Manuscript accepted on :08-12-2021
Published online on: 28-12-2021
Plagiarism Check: Yes
Reviewed by: Dr. Mohanraj Subramanian
Second Review by: Dr. Hanefi ÖZBEK
Final Approval by: Dr. Ayush Dogra
Faizan Naeem Razali* , Nur Syahirah Izzati Rani
, Nur Syahirah Izzati Rani , Muhammad Imran Kamil Mazian, Ahmad Naeem Mohd Nafi and Siti Hajar Musa
, Muhammad Imran Kamil Mazian, Ahmad Naeem Mohd Nafi and Siti Hajar Musa
Faculty of Pharmacy and Health Sciences, Universiti Kuala Lumpur-Royal College of Medicine Perak, (UniKL-RCMP), 30450 Ipoh, Perak, Malaysia
Corresponding Author E-mail: faizan@unikl.edu.my
DOI : https://dx.doi.org/10.13005/bpj/2310
Abstract
The polysaccharide isolated from Solanum nigrum was proven to possess an immunomodulatory effect and able to suppress the progression of tumor cells by proxy. However, data on the toxicity profile is still limited. The present preclinical study was conducted to investigate the toxicity potential of the crude polysaccharide sample. The acute toxicity experimental design was adapted from OECD 423 guideline. Nine female BALB/c mice were randomly divided into 3 groups, 3 mice per group (n=3). Mice in group A (first-step treatment) were orally administered with a single treatment of crude polysaccharide sample at concentration 2,000 mg/kg/bw (300 µL). Mice in group B (second-step treatment) were received the single treatment after 24 hours, depending on the observation of mice in group A. Mice in group C served as control. Mortality and clinical signs associated with toxicity were observed within 24 hours of treatment session and for the subsequence 14 days for delay-death detection. Mice body weight was recorded starting at day-0 until day-14 prior to sacrificing at day-15. Blood, liver, and kidney were harvested for toxicology assessment. Within 24 hours of treatment, 1 mouse in group A was found to died, while no mortality and delay-death were observed in groups B and C. Referring to OECD 423, it was estimated that the LD50 of the treated sample was 2,500–5,000 mg/kg/bw. No significant changes (p<0.05) were detected in terms of body weight and organ weight indexes of the treated mice as compared to control. The polysaccharide treatment also revealed no significant elevation in mice serum glucose levels. The present findings indicated that the treatment of crude polysaccharide sample exerted a very mild acute toxicity effect when orally administered at 2,000 mg/kg/bw, with no delay-death.
Keywords
Acute toxicity; OECD Guideline; Plant Polysaccharide; Solanum Nigrum; Toxicology
Download this article as:| Copy the following to cite this article: Razali F. N, Rani N. S. I, Mazian M. I. K, Mohd-Nafi A. N, Musa S. H. Preclinical Acute Toxicity Assessment of a Crude Polysaccharide Isolated from the Stem of Solanum Nigrum: A Preliminary Analysis. Biomed Pharmacol J 2021;14(4). |
| Copy the following to cite this URL: Razali F. N, Rani N. S. I, Mazian M. I. K, Mohd-Nafi A. N, Musa S. H. Preclinical Acute Toxicity Assessment of a Crude Polysaccharide Isolated from the Stem of Solanum Nigrum: A Preliminary Analysis. Biomed Pharmacol J 2021;14(4). Available from: https://bit.ly/3EwX5Gs |
Introduction
Herbal medicines have been utilized and are still being used in developing countries as the primary source of medicinal treatment. Plenty of scientific evidence has proven the potential of terrestrial plant extracts for the preparation of evident-based treatments against various diseases 1. Even though many believe that the utilization of medicinal plant products recognized as ‘natural’ as treatments for diseases, it does not mean that it is entirely safe to be consumed. In medicinal production, secondary metabolites that have been extracted from plants such as saponins, terpenoids, cyanogenic, tannins, toxic amino acids, glycosides, and alkaloids were widely used as a part of active ingredients in pharmaceutical formulation 2. Toxicology studies need to be conducted as it may provide knowledge on the plant in terms of their toxicity capability and precaution that need to be taken for the utilization pharmaceutical industry3.
