Sally A El Awdan and Gihan F. Asaad*
and Gihan F. Asaad*
Pharmacology Department, National Research Center, ElBohoos St. Dokki, Giza 12622, Egypt
Corresponding Author E-mail:dr_g.asaad@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2283
Abstract
Liver fibrosis is considered: “a pathological repairing process in liver injuries leading to extracellular cell matrix (ECM) accumulation evidencing chronic liver diseases”. Chronic viral hepatitis, alcohol consumption, autoimmune diseases as well as non-alcoholic steatohepatitis are from the main causes of liver fibrosis (Lee et al., 2015; Mieli-Vergani et al., 2018). Hepatic stellate cells (HSCs) exist in the sinus space next to the hepatic epithelial cells as well as endothelial cells (Yin et al., 2013). Normally, HSCs are quiescent and mainly participate in fat storage and in the metabolism of vitamin A. HSCs are produced during liver injury and then transformed into myofibroblasts. The activated HSCs resulted in a sequence of events considered as marks fibrosis. The activation of HSCs mostly express alpha smooth muscle actin (α-SMA). Moreover, ECM is synthesized and secreted by HSCs that affects markedly the structure and function of the liver tissue leading to fibrosis (Tsuchida et al., 2017; Han et al., 2020). Hence, activated HSCs are attracting attention as potential targets in liver fibrosis. Many signaling molecules are involved in HSCs activation first and foremost, platelet-derived growth factor (PDGF) and transforming growth factor-beta (TGF-β) (Tsuchida et al., 2017; Wang et al., 2020c) as interfering the PDGF or TGF-β signaling pathways is a growing field for liver fibrosis treatment.
Keywords
Liver fibrosis; Hepatic stellate cells; PDGF; TGF-β; α-SMA
Download this article as:| Copy the following to cite this article: El Awdan S. A, Asaad G. F. Liver Fibrosis: Underlying Mechanisms and Innovative Therapeutic Approach. A Review Article. Biomed Pharmacol J 2021;14(4). |
| Copy the following to cite this URL: El Awdan S. A, Asaad G. F. Liver Fibrosis: Underlying Mechanisms and Innovative Therapeutic Approach. A Review Article. Biomed Pharmacol J 2021;14(4). Available from: https://bit.ly/3mxJkky |
Introduction
The ECM proteins’ – particularly collagen type 1, – accumulation distorts the hepatic architecture through the formation of a fibrous wound, followed by the expansion of spherical areas of cells called nodules then hardening of the tissue. A series of events follows resulting in cirrhosis. Liver cirrhosis causes hepatocellular dysfunction, hepatocellular carcinoma (HCC) and hence, hepatic failure (Giannitrapani et al., 2014).
Liver fibrosis involves parenchymal, non-parenchymal liver cells and infiltrating immune cells. Cell death activates the inflammatory and pro-fibrogenic pathways that triggers fibrosis progression, as well as the possibility of reversing the process that aids in fibrosis resolution (Lee et al., 2015).
Hepatic macrophages, injured hepatocytes, lymphocytes and endothelial cells leads to the induction of HSC. The dead hepatocytes release of reactive oxygen species, damage-associated molecular patterns (DAMPs) which stimulate macrophages (Kupffer cells) to release proinflammatory biomarkers and pro-fibrogenic markrs, (Yang et al., 2012).
Other pro-inflammatory factors include gut-derived pathogen-associated molecular patterns (PAMPs) as well as chemokines like CC-chemokine ligand-2 CCL2. CCL2, also known as monocyte chemotactic peptide-1 (MCP-1), it is considered a member of the beta (C-C) chemokine family. It is expressed in many cell types, including hepatocytes, stellate cells and inflammatory cells (Degre et al., 2012). C-C chemokine receptor type 2 (CCR2) has been found to be the known receptor for CCL2 and it is usually expressed on monocytes, T lymphocytes and basophils (Xu et al., 2019).
From the factors of chronic wound healing is the toll-like receptor 4 (TLR4) activation enhancing TGF-β-dependent HSC activation (Liu et al., 2014). Moreover, oxidative stress plays a very important role in all fibrogenic changes as shown by the overexpression of critical genes related to inflammation and chronic damage of tissues (de Castro Bras et al., 2020).
The increased activity of free radicals, together with the decreased antioxidant defenses result in the occurrence of oxidative stress that significantly contributes to fibrogenesis and excessive tissue remodeling (de Alwis et al., 2008).
