Manuscript accepted on :18-12-2021
Published online on: 27-12-2021
Plagiarism Check: Yes
Reviewed by: Dr Nurul Diyana Sanuddin
Second Review by: Dr. de Souza Zanotta Dumit, Sara
Final Approval by: Dr. Ian James martin
Reda Hallab1* , Nouzha Ben Raïs Aouad2, khalida Eddaoui2and Hmad Ouabi3
, Nouzha Ben Raïs Aouad2, khalida Eddaoui2and Hmad Ouabi3
1Doctoral Study Center for Life and Health Sciences, Faculty of Medicine and Pharmacy of Rabat, Impasse Souissi, Rabat 10100, Morocco.
2Nuclear Medicine Department, IBN SINA Hospital - Ibn Sina Rabat University Hospital Center, Avenue Abderrahim Bouabid, Rabat.
3PET SCAN RABAT, Al Azhar Oncology Center, Angle, Rue Ouezzane, Rue Idriss Al Azhar, Rabat.
Corresponding Author E-mail: reda_hallab@um5.ac.ma
DOI : https://dx.doi.org/10.13005/bpj/2284
Abstract
The quality assurance program ensures that the entire radiological system and associated equipment are functioning properly and optimally. To this end, it is essential that a quality assurance program be in place in each medical facility where ionizing radiation sources are used, to verify the proper functioning of these instruments as well as the radionuclides measured in nuclear medicine. In addition, the procedures of the quality assurance program must comply with regulatory requirements and international recommendations. The method of this study is to review the regulatory requirements adopted by different countries regarding the quality assurance program procedures as well as various recent scientific works and those published by the International Atomic Energy Agency. In addition, to compare the radiation protection requirements of the procedures of the mentioned works; exposure justification and optimization, quality control, registration system, professional training and audit system and to suggest improvements. The result of the review study, add a procedure to the quality assurance program, so that the quality assurance attempts to cover all procedures involving sources of ionizing radiation, thus ensuring compliance with the standards of radiological safety in nuclear medicine facilities.
Keywords
Nuclear Medicine; Quality assurance program; Radiation protection; Radiation safety; Regulatory requirements
Download this article as:| Copy the following to cite this article: Hallab R, Aouad N. B. R, Eddaoui K, Ouabi H. Regulatory Requirements of Quality Assurance Program in Nuclear Medicine – Review of the Procedures. Biomed Pharmacol J 2021;14(4) . |
| Copy the following to cite this URL: Hallab R, Aouad N. B. R, Eddaoui K, Ouabi H. Regulatory Requirements of Quality Assurance Program in Nuclear Medicine – Review of the Procedures. Biomed Pharmacol J 2021;14(4) . Available from: https://bit.ly/3FIuxLe |
Introduction
Quality assurance is defined as all planned and methodical actions necessary to assure that a structure, system or component will work successfully in service according to the International Organization for Standardization (ISO); indeed, satisfactory performance in diagnostic nuclear medicine implies optimal quality of the entire process1. Indeed, the regulatory requirements at the national level, in particular Law 142-122, encourage the obligation to define and implement a quality assurance program in an installation using sources of ionizing radiation. During this study, we have reviewed various regulatory requirements from different countries (France, Belgium, and Switzerland) and the published scientific work of the International Atomic Energy Agency (IAEA), which states that an optimal quality of the whole process and the procedures of the quality assurance program ensure satisfactory performance in nuclear medicine, particularly in diagnostic and therapeutic areas1. Review of the procedures made it possible to have a global perspective of radiation protection requirements in a nuclear medicine facility and to try to find avenues for improvement for the procedures of the quality assurance. During this research, recommendations were made to encompass the full range of quality assurance in nuclear medicine.
Procedures of Quality Assurance Program
Justification
Various works reviewed in this paragraph encourage that the principle of justification be present in the quality assurance program, specifically; the procedures implementing the principle of justification are present in the said program: the stages from receipt of the request act until the decision to execute it3. According to the principle of justification, a practitioner in a medical establishment (nuclear medicine) can only practice if it is justified4. Various points are identified while prescribing an examination in a medical facility (nuclear medicine), such as the patient’s identity, the assessment of the advisability of executing the radiological act, the presence of any contraindications, and good patient preparation5. Medical applications of ionizing radiation must be justified in terms of radiation protection and safety by comparing the advantages of exposure against the risk of radiation harm and examining alternative procedures that do not entail medical exposure6.
Optimisation
The application of the requirements of the optimization principal procedures is present in the quality assurance program, specially:
Identification the roles and responsibilities of each function of the stakeholders involved in the implementation of optimization procedures3;
The written procedures by type of act as well as the methods of their preparation3;
The processes for treating high-risk patient groups, such as women of childbearing age, pregnant women, and children, as well as persons with problems that necessitate periodic examinations3. The essential precautions about the importance of notifying the existence or possibility of pregnancy in a woman undergoing a radiological examination, or in the event of the administration of radioactive products to the nursing woman4.
Quality control
The protocols for checking medical devices after receipt and before use, as well as the procedures for performing medical device maintenance and quality control, included when a change in software version has an impact on the dose or image quality3. The quality assurance program, in accordance with international standards and recommendations, must include all quality control modalities, including radiopharmaceuticals7. Quality control procedures must be performed on a regular and planned basis, and they must include acknowledged useful technologies. It is important that the facilities using ionizing radiation sources adhere to applicable requirements instituted from regulatory bodies, the purpose it’s to be in compliance at all times8. Quality control is a critical for routine nuclear medicine practice9.