Solanum nigrum is an herbal plant that widely grows and is distributed throughout temperate climate zones to the tropical region of Asia and the Southern hemisphere, from sea level to altitudes over 3,500 meters 4. S. nigrum is commonly used as traditional folk medicine and is believed to have various biological activities such as anti-cancer, anti-septic, anti-dysenteric and wound healing properties 5. In the previous studies, the S. nigrum polysaccharide fraction, SN-ppF3 was proven to have immunomodulatory activities where it could classically activate macrophage cells through the NF-κB transduction signaling pathway and indirectly suppressed the proliferation of breast cancer cells in tumor-induced BALB/c mice 6,7,8. These documented health benefits of the S. nigrum plant products can be utilized in various pharmaceutical applications. However, information regarding toxicity capability is still limited. Although natural source phytoconstituents are familiar to have little to no toxicity effect, the continuous consumption of the compounds could draw follow-up health implications. Thus, this study was carried out to deduce the sub-acute toxicity information of the S. nigrum crude polysaccharide extract at the preclinical level.
Materials and Methods
Materials
General chemicals used in this study were analytical standard grade and were purchased from Sigma-Aldrich company unless specifically stated. Information on the instruments used was provided in the paragraph.
Methods
Sample preparation
Fresh plants of Solanum nigrum L. nigrum were purchased from the local market located at Bangi, Selangor, Malaysia. The S. nigrum plant was previously identified and authenticated by Dr. Sugumaran Manickam from Institute of Biological Sciences, Faculty of Science, University of Malaya, Kuala Lumpur, Malaysia, and a voucher specimen was deposited at the Rimba Ilmu Herbarium (Herbarium number: KLU 47872). The crude polysaccharide samples were extracted according to the previously described method 6. Briefly, polysaccharide was extracted from dried, ground stems of S. nigrum by refluxing the sample with 2 L of petroleum ether (60°C–80°C), followed with 2 L of 80% ethanol. The residue was then boiled in 2 L of 95°C water for 5 hours. The polysaccharide in the filtrate of the boiled mixture was precipitated out with an equal volume of 70% ethanol, overnight at 4°C. Next, the crude polysaccharide was collected through centrifugation at 2,300 ×g for 5 minutes. After that, the crude polysaccharide sample was washed twice with 95% ethanol. The crude polysaccharide was allowed to dry in a desiccator for approximately 7 days or until it completely dried. The crude polysaccharide was stored at 4°C for further use.
Estimation of carbohydrate content
The phenol-sulfuric acid assay was carried out to detect and estimate the carbohydrate content in the crude polysaccharide samples. Exactly 1,000 µL of 1 mg/mL of crude polysaccharide sample was pipetted into a glass test tube. Then, the sample was mixed with 1,500 µL sulfuric acid and 300 µL of 5% phenol. The solution was heated in a 95°C water bath for 5 minutes. The presence of carbohydrates was indicated as the appearance of a yellow color solution. To determine the amount of carbohydrate content, the solution was assessed through a spectrophotometer at 490 nm wavelength. Then, the absorbance value was extrapolated in a standard curve of D-glucose 9. The chemical test was conducted at least in triplicates (n=3).
Estimation of protein content
The protein content in the crude polysaccharide sample was estimated by using the Bradford assay. Basically, 1,000 µL of a 1 mg/mL crude polysaccharide sample was mixed with 500 µL of Bradford reagent in a glass test tube. The solution was incubated in a dark ambiance for 10 minutes, allowing the reaction to happen. The presence of protein was indicated as the appearance of the blue color solution. A spectrophotometer was used to measure the absorbance at a spectrum wavelength of 595 nm. The protein content in the crude polysaccharide of S. nigrum was estimated by extrapolating the absorbance value in a standard curve of bovine serum albumin. The chemical test was conducted at least in triplicates (n=3).