Prevalence of Liver Fibrosis in Egypt
Liver diseases with many types lead to liver fibrosis, which leads to further sedimentation of extracellular matrix followed by more complicated liver conditions that leads to death. According to the WHO data in 2018, Liver Disease Deaths in Egypt reached 12.40% of total deaths that ranks Egypt #1 worldwide (WHO, 2018). This report draws the scientists’ attention to explore more new solutions to alleviate liver fibrosis.
Nanomedicine
Nanomedicine is concerned with nanoparticles’(NPs) designing and application in order to diagnose and treat diseases (Silva et al., 2019; Pucek et al., 2020). Nanomedicine is a critical field of nanotechnology research that has greatly affected and aided biomedicine through the past years. These nano formulations have been designed and adjusted as effective therapeutic agents to alleviate liver fibrosis targeting specific sites (Poilil Surendran et al., 2017). Moreover, nanostructures were designed as nano agents for contrast enhancement as nanoprobes to diagnose liver fibrosis (Sheng et al., 2018).
Numerous nanoparticles (NPs) have been intensively scouted for diagnosing and curing hepatic fibrosis such as metal oxide NPs (Sheng et al., 2018), lipid NPs (Jimenez Calvente et al., 2015), polymer NPs (Li et al., 2010), metal NPs (Duong et al., 2015), and protein NPs (Melgert et al., 2000).
The wide-range composition, size, shape and changeable surface properties of NPs makes them unique, as controlled drug release, high divergence, extended bioavailability, reduction of toxic side effects and improvement of the pharmacokinetics of drugs (Petros et al., 2010). An interesting and beneficial significance of nano systems is the possibility to diagnose and treat at the same time (Nagorniewicz et al., 2019).
Human diseases and Nanotechnology
Medical NPs are designed nano-structures from drugs, peptides, proteins, or nucleic acids and are loaded in carriers. Hereafter, NPs are used to resolve the conflict of barriers that conquer the delivery of drugs and imaging labels to targets. These barriers may be attributed to biological, biophysical, and biomedical problems. Transforming the drugs and genes into NPs to target specific cells or tissues via coating or attaching ligands, may alter the therapeutic hypothesis, thus therapeutic agents can reach directly the needed site.
There are marked benefits to use NPs delivery systems as:
Avoiding the degradation of the therapeutic agent by being inactivated or being broke down until it scopes the site of action;
optimizing the pharmacological effects that result in enhancing drugs’ bioavailability
possibility of integration of hydrophobic and hydrophilic molecules;
reduction of drug blood level fluctuations that may subject patients to lower risk of noneffective or toxic doses
decreasing the side effects and toxicity of drugs
controlled drug release that is needed in many pathological conditions;
using different routes of administration
due to the high affinity between the site of interest and the nano drug, active targeting is fulfilled
Liver and spleen are considered to be the chief organs of accumulation of NPs (Moghimi et al., 2001) owing to help them reach the blood and high number of tissue-resident phagocytic cell (Giannitrapani et al., 2014; Mohammadpour et al., 2020).
Common NPs types recruited to treat liver diseases
The NPs used in drug delivery and biomedical studies is promptly increasing. NPs can be categorized into two main categories: inorganic and organic NPs and inorganic NPs have outstanding properties that made them of great interest.
Generally, inorganic NPs constitute a metal oxide as titanium oxide and iron oxide, or metal central core with a surface protective organic layer. This outer layer plays a crucial role in preventing the degradation of the inside content and permitting the pairing of biomolecules with the reactive groups as thiols and amines in order to link proteins, peptides, and folic acid.
Inorganic NPs have significant attention in recent years as they possess many physicochemical properties which are attributed to their different size and material, as compared to organic NPs. They possess unique magnetic, electronic, and optical properties which can be customized by regulating the size, composition, structure, and shape. Due to their inertness, easy functionalization, and stability, they have been considered as an attractive alternative over organic NPs for imaging specific tissues and drug delivery (Ketabat et al., 2019).
Organic Polymeric NPs include numerous sorts of nanoparticles for example; liposomes, solid lipid nanoparticles (SLN), and nanostructured lipid carriers (NLCs) (Figure 1). These biocompatible and biodegradable polymeric NPs are now of great importance as possible drug delivery preparations which can be used as carriers of DNA in gene therapy, administered in drug targeting to particular organs or tissues, and they can deliver peptides and proteins orally.
