-Based Complementary and Alternative Medicine, 4(SUPPL. 1), 37–40.
CrossRef - Mustafa, N. H., Ugusman, A., Jalil, J., & Kamisah, Y. (2018). Anti-inflammatory property of Parkia speciosa empty pod extract in human umbilical vein endothelial cells. Journal of Applied Pharmaceutical Science, 8(1), 152–158.
- Razak, R. N. H. A., Rahman, S. A., Hamdan, A. H., Ramli, R., Isa, M. L. M., Muhammad, H., & Hassan, N. F. N. (2019). Evaluation of acute and sub-acute oral toxicity of the aqueous extract of Aquilaria malaccensis leaves in Sprague Dawley rats. Asia-Pacific Journal of Molecular Biology and Biotechnology, 27(1), 20–32.
CrossRef - Naidu, J. R., Ismail, R., & Sasidharan, S. (2014). Acute oral toxicity and brine shrimp lethality of methanol extract of Mentha Spicata L (Lamiaceae). Tropical Journal of Pharmaceutical Research, 13(1), 101-107.
CrossRef - Awad, A. B., Chinnam, M., Fink, C. S., & Bradford, P. G. (2007). β-Sitosterol activates Fas signaling in human breast cancer cells. Phytomedicine, 14(11), 747–754.
CrossRef - Bin Sayeed, M. S., & Ameen, S. S. (2015). Beta-Sitosterol: A Promising but Orphan Nutraceutical to Fight Against Cancer. Nutrition and Cancer, 67(8), 1216–1222.
CrossRef - Kangsamaksin, T., Chaithongyot, S., Wootthichairangsan, C., Hanchaina, R., Tangshewinsirikul, C., & Svasti, J. (2017). Lupeol and stigmasterol suppress tumor angiogenesis and inhibit cholangiocarcinoma growth in mice via downregulation of tumor necrosis factor-α. PLoS One, 12(12), e0189628.
CrossRef - Chen, Y. F., Ching, C., Wu, T. S., Wu, C. R., Hsieh, W. T., & Tsai, H. Y. (2012). Balanophora spicata and lupeol acetate possess antinociceptive and anti-inflammatory activities in vivo and in vitro. Evidence-Based Complementary and Alternative Medicine, 2012.
CrossRef - Naziri, E., Mantzouridou, F., & Tsimidou, M. Z. (2011). Squalene resources and uses point to the potential of biotechnology. Lipid Technology, 23(12), 270–273.
CrossRef - Chen, J., Wang, Z., Lu, Y., Dalton, J. T., Miller, D. D., & Li, W. (2008). Synthesis and antiproliferative activity of imidazole and imidazoline analogs for melanoma. Bioorganic & medicinal chemistry letters, 18(11), 3183–3187.
CrossRef - Chen, A. Y., & Chen, Y. C. (2013). A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chemistry, 138(4), 2099–2107.
CrossRef - Wang, X., Yang, Y., An, Y., & Fang, G. (2019). The mechanism of anticancer action and potential clinical use of kaempferol in the treatment of breast cancer. Biomedicine and Pharmacotherapy, 117 (May), 109086.
CrossRef - Kabubii, Z. N., Mbaria, J., & Mathiu, M. (2015). Acute toxicity studies of Myrsine africana aqueous seed extract in male Wistar rats on some hematological and biochemical parameters. Clinical Phytoscience, 1(1), 1-4.
CrossRef - Gv, N., Pullaiah, C. P., S, D., & Reddy, D. (2017). Acute Toxicity Studies of Aqueous Seed Extract of Vigna Unguiculata in Albino Rats. Innovare Journal of Ayurvedic Sciences, 5(February), 1.
- Ekperikpe, U. S., Owolabi, O. J., & Olapeju, B. I. (2019). Effects of Parkia biglobosa aqueous seed extract on some biochemical, haematological and histopathological parameters in streptozotocin induced diabetic rats. Journal of Ethnopharmacology, 228, 1–10.
CrossRef - Al Batran, R., Al-Bayaty, F., Jamil Al-Obaidi, M. M., Abdualkader, A. M., Hadi, H. A., Ali, H. M., & Abdulla, M. A. (2013). In Vivo Antioxidant and Antiulcer Activity of Parkia speciosa Ethanolic Leaf Extract against Ethanol-Induced Gastric Ulcer in Rats. PLoS ONE, 8(5), 2–12.
CrossRef - Wattanathorn, J., Uabundit, N., Itarat, W., Mucimapura, S., Laopatarakasem, P., & Sripanidkulchai, B. (2006). Neurotoxicity of Coscinium fenestratum stem, a medicinal plant used in traditional medicine. Food and chemical toxicology, 44(8), 1327-1333.
CrossRef - Raza, M., Al-Shabanah, O. A., El-Hadiyah, T. M., & Al-Majed, A. A. (2002). Effect of prolonged vigabatrin treatment on hematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Scientia Pharmaceutica, 70(2), 135–145.
CrossRef - Lyoussi, B., Tangi, K. C., Morel, N., Haddad, M., & Quetin-Leclercq, J. (2018). Evaluation of cytotoxic effects and acute and chronic toxicity of aqueous extract of the seeds of Calycotome villosa (Poiret) Link (subsp. intermedia) in rodents. Avicenna journal of phytomedicine, 8(2), 122.
- Arika, W. M., Nyamai, D. W., Musila, M. N., Ngugi, M. P., & Njagi, E. N. M. (2016). Hematological markers of in vivo toxicity. Journal of Hematology & Thromboembolic Diseases.
- Dioka, C., Orisakwe, O. E., Afonne, O. J., Agbasi, P. U., Akumka, D. D., Okonkwo, C. J., & Ilondu, N. (2002). Investigation into the haematologic and hepatotoxic effects of rinbacin in rats. Journal of Health Science, 48(5), 393–398.
CrossRef - Fatima, N., & Nayeem, N. (2016). Toxic effects as a result of herbal medicine intake. Toxicology-New Aspects to This Scientific Conundrum. London, UK: InTech Open, 193-207.
CrossRef