Preparation of preclinical analyses
Nine female BALB/c mice (5–6 weeks old) weighing around 28–30 g were purchased from Sinar Scientific Sdn. Bhd. located in Sri Kembangan, Selangor, Malaysia. Mice were acclimatized for at least 14 days prior to the experiment. Mice were kept in cages under controlled environmental conditions of temperature 25±1°C and 12 hours/12 light/ dark cycle and supplied with standard food pellets and tap water, ad libitum. The principles and guidelines for animal care, research and animal sacrificed protocols were in accordance with animal ethical clearance approved by the Universiti Kuala Lumpur Institute of Medical Science Technology (UniKL-MESTECH) Animal Ethics Committee (Ref.: AEC/MESTECH-UNIKL/2020/001/MAY-2020-NOV-2022).
Acute toxicity study
The acute toxicity study was carried out in accordance with OECD 423 guidelines. Nine healthy mice were randomly divided into 3 groups consisting of 3 mice in each group (n=3). Mice in group A were single-time treated with 300 µL of 2,000 mg/kg crude polysaccharide as the first-step treatment 10. For the second-step treatment, mice in group B were receiving a similar treatment regimen as in group A, if there is 0 mortality or only 1 mouse died. The treatment dose will be adjusted if mortality of more than 1 mouse is recorded. Mice in group C were served as control that received only the vehicle, 300 µL of normal saline. The overall treatment design was referred to a workflow as suggested by OECD 423 guideline. The effect of the treatment sample was determined within the first 24 hours of the treatment session and alive animals continue to be observed (without treatment) within 14 days for delay-death detection. At the end of the session, the LD50 of the sample dose was determined.
Behavioral observation of the treated mice
The morbidity and mortality of the treated mice were observed every 1 hour within 12 hours after the first-step treatment session and continued to observe for the next 24 hours. This observation was conducted to detect possible toxicological symptoms as suggested by OECD 423 guideline. The weight of each mouse was recorded daily starting on day-0 until day-14. The physical observation was carried out to indicate any sign associated with toxicity that arises in terms of survival, food intake, fur, skin, eyes, and any bizarre behaviors. On day-15, mice were sacrificed through cervical dislocation. Blood and vital organs (kidney and liver) were harvested for the next subsequence toxicology assessments.
Hematology assessment on serum glucose level
The blood sample was withdrawn through cardiac puncture and a portion of the collected blood was assessed in terms of its serum glucose level by using a standard glucometer. Index of serum glucose level for treated mice was calculated and compared to the control group.
Vital organ assessment
The harvested liver and kidney were weighted using a weighing balance. The weight for each vital organ was recorded and the organ weight indexes were calculated based on the following formula:
Next, the calculated organ weight index of group A and B were compared to the control group mice. The harvested organs were then preserved in 15 mL of 10% formalin solution for further histopathological analysis.
Statistical analysis
The one-way analysis of variance (ANOVA) was used to analyze all collected data, with the significant difference between data means determined by Duncan’s multiple range test at 95% confidence level (p<0.05) with a minimal number of replication (n=3) using SPSS 17.0 Statistic software. All graphs and standard curves were constructed by using GraphPad Prism 5 software.
Results and Discussion
Percentage of extraction yield of crude polysaccharide
The crude polysaccharide was extracted by using the Soxhlet extraction method with two different solvents in a separate extraction process. Soxhlet extraction method utilizes the principle of reflux and siphoning to continuously extract the crude with clean solvent. Table 1 shows the yield of crude polysaccharides obtained from 107.44 g of dry stems of S. nigrum. The total weight of crude polysaccharides was 1.98 g. Throughout the process, 1.84% of crude polysaccharides were successfully be extracted. The yield percentage of isolated crude polysaccharide was higher as compared to another study that only yielded around 1.30% of crude polysaccharides via maceration method 11. The difference is probably due to the type of extraction technique applied. The Soxhlet extraction method is an automatic continuous extraction technique that can produce larger yields that requires less time and smaller solvent consumption compared to other conventional methods such as maceration 12. It was suggested that the Soxhlet extraction method is the preferable method used to extract crude polysaccharides as compared to the maceration technique due to high extraction efficiency.