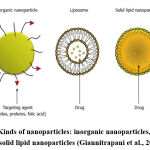 |
Figure 1: Kinds of nanoparticles: inorganic nanoparticles, liposomes and solid lipid nanoparticles (Giannitrapani et al., 2014). |
Biodegradable polymers of natural origin include chitosan, rosin, gelatin and sodium alginate. On the other hand, synthetic polymers include “polylactic acid (PLA), polycaprolactones (PCL), polycyanoacrylates and polyaminoacid conjugates” (Nie et al., 2020; Mohamed et al., 2021).
The US Food and Drug Administration had approved PLA and PLGA biodegradable polymeric nanoparticles. Liposomes are synthetic sphere-shaped vesicles that contains one or more phospholipid bilayers enfolding an aqueous compartment. A variety of hydrophobic and hydrophilic medications can be encapsulated in the dual compartment structure of liposomes where hydrophilic drugs inside the internal aqueous core shaped by the lipid membrane, while hydrophobic drugs can be fused into the bilayer.
Liposomal NPs are the simplest form of NPs that have several advantages as good biocompatibility, easy preparation, increased uptake and reduced systemic toxicity (Datta et al., 2020; Wang et al., 2020a).
Conventional liposomes are immediately removed from the blood circulation and that is rendered to their high affinity for the reticuloendothelial system (RES), that is why they are coated with hydrophilic molecules linked to the liposomal formulation by a lipid anchor so as to prolong liposome circulation over time (Immordino et al., 2006). Consequently, the pharmacological potency has been improved as well as reduction in the used dose.
Solid lipid nanoparticles (SLNs), were introduced in the 1990s (Muller et al., 2000) and they were considered a new carrier system other than liposomes, polymeric nanoparticles and emulsions.
The solid lipid core can include triglycerides, fatty acids, steroids, waxes and glyceride mixtures. SLNs have advantages over the traditional systems as it avoids some of the disadvantages of other types since they are produced easily without the usage of organic solvents, and they can be synthesized on a large scale at low cost (Bondi et al., 2003). Moreover, they do not cause biodegradability problems or toxicity (Puri et al., 2009; He et al., 2020).
NLCs were introduced by late 1990s to improve the capabilities of other previous SLNs generations (Pardeshi et al., 2012; Lin et al., 2021). NLCs are formed by blending liquid lipids and solid lipids. Both SLNs and NLCs are made of biodegradable, physiological and biocompatible lipids and surfactants. NLCs have a higher drug loading capacity when compared with SLNs. Moreover, NLCs possess lower water content, longer physical stability and reduced drug expulsion during storage (Pardeshi et al., 2012; Aggarwal et al., 2021).
Potential Targets of Liver Fibrosis
Choosing the target and the carrier while targeting hepatic ailments should be conferring to the type of the ailment. The hepatoprotective drugs should be directed at the liver cells, while in autoimmune and inflammatory conditions, drugs should be delivered to the Kupffer cells. Moreover, antifibrotic drugs perform their action via targeting the hepatic stellate cell. Erroneous drugs uptake lead to ineffectiveness as well as possible detrimental effects.
Triggered HSCs primarily elaborate during inflammation, angiogenesis, and fibrogenesis responses for the progress of liver fibrosis. Approaches to target HSCs may potentially treat liver fibrosis due to their involvement in the development of the disease. Another strategy includes using anti-inflammatory drugs or collagen deposition inhibitors.
Hepatic stellate cells (HSCs)
Primary modulations in the genetic expression as well as phenotypic changes in HSCs are the initial stage in the development of liver fibrosis. These modulations and changes are rendered to cytokine and chemokine stimulation (Figure 2).
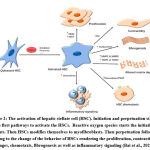 |
Figure 2: The activation of hepatic stellate cell (HSC). Initiation and perpetuation stages are the first pathways to activate the HSCs. |
Cytokines secreted by Kupffer cells play a chief role in the activation of HSCs (Pradere et al., 2013).