Training of professionals
According to some of the work reviewed, professional training is critical in the quality assurance program, specifically: the training and qualification procedures for professionals are specified in the quality assurance program for any newcomer when changing their position, workstation, or medical equipment3. Implementation of appropriate training for all professionals with responsibility for quality assurance. It also includes the implementation of continuing education sessions to keep employees up to date for the benefit of professionals participating in the medical facility’s quality assurance8.
Record system
The quality assurance program provides for the development of a recording system, which will be beneficial in the event of a material, human, or organizational incident that is likely to result in the accidental or unintentional exposure of individuals during a medical imaging procedure3. The recording system includes the dates of detection and recording of the incident, a description of the event, the circumstances surrounding its occurrence and its consequences, and mechanisms for notifying the exposed person or his representative3. The system also provides records of equipment performance, radiopharmaceutical and nuclear medicine equipment monitoring results, any performance deviations or difficulties detected as well as the established corrective actions8.
Audit system
The quality assurance program should include the audit system1. Il is divided into three phases: self-assessment, internal clinical audit, and external clinical audit, the clinical audit must be documented at each step4. Quality Management Audits in Nuclear Medicine (QUANUM) is a program developed by the International Atomic Energy Agency to assist its member states in auditing the status of their nuclear medicine practices and their adherence to international standards, the program covers all aspects of nuclear medicine, in particularly quality assurance and control of instrumentation10.
Review of the procedures to the assurance quality program
Reviewing the procedures of the quality assurance program for the different regulatory requirements and scientific work. This revealed that most of the procedures are similar for the different works; it is suggested to add the review and assessment phase in the quality assurance program as a step that assesses all the program procedures8. To do this, an organization chart is proposed listing all the procedures of the quality assurance program:
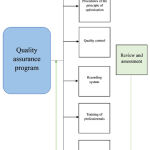 |
Figure 1: Flow Chat of procedures of the quality assurance program |
Conclusion
The adoption of a quality assurance system allows the establishment of a radiation protection culture, thus ensuring radiation protection within a nuclear medicine or radiography facility. Furthermore, this is in accordance with regulatory requirements and international recommendations. During our review of the various regulatory requirements of the procedures of the quality assurance program and the scientific work as well as the evaluation of all the procedures of the said program. It was concluded that most of the reviewed works take into account most of the radiation safety and radiation protection requirements of the procedures of the quality assurance program. The result was the addition of the review and evaluation phase to the said program (flowchart established) according to the reviewed works. Its importance lies mainly in the improvement of medical procedures using ionizing radiation sources in nuclear medicine. This improvement must be continuous, which will minimize the risk of adverse events occurring in a nuclear medicine facility. For future research, we recommend that the regulatory requirements for quality assurance of radioactive waste generated by nuclear medicine facilities (imaging and therapy) be reviewed.
Acknowledgement
I thank all the contributors who helped me in this work; Hmad OUABI, Khalida Eddaoui, Pr. Nouzha Benraiss Aouad
I am asking for some of your precious time to inform you of the submission of my scientific article, to participate in the development of medical science in general and nuclear medicine in particular.
It will be a great pleasure and a great honor for me to publish in your prestigious journal.
Pending a favorable response, please accept, dear organizers, the expression of my sincere thanks.
Conflict of Interest
All authors declare that there is no conflict of interest
Funding Sources
There are no funding source.
References
- Ortiz López P. Applying Radiation Safety Standards in Nuclear Medicine. Safety Reports Series No. 40. IAEA VIENNA. 2005 ;
- Government of Kingdom of Morocco. Law 142-12, Related to Nuclear and Radiological Safety and Security and the Creation of Moroccan Agency for Nuclear and Radiation Safety and Security. 2014;
- French Republic. Nuclear Safety Authority. Quality assurance obligations in medical imaging using ionizing radiation. DC-0660. 2019;
- Federal Agency for Nuclear Control. Medical exposures and exposures for non-medical imaging purposes with medical radiological equipment. Royal Decree. 2020;
- Lucio M. Quality assurance and patient safety in nuclear medicine. International Journal of Radiology Research. Volume 2; Issue 1; 2020. Page No. 11-13;
- Sang Hyun P, Min Kyung K, Sang-eun H et al. Quality control to manage unsealed radioactive sources in nuclear medicine. Progress in Nuclear Science and Technology. Volume 6, (2019), pp. 26-29;
CrossRef - Skovorodko K, Urbanavičiūtė R. Implementation of a quality assurance program and quality control results of radiopharmaceuticals. International Atomic Energy Agency (IAEA). 2020. INIS Volume 52. INIS Issue 04 ;
- Phyllis S, Donald R. Recommendations for Quality Assurance Programs in Nuclear Medicine Facilities. Radiation Recommendations Series. 1984 Oct. p 4 – 8;
- Kheruka S. C. Importance of quality control in nuclear medicine. International Atomic Energy Agency (IAEA). 2020. INIS Volume 52. INIS Issue 23;
- Maurizio D, Leonel T, Mario M, et al. Comprehensive Auditing in Nuclear Medicine Through the International Atomic Energy Agency Quality Management Audits in Nuclear Medicine Program. Part 2: Analysis of Results. Semin Nucl Med. Elsevier Inc. 2017 Feb. 0001-2998.