Table 1: Yield of crude polysaccharide extracts isolated from the stem of Solanum nigrum.
| Weight of dry stem (g) | Weight of crude polysaccharide (g) | Percentage extraction yield (%) |
| 107.44 | 1.98 | 1.84 |
Carbohydrate content in crude polysaccharide sample
The phenol-sulfuric acid method is frequently used colorimetric approach to detect the presence of sugar compound and estimate the carbohydrate level contained in the sample 13. The amount of carbohydrate can be quantitatively estimated through the color intensity detection by using a spectrophotometer and extrapolation against the standard curve of D-glucose 9. Referring to Table 2, the estimated percentage of the carbohydrate content of crude polysaccharides was around 46.30%. The percentage of carbohydrate content was considered low as compared to another study, which estimated around 63.60% 14. However, the present finding is assumed to reveal a decent amount of carbohydrate content as it was reported that the carbohydrate content ranging in between 8.38% to 67.17% is categorized under the polysaccharide group for a crude sample that belongs to the family of Solanaceae plant 14. Therefore, the crude polysaccharide of S. nigrum is assumed to be majorly composed of carbohydrates. In an effort of obtaining a greater percentage of the carbohydrate content in the crude polysaccharide sample, it was suggested that reducing the boiling time of the sample could elevate the carbohydrate content. The influence of boiling contributes to the breakage of weak bonds between polysaccharides and the cleavage of glycosidic bonds, which may result in the solubilization of low molecular weight carbohydrates due to thermal degradation 15.
Table 2: Estimation of carbohydrate content in Solanum nigrum crude polysaccharide sample.
| Concentration of crude polysaccharide (µg/mL) | Absorbance (OD) | Concentration of carbohydrate (mg/mL) | Mean ± SD (mg/mL) | Percentage of carbohydrate content (%) |
| 1,000 | 1.027 | 389.42 | 462.9 ± 108.1 | 46.3% |
| 1.582 | 587.00 | |||
| 1.091 | 412.21 |
Data expressed was mean ± standard deviation (n=3).
Protein content in crude polysaccharide sample
The protein content from the isolated crude polysaccharide was estimated by using the Bradford assay. As a positive indication, the solution turned into the blue color indicating the presence of protein 16. Data in Table 3 revealed that the protein content in the crude polysaccharide sample was estimated at around 34.7%. The result was higher as compared to the other study that shows only 3.26% of crude protein content. According to the 14, the crude protein contents are commonly varied from 2.39% to 6.45% in the reproductive stage among Solanaceae. Thus, the crude polysaccharide was considered higher in protein content. This is due to the crude polysaccharides being not purely polysaccharides and may contain glycoproteins and some other polar components. It was suggested that further sample purification can be performed to remove protein and some other unwanted components by using ion-exchange column chromatography. Crude sample can be resolved through diethylaminomethyl cellulose as the column stationary phase, and it was showed that the total content of protein in semi-purified polysaccharide fractions were less than 1% and indicating the absent of glycoproteins 6. The crude polysaccharide sample is expected to possess the very least protein amount, concerning protein derivatives such as glycoproteins in most Solanaceae plant families could exert significant toxicity capability.
Table 3: Estimation of protein content in Solanum nigrum crude polysaccharide sample.
| Concentration of crude polysaccharide (µg/mL) | Absorbance (OD) | Concentration of protein (mg/mL) | Mean ± SD (mg/mL) | Percentage of protein content (%) |
| 1,000 | 0.464 | 0.683 | 0.495 ± 0.211 | 34.6% |
| 0.182 | 0.268 | |||
| 0.364 | 0.535 |
Data expressed was mean ± standard deviation (n=3).