When HSCs become activated, they release reactive oxygen species (ROS), NF- κβ and damage-associated molecular patterns (DAMP) which trigger the innate immune response. Thus, leukocytes release lipid peroxides and TNF-related apoptosis inducing ligand (TRAIL) and other ligands (Lan et al., 2020). The activation of the HSCs, results in matrix synthesis, proliferation, and loss of retinoids and all these changes ends by liver fibrosis. As an alternative of causing injury to normal liver cells, those activators enhance the inactive HSCs into activated form. Liver damage induced by carbon tetrachloride in rodent stimulates the HSC and liver fibrosis mechanism (Miao et al., 2019). TGF-β and PDGF are two main cytokines that lead to HSC activation followed by activation of the cells during the progression of liver fibrosis.
Transforming Growth Factor Beta (TGF-β)
Transforming Growth Factor Beta (TGF-β) is: “one of the most potent cytokines to induce fibrogenesis as it activates HSCs”. It has been found that different miRNAs are indulged in the events of liver fibrosis and they control many critical steps in its development. From the intracellular pivotal effectors that has been discussed in affecting TGF-β are the SMAD proteins. (Hellerbrand et al., 1999; Li et al., 2018).
Concerning liver fibrosis SMAD2 and SMAD7 are hepatoprotective while SMAD3 and SMAD4 are profibrotic proteins. The interference in the pathway of SMAD3 blocks the epithelial myofibroblast transition and stops the expression of collagen type 1. Moreover, SMAD4 enhances the responsive promoter effect of SMAD3 resulting in intensifying liver fibrosis.
On the other hand SMAD7 inhibits SMAD3 fibrogenesis so it is an evidence now that various miRNAs participate in the hepatic fibrotic disease and hence targeting TGF-β/SMAD signaling could be an attractive crucial target in alleviating liver fibrosis (Zhang et al., 2020) (Figure 3).
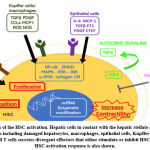 |
Figure 3: Events of the HSC activation. |
Abbreviations: “α-SMA, alpha smooth muscle actin; CTCF, connective tissue growth factor; DAMP, damage associated molecular patterns; ECM, extracellular matrix; CCL2, C-C motif chemokine 2; EMP, extracellular matrix proteins; ERK, extracellular signal-regulated kinase; MCP1, monocyte chemoattractant protein 1; ET1, endothelin 1; HSC, hepatic stellate cell; IFNg, interferon gamma; IGF1, insulin-like growth factor 1; IL-6, interleukin 6; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; ncRNA, noncoding ribonucleic acid; Hh, hedgehog; ROS, reactive oxygen species; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; NOS, nitric oxide synthase; PDGF, platelet-derived growth factor; TGFb, transforming growth factor beta; VEGF, vascular endothelial growth factor” (Chan et al., 2020).
The mitogen-activated protein kinase (MAPK) pathway is also affected by the TGF-β, with extracellular signal-regulated kinase (ERK) and c-jun N-terminal kinase (JNK) changes (Hanafusa et al., 1999). Alpha smooth muscle actin (a-SMA), proteoglycans, as well as collagen with its two types; types I and III are upregulated through induced transcription after their activation (Breitkopf et al., 2006).
The level of both α-SMA as well as the levels of collagen I and III are from the important markers that indicate HSCs activation and the degree of liver fibrosis so as to follow up and monitor the case. The elevation of such markers indicates the aggravation of the liver fibrosis (Tomasek et al., 2002; Schuster-Gaul et al., 2020).
Cells can receive external signals and respond appropriately via the MAPK pathway mainly throughout the epithelial-mesenchymal transition (EMT) (Gui et al., 2012) EMT is an important process during the development of the embryo, fibrosis, and tumor progression in which the epithelial cells attain mesenchymal, fibroblast-like properties and display low intercellular adhesion and increased motility.
TGF-β signaling is the preliminary pathway to trigger EMT, and its relationship with the Smad family is well considered. Previous studied showed that the MAPK and TGF-β signaling pathways relate together and exert a synergistic effect on the release of growth factors and cytokines that endorse EMT” (Gui et al., 2012).
Platelet-Derived Growth Factor (PDGF)
From the most important roles of mitogen PDGF and its receptor PDGF receptor-β (PDGFRβ) is acting on HSCs leading to their proliferation and migration (Wong et al., 1994; Kostallari et al., 2018).
Patients suffering from chronic liver diseases can be monitored and followed up by checking the inflammatory extent of their case which is a reflection and it is linked to the expression of PDGF (Zuo et al., 2019). It I to be noted that in spite of the expression of PDGFRβ mRNA expression is found to be in both quiescent and activated HSCs, only protein production was limited to the activated HSCs cells (Henderson et al., 2013).