Acute toxicity evaluation
Behavioral observation and mice body weight
Behavioral changes and body weight are preliminary standard signs of toxicity caused by numerous chemicals and drugs 17. Within 24 hours of the first-step treatment session, one mouse expressed changes in its behavior such as less active, sleepy eyes, fast breathing compared to control groups. That mouse was reported dead (Table 4). The remaining mice were behaved normally as no signs of changes in the fur, salivation, and diarrhea, indicating no toxicity implication 18. Mice in second-step treatment did not show any kind of abnormal behaviors within 24 hours after the administration of the treatment with crude polysaccharide. No significant changes in general appearance or behavioral pattern were observed till the end of 14 days experimental period (Table 5).
Table 4: Acute toxicity potential on mortality of treated mice.
| Group | Dose (mg/kg/bw) | Number of mice in cage | +Number of dead mice | *Mortality rate (%) |
| A (SN-CP) | 2,000 | 3 | 1 | 33.3 |
| B (SN-CP) | 2,000 | 3 | 0 | 0 |
| C (Control) | – | 3 | 0 | 0 |
+Within 24 hours of treatment session. *Mortality rate (%) = (Number of dead mice after treatment / number of treated mice) × 100. SN-CP = S. nigrum crude polysaccharide. (A): First-step treatment. (B): Second-step treatment.
Table 5: Behavioral observations for control and polysaccharide-treated groups.
| Groups | First-step treatment
(SN-CP 2,000 mg/kg/bw) |
Second-step treatment
(SN-CP 2,000 mg/kg/bw) |
Control | |||
| *Observation | 24 hours | 14 days | 24 hours | 14 days | 24 hours | 14 days |
| Activity | Inactive | +Active | Active | ++Active | Active | ++Active |
| Eyes | Sleepy | +Normal | Normal | ++Normal | Normal | ++Normal |
| Appetite | Normal | +Normal | Normal | ++Normal | Normal | ++Normal |
| Fur | Normal | +Normal | Normal | ++Normal | Normal | ++Normal |
| Breathing | Normal | +Normal | Normal | ++Normal | Normal | ++Normal |
| Salivation | Absent | +Absent | Absent | ++Absent | Absent | ++Absent |
| Diarrhea | Absent | +Absent | Absent | ++Absent | Absent | ++Absent |
One mouse was found died within 24 hours of first-step treatment. Observation was continued to day-14 for the remaining alive tested mice, +n=2 and ++n=3. *Observation of clinical signs suggested in OECD 423 Guideline. SN-CP: S. nigrum crude polysaccharide.
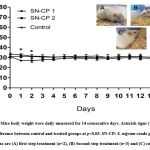
The change in body weight is an indicator of mice’s health deterioration after the initial sample administration. Dramatical decline greater than 10% of the initial body weight is imposed to toxicity indication 19. In this study, mice body weights were measured from day of dosing (day-0) until day-14. There was a significant difference in the changes of the body weight from day 0 to day-2 in the first-step treatment due to one mouse was found dead on that day. The bodyweight showed a slight reduction in the first two days and then it was maintained until the end of the treatment (Figure 1). A documented literature stated that herbal extracts can help to suppress the appetite of animals and thus reduce animal body weight 19. Reduction in body weight is often correlated with normal physiological body responses towards plant extract compounds 20. Overall, there were no significant differences in the in changes of the body weight between treated mice and control mice until the end of the observation. Therefore, it was suggested that the treatment of S. nigrum crude polysaccharide exerted almost non-toxic and did not induce any harmful changes on the mice to body weights.