Vascular Endothelial Growth Factor (VEGF)
The vascular endothelial growth factor (VEGF) was documented as vascular permeability factor that is formed by fibroblasts and it is well-thought as a signal protein responsible for the initiation and stimulation of blood vessels. They are involved with other mediators in both the de novo formation of the embryonic circulatory system and angiogenesis (Yang et al., 2014).
In liver fibrogenesis, VEGF stimulates the cell proliferation of HSCs, which includes angiogenesis in the distorted liver tissue. VEGF has a multifaceted role in fibrogenesis, liver tissue repair and reversal of fibrosis (Kantari-Mimoun et al., 2015). VEGF also controls the migration of monocytes, penetrability of liver sinusoidal and scar-associated macrophage function, which are considered a fibrotic resolution and tissue repair processes (Yang et al., 2014).
Connective Tissue Growth Factor (CTGF)
Huang et al., 2012 stated that “the connective tissue growth factor (CTGF) is markedly expressed in the liver suffering from fibrosis when it is compared to the normal liver and that it is a potent fibrogenic cytokine that is similar to PDGF and it contributes to the accumulation of ECM resulting in a series of hepatic fibrogenic actions” (Huang et al., 2012). CTGF sensitizes HSCs that mainly produces it. CTGF is considered to be one of the main critical effectors that pushes the production of collagen and its expression is associated with the severity and degree of liver fibrosis (Chen et al., 2015; Choi et al., 2020).
Hedgehog Pathway
Omenetti et al. 2011, indicated that: “The hedgehog (Hh) pathway is an important system in the regulation of progenitor cells’ fate in liver fibrosis and smoothened homolog (SMO) is released and activated by the upregulation of Hh ligands and it drives the epithelial regeneration by promoting mesenchymal-to-epithelial transitions of the myofibroblasts originated from HSCs” (Omenetti et al., 2011). Various studies documented that interfering or targeting the Hh pathway can hinder the liver fibrosis (Greenbaum et al., 2011). The Hh pathway could possess the possible targets of fibrotic treatment (Yan et al., 2020; Deng et al., 2021)
Toll-Like Receptor
“Toll-Like Receptor Dietary or free cholesterol in the liver activates HSCs leading to worsening of liver fibrosis”. The elevated intracellular cholesterol level in HSCs leads to the signaling of Toll-like Receptor (TLR) 4 (Zhang et al., 2021a) leading to the sensitization of HSC to TGF-β-activation (Tomita et al., 2014) and that is why cholesterol-lowering drugs could help alleviate the fibrosis by slowing down the free cholesterol accumulation (Van Rooyen et al., 2013).
Nanomedicine for Liver Fibrosis Therapy
NPs as Therapeutic Agents
The inorganic NPs own a distinct properties that made them as superior medications for targeting the liver fibrosis (Anselmo et al., 2015; Tee et al., 2019). Peng et al., 2018 reported that “titanium dioxide NPs (TiO2 NPs) and silicon dioxide NPs (SiO2 NPs) can prevent the expression of collagen I and α-SMA as well as facilitating the degradation of collagen I via upregulating the matrix metalloproteinases (MMPs) and downregulating the tissue inhibitors of metalloproteinases (TIMPs), leading to the possible antifibrotic activities of these NPs” (Peng et al., 2018).
These NPs also possess an anti-migratory and anti-adhesive effects via regulating gene expression of the epithelial mesenchymal transition (EMT) and work on the reversal of TGF-β-activated HSCs to the inactive state (Figure 4).
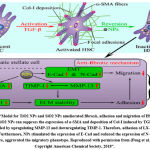 |
Figure 4: Model for TiO2 NPs and SiO2 NPs ameliorated fibrosis, adhesion and migration of HSCs. |
The anti-inflammatory effect of Cerium oxide NPs causes the decrease of liver steatosis, liver fibrosis and portal hypertension (Oro et al., 2016). Oral administration of trimanganese tetraoxide Mn3O4 NPs showed an mitigation of CCl4 induced liver fibrosis (Adhikari et al., 2016).
Some studies indicated that Zinc oxide (ZnO) NPs reduced liver fibrosis induced by dimethylnitrosamine through more than one mechanism, namely, reducing oxidative stress, lipid peroxidation, and inflammation (Rani et al., 2018).
Further types of inorganic NPs are used to ameliorate liver fibrosis other than metal oxide NPs. Gold NPs reduced liver fibrosis methamphetamine- and ethanol induced liver injury in rats via hindering Kupffer cells and HSCs activities (de Carvalho et al., 2018). This mechanism includes the regulation of phosphatidylinositol 3-kinase/Akt (AKT/PI3K) and MAPK signaling pathways by gold NPs, thus reducing both pro-inflammatory cytokine release and oxidative stress. Besides, Bai et al., 2020 stated that “vitamin E-modified selenium NPs can reduce liver fibrosis by ameliorating of the oxidative stress” (Bai et al., 2020a).
It is prominent that the dynamic targeting is imperative for the targeting the distorted tissues as well as avoiding the normal tissues, thus, increasing the efficacy of these drugs and limiting their detrimental effects. Hence, “Active targeting is a way to increase the quantity of drug brought to the target cell when compared to free drug or nano drugs passively targeted”.
NPs as Drug Transporters without Targeting Ligand for the Treatment of Liver Fibrosis
The liver is the central metabolic and excretory organ in the body and NPs are used as drug carriers due to their dimensions which can accumulate and target liver cells for the treatment of liver fibrosis.
Lipid-based NPs
Lipid-based NPs are the most significant vehicles due to their good biocompatibility and low toxicity (Bottger et al., 2020). Reebye et al., 2018 reported that: “CCAAT/enhancer-binding protein alpha (CEBPA), a master transcriptional factor in the liver can reduce fibrosis and restore liver function via resetting the hepatocyte natural gene regulatory mechanism. They also stated that the small activating RNA oligonucleotide therapy (CEBPA-51) formulated in liposome NPs can upregulate CEBPA expression, thus ameliorating the fibrosis” (Reebye et al., 2018).
In another study, authors reported a depletion in inflammatory cytokines, α-SMA and collagen after administration of nanostructured lipid curcumin carriers (Cur-mNLCs), on the other hand the hepatocyte growth factors (HGF) and Matrix metalloproteinase-2 (MMP2) were significantly elevated
Polymer-based NPs
Polymer-based NPs have been used as drug carriers for liver fibrosis treatment. Leber et al., 2017, mentioned that the ketal cross-linked cationic nanohydrogel particles were synthesized to deliver Cy5-labeled anti-col1α1 siRNA, leading to carrier enhancement increase accumulation in the fibrotic tissues and so preventing the progression of fibrosis. Dfgguop[(Leber et al., 2017; Kaps et al., 2020).
PLGA and eudragit were used as drug carriers. Phyllanthin was carried by PLGA to reduce the biochemical markers of liver fibrosis and the researchers found that it significantly depleted various liver enzymes mainly, aspartate aminotransferase and alanine aminotransferase and much more interesting, was the reduction in the collagen deposition (Krithika et al., 2015; You et al., 2021). Other studies showed that administering of silymarin which was carried by eudragit NPs for the liver fibrosis handling had marked effects manifested by declining the expression of TGF-β, TNF-α, TIMP-1 and Cytokeratin 19 (CK-19). In addition, nano formulations were found to rise MMP-2 expression and the MMP-2/TIMP-1 ratio (Younis et al., 2016).
Inorganic NPs
Silica-based NPs are used as drug carriers due to their porous structure. “Salvianolic acid B (SAB) loaded rhodamine B covalently grafted mesoporous silica NPs (SAB@MSNs-RhB) were prepared for liver fibrosis therapy” (He et al., 2010; Tao et al., 2021).
The SAB@MSNs-RhB formulation revealed improved cellular uptake, enhanced efficacy and continual drug release in hepatic fibrosis. Mesoporous silica NPs reduces the secretion of inflammatory cytokines via inhibition of TnC expression, as well as hepatocyte migration (Vivero-Escoto et al., 2019). Hesperetin loaded on PEGylated gold NPs showed significan antioxidant, anti-inflammatory and anti-proliferative when compared to hesperetin alone in hepatocarcinogenesis induced by diethylnitrosamine in rats (Krishnan et al., 2017).