Treatment effect on mice survival
Acute toxicity test measures the adverse effects that occur within a short period of time, commonly 24 hours after a single dose of a substance is administered. For this acute toxicity study, the crude polysaccharide of S. nigrum was administered according to the suggested experimental design of OECD 423 guideline. Referring to Table 4, one mouse was found dead at the tested sample dose of 2,000 mg/kg within 24 hours of sample administration. The study was continued with the second-step treatment, where group B mice (n=3) were administered with crude polysaccharides at a similar sample dose as in the first-step treatment. After 24 hours of the treatment session, no mortality was recorded. No delay death was also recorded as mice remained alive until 14 days of the treatment session. In this study, the LD50 of the treatment dose was determined as in between 2,500 to 5,000 mg/kg/bw. The toxicity level can be categorized as non-toxic or harmless as it executed no acute toxicity effect toward tested animals 21. The claim was also supported by the chemical labelling and classification of acute systemic toxicity based on oral LD50 values; extremely toxic, < 5 mg/kg body weight; toxic, > 5 < 50 mg/kg; harmful, > 50 < 500 mg/kg; and no label, > 500 < 2,000 mg/kg 22. Thus, the lethal effect presented in this treatment suggested that the crude polysaccharide of S. nigrum implied very mild toxicity given orally at a dose of 2,000 mg/kg.
Vital organ assessment
The vital organs were harvested after 14 days of treatment and the data in Table 6 has shown no significant difference (p<0.05) of the organ weight indexes for mice in the crude polysaccharide-treated group as compared to the control group. Significant changes towards organ weight index indicate toxicity implication as the treatment could cause organ damage and series of inflammation. Significant increment of mice liver weight index was reported when exposed to toxicants 23,24. The present findings suggested that oral treatment of S. nigrum crude polysaccharide was not causing deterioration of vital organs (liver and kidney) weight indexes. However, further histopathology assessment is necessary to be conducted to confirm the preliminary claim.
Table 6: Vital organ weight indexes of crude polysaccharide treated mice.
| Group | Dose
(mg/kg/bw) |
**Body weight (g) | *Liver weight index (mg/g) | *Kidney weight index (mg/g) |
| A (SN-CP) | 2,000 | +29.50 ± 1.50a | 35.93 ± 0.19b | 12.54 ± 0.05c |
| B (SN-CP) | 2,000 | ++30.39 ± 3.40a | 31.26 ± 0.32b | 11.85 ± 0.04c |
| C (Control) | – | ++30.57 ± 0.85a |
43.18 ± 0.13b |
11.45 ± 0.04c |
*Organ body index (mg/g) = organ weight (mg) / body weight (g). Data expressed were mean ± standard deviation (+n=2, ++n=3). Superscripted letters (a, b and c) across row indicated no significant differences at p<0.05. **Body weight of mice was measured at the last day-14. SN-CP: S. nigrum crude polysaccharide.
Effect of treatment on serum glucose level
It was reported earlier that the polysaccharide sample composed of high carbohydrate content (Table 2) and prolonged consumption might affect the regulation of serum glucose level. Mice serum glucose level was measured by a glucometer at day-15, and data in Table 7 has shown no significant differences (p<0.05) of serum glucose level index of sample-treated groups as compared to the control group. It was suggested the single treatment of crude polysaccharide samples at doses of 2,000 mg/kg did not affect the regulation of serum glucose levels. However, as the current study was conducted onto healthy test subject with no diabetic history, further investigation is necessary to be performed on test subjects with the diabetic conditions to confirm the effect of crude polysaccharide samples on serum glucose regulation 25.
Table 7: Serum glucose index of polysaccharide-treated mice.
|
Group |
Treatment dose
(mg/kg/bw) |
*Serum glucose level index (mmol/L/g) |
| A (SN-CP) | 2,000 | +0.24 ± 3.39d |
| B (SN-CP) | 2,000 | ++0.24 ± 2.79d |
| C (Control) | – | ++0.28 ± 0.07d |
*Index of serum glucose to mice body weight at day-15. Data expressed were mean ± standard deviation. Superscripted letter (d) across row indicated no significant differences at p<0.05 (+n=2, ++n=3). SN-CP: S. nigrum crude polysaccharide.
Conclusion
Data gathered in the present study revealed that a single treatment of crude polysaccharide isolated from the stem of S. nigrum at dose 2,000 mg/kg/bw was not exerting acute toxicity towards BALB/c mice. It was reported that only one mouse was found dead in the first-step treatment, while the rest of the test subjects were alive with no delay-death recorded. Referring to the study workflow suggested by OECD 423 guideline, LD50 of the crude polysaccharide sample was determined between 2,500 to 5,000 mg/kg/bw. The single treatment caused insignificant changes in mice behavior, body weight index and vital organ indexes as compared to the control group.
Acknowledgment
The authors were grateful to the Ministry of Higher Education (MOHE), Malaysia for funding this present research under the Fundamental Research Grant Scheme (FRGS) (Ref: FRGS/1/2019/STG04/UNIKL/02/1). The authors also thank Universiti Kuala Lumpur, Royal College of Medicine Perak (UniKL-RCMP) for providing research facilities.
Conflict of interest
The authors wish to confirm that there is no conflict of interest associated with this publication.
Funding source
The present study was funded by the Ministry of Higher Education (MOHE), Malaysia under the Fundamental Research Grant Scheme (FRGS) (Ref: FRGS/1/2019/STG04/UNIKL/02/1).
References
- Sivaraj, R., Rahman, P. K., Rajiv, P., Narendhran, S. and Venckatesh, R. Biosynthesis and characterization of Acalypha indica mediated copper oxide nanoparticles and evaluation of its antimicrobial and anticancer activity. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy., 2014; 129: 255–258.
CrossRef - Dai, J. and Mumper, R. J. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules., 2010; 15(10): 7313–7352.
CrossRef - Mounanga, M.B., Mewono, L. and Angone, S. A. Toxicity studies of medicinal plants used in sub-Saharan Africa. Journal of Ethnopharmacology., 2015; 174: 618–627.
CrossRef - Huang, H. C., Syu, K. Y. and Lin, J. K. Chemical composition of Solanum nigrum linn extract and induction of autophagy by leaf water extract and its major flavonoids in AU565 breast cancer cells.Journal of Agricultural and Food Chemistry., 2010; 58(15): 8699–
CrossRef - Edmonds, J. M. and Chweya, J. A. Black nightshades: Solanum nigrum L. and related species, in: Promoting the conservation and use of underutilized and neglected crops, Vol. 15. Rome: Institute of Plant Genetics and Crop Plant Research, Gatersleben / International Plant Genetic Resources Institute (1997).
- Razali, F. N., Ismail, A., Abidin, N. Z. and Shuib, A. S. Stimulatory effects of polysaccharide fraction from Solanum nigrum on RAW 264.7 murine macrophage cells. PloS One., 2014; 9(10): e108988.
CrossRef - Razali, F. N., Sinniah, S. K., Hussin, H., Abidin, N. Z. and Shuib, A. S. Tumor suppression effect of Solanum nigrum polysaccharide fraction on breast cancer via immunomodulation. International Journal of Biological Macromolecules., 2016; 92: 185–193.
CrossRef - Razali, F. N., Shuib, A. S. and Abidin, N. Z. Prediction of signaling pathway induced by solanum nigrum polysaccharide fraction, SN-ppF3 in activating RAW 264.7 macrophage cells. Malaysian Journal of Biochemistry and Molecular Biology., 2019; 22(2): 6–11.
- Chen, J. R., Yang, Z. Q., Hu, T. J., Yan, Z. T., Niu, T. X., Wang, L., Cui, D. A. and Wang, M. Immunomodulatory activity in vitro and in vivo of polysaccharide from Potentilla anserina. Fitoterapia., 2010; 81(8): 1117–1124.