Protein-based NPs
Due to the biocompatibility and low immunogenicity of protein-based NPs, they were the most suitable drug carriers to be studied in the treatment of liver fibrosis (Hawkins et al., 2008; Jimenez-Rosado et al., 2021). Curcumin-loaded zein nanospheres exerted a significant potency high effectiveness in decreasing the hepatic collagen I gene expression, TGF-β and as MMP2 inhibitor (Algandaby et al., 2016).
Moreover, a study proved that berberine entrapped in glucose-modified albumin NPs compared with free berberine repressed the growth of the human hepatic stellate cell line LX-2 and hence, abridged liver fibrosis more efficiently in vivo (Guo et al., 2019; Bai et al., 2020b).
Liver fibrosis is attenuated using free dexamethasone treatment. Human serum albumin-dexamethasone NPs have been developed to transport dexamethasone to the cells playing a crucial role in the pathogenesis of liver fibrosis, namely non-parenchymal hepatic cells. Such modification leads to significant inhibition of TNF-α that attenuated liver fibrosis (Melgert et al., 2000).
NPs as Drug Transporters with Targeting Ligands for the Treatment of Liver Fibrosis
Actually, the random drug delivery systems used in attenuating liver fibrosis are of low clinical effectiveness specially when compared to the target designed ones. The most efficient systems that can be used as a way to deliver drugs to a certain place are the nano formulations. Many studies in recent years showed advanced way of using nanoparticles in targeting hepatic fibrotic cells to alleviate liver fibrosis. It is to be noted as a rule that: “various receptor types that are expressed/over-expressed on HSCs have been targeted using the suitable targeting ligands that are conjugated at the surface of liposomes” (Figure 5) (Azzam et al., 2020; Hong et al., 2020; Kurniawan et al., 2020; Wang et al., 2020b; Zhang et al., 2021b).
“A drug-carrier construct basically consists of at least three elements: a carrier (or core molecule), a homing device, and a drug”.
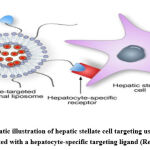 |
Figure 5: Schematic illustration of hepatic stellate cell targeting using a PEGylated liposome decorated with a hepatocyte-specific targeting ligand (Reddy et al., 2011). |
Targeting high-affinity membrane receptor for retinol binding protein (rRBP)
HSCs, precisely being retinoid storing cells in the liver, express retinol binding protein (rRBP) receptors that plays an important role in binding and taking-up vitamin A (VA). Thus, vitamin A-conjugated liposomes that are loaded with “siRNA against gp46 (the rat homolog of human heat shock protein 47)” that is involved in inhibition of collagen secretion, were injected intravenously into the fibrotic livers in rats.
Extensive decrease of collagen secretion and lessening in fibrosis was revealed after such drug treatment. It is to be noted that in normal physiological conditions, VA storage cells play pivotal roles in vitamin A homoeostasis regulation. On the other hand, in pathological conditions, such as liver fibrosis or cirrhosis, HSCs VA storage cells lose vitamin A and instead, synthesize a large number of components of extracellular matrix including proteoglycan, collagen, adhesive glycoproteins and glycosaminoglycan. The morphology of HSCs changes from the star-shaped stellate cells to that of fibroblasts or myofibroblasts (Senoo et al., 2010). Thus, targeting hepatic HSCs (VA storage cells) with nanoparticles is a crucial target in liver fibrosis therapy.
In one study, VA-coupled liposomes were fabricated to deliver imatinib. Interestingly, hepatic accumulation of imatinib increased by 13.5-fold when compared with free imatinib (El-Mezayen et al., 2017). The nano formulations depleted the expression of phosphorylated PDGFR-β and moreover, depleted the expression of profibrotic mediators such as TGF-α, hydroxyproline, and MMP2.
El-Mezayen et al., 2018 showed that: “the nano formulations VA-coupled liposomes used to deliver valsartan which is an angiotensin II receptor antagonist significantly increased the expression of hepatic Mas-receptor and PPAR-β. Moreover, it potently normalized the fibrogenic mediators’ level by improving the permeability and bioavaiability of valsartan” (El-Mezayen et al., 2018).
A study used retinol binding protein receptor and “collagenase I co-decorated polymeric micelles” based on “PLGA-b-poly (ethylene glycol)-maleimide (PLGA-PEG-Mal)” (named CRM) as HSC-targeting nanodrug delivery systems for treatment of liver fibrosis (Fan et al., 2020). In this experiment, the decoration of collagenase I enabled the nanocarrier penetration to the fibrotic tissue. In parallel to this outcome, CRMs potently degraded pericellular collagen I and successfully accumulated in the fibrotic liver hence, targeting activated HSCs (Fan et al., 2020).