CrossRef - Roy, S., Ukil, B. and Lyndem, L. M. Acute and sub-acute toxicity studies on the effect of Senna alata in Swiss Albino mice. Cogent Biology., 2016; 2(1): 1–11.
CrossRef - Mazher, M., Anjum, M., Mushtaq, W., Noshad, Q. and Malik, N.Z. Antifungal assay of Solanum nigrum Linn. fruit, leaves and stems extracts in different solvents. International Journal of Biosciences., 2017; 10(4): 380–385.
CrossRef - Handa, S. S. An overview of extraction techniques for medicinal and aromatic plants. Extraction Technologies for Medicinal and Aromatic Plants., 2008; 1: 21–40.
- Sadasivam, S. and Manickam, A. Phenol sulphuric acid method for total carbohydrate. Biochemical Methods., 2005.
- Hameed, I. and Hussain, F. Proximate and elemental analysis of five selected medicinal plants of family Solanaceae. Pakistan Journal of Pharmaceutical Sciences., 2015; 28(4): 1203–1215.
- Svanberg, S. M., Nyman, E. M. G. L., Andersson, R. and Nilsson, T. Effects of boiling and storage on dietary fibre and digestible carbohydrates in various cultivars of carrots. Journal of the Science of Food and Agriculture., 1997; 73(2): 245–254.
CrossRef - Kielkopf, C. L., Bauer, W. and Urbatsch, I. L. Bradford assay for determining protein concentration. Cold Spring Harbor Protocols., 2020; 2020(4): 102269.
CrossRef - Ezeja, M. I., Anaga, A. O. and Asuzu, I. U. Acute and sub-chronic toxicity profile of methanol leaf extract of Gouania longipetala in rats. Journal of Ethnopharmacology., 2014; 151(3): 1155–1164.
CrossRef - Rajeh, M. A. B., Kwan, Y. P., Zakaria, Z., Latha, L. Y., Jothy, S. L. and Sasidharan, S. Acute toxicity impacts of Euphorbia hirta L extract on behavior, organs body weight index and histopathology of organs of the mice and Artemia salina. Pharmacognosy Research., 2012; 4(3): 170–177.
CrossRef - Raza M., Al-Shabanah O. A., El-Hadiyah T. M. and Al-Majed A. A. Effect of prolonged vigabatrin treatment on hematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Scientia Pharmaceutica., 2002; 70(2): 135–145.
CrossRef - Rosidah, Y., Sadikun, A., Ahmad, A., Akowuah, G. A. and Asmawi, M. Z. Toxicology evaluation of standardized methanol extract of Gynuraprocumbens. Journal of Ethnopharmacology., 2009; 123(2): 244–249.
CrossRef - Organisation for Economic Co-Operation and Development (OECD) 423. OECD Guidelines for Testing of Chemical: Acute Oral Toxicity-Acute Toxic Class Method., 2008; 1–14.
- Kausar, S. H. and More, V. R. Determination of medium lethal dose (LD50 value) for oral acute toxicity of royal jelly. World Journal of Pharmacy and Pharmaceutical Sciences., 2019; 8(6): 475–481.
- Chebaibi, M., Bousta, D., Chbani, L., Iken, I. and Achour, S. Evaluation of acute toxicity of plants’ mixture used in traditional treatment of kidney diseases in Morocco. Pharmacognosy Research., 2019; 11(2): 155–161.
CrossRef - Kaware, M. Changes in liver and body weight of mice exposed to toxicant. International Research Journal of Science and Engineering., 2013; 3(1): 92–95.
- Zhang, L., Liu, Y., Ke, Y., Liu, Y., Luo, X., Li, C., Zhang, Z., Liu, A., Shen, L., Chen, H. and Hu, B. Antidiabetic activity of polysaccharides from Suillellus luridus in streptozotocin-induced diabetic mice. International Journal of Biological Macromolecules., 2018; 119: 134–140.
CrossRef