A second-generation tyrosine kinase inhibitor, nilotinib (NIL) was loaded on CRM forming CRM/NIL. Such formulation was administered for the treatment of liver fibrosis and it exhibited outstanding antifibrotic effect (Fan et al., 2020) (Figure 6).
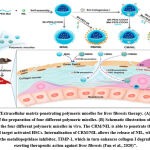 |
Figure 6: Extracellular matrix-penetrating polymeric micelles for liver fibrosis therapy. |
In addition, polymeric micelles (PVMs) designed with PLGA-polyspermine-PEG-VA were prepared to target HSCs and transport the silibinin and genetic drug siCol1α1 to the hepatic fibrotic tissue (Qiao et al., 2018). It significantly depleted collagen I and attenuated liver fibrosis (Luo et al., 2019). Other than polymeric micelles, other polymeric nano formulations have been designed for drug, nucleic acid and other therapeutic moieties delivery to particular sites for the treatment of liver fibrosis.
Hassan et al. reported that “chitosan NPs loaded with atorvastatin and JQ1 (a thienotriazolodiazepine) could ameliorate the cytokine-induced activation of HSCs and hence, reverse fibrotic response in rodent models and further conjugated with retinol leading to targeting and preventing HSC activation” (Hassan et al., 2019).
Targeting retinoic acid receptor
Chondroitin sulfate micelles that are coupled with retinoic acid and doxorubicin had a specific action as they were taken up by activated HSCs selectively and not by normal hepatocytes (Luo et al., 2019). These micelles specifically gathered in the Golgi apparatus and triggered the destruction of the Golgi structure, as a result, collagen I production was depleted and this manifested antifibrotic effects in fibrotic rats (Luo et al., 2019).
Targeting PDGFRβ on HSCs
A study was accomplished to explore the effects of a new TRAIL (TNF‐related apoptosis‐inducing ligand) formula that can target activated HSCs to alleviate liver fibrosis and explore the underlying mechanism.
Li et al., in 2020 stated that: “Platelet-derived growth factor receptor β (PDGFRβ) was co-expressed with TRAIL receptor 2 (DR5) in the activated HSCs and that the ZPDGFRβ affibody with high affinity for PDGFRβ could bind the activated HSCs and, thus, accumulate in the fibrotic liver (Figure 7).
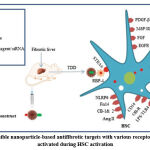 |
Figure 7: Possible nanoparticle-based antifibrotic targets with various receptors and ligands activated during HSC activation |
ZPDGFRβ was fused to hTRAIL to produce the fusion protein Z-hTRAIL. Compared to hTRAIL, Z-hTRAIL showed greater in vitro cell binding and apoptosis-induction in aHSCs. In addition, Z-hTRAIL induced apoptosis of activated HSCs but spared other normal liver cells. In vivo, Z-hTRAIL accumulated preferentially in fibrotic livers and exerted greater effects than hTRAIL in inducing activated HSCs apoptosis and reducing extracellular matrix (ECM) deposition. These results demonstrated that the antihepatofibrotic effect of hTRAIL was improved by PDGFRβ-targeted delivery (Li et al., 2020)”.
The cyclic peptide pPB can predominantly recognize PDGFRβ on the HSCs surface. Li et al., 2019 stated that pPB-modified liposomes was administered as a way to deliver recombinant human tumor necrosis factor-related apoptosis-inducing ligand (rhTRAIL) to the activated HSC membrane, showed prolonging rhTRAIL presence and circulation in vivo and hence, alleviating fibrosis” (Li et al., 2019a).
Recently, pPB coupled to albumin was used to deliver the Rho-kinase inhibitor Y27632 to HSC leading to beneficial effects in vivo (Klein et al., 2019). The same peptide was used to deliver lipid nanoparticles containing siRNA to HSC (Jia et al., 2018). In addition, a cyclic-RGD-based peptide was coupled to liposomes for the delivery of the hedgehog inhibitor vismodegib (Li et al., 2019b). This latter construct was quite effective in reducing fibrosis in two experimental animal models of liver fibrosis in mice”.
Targeting CXCR4
“In recent years, another receptor recognizing peptide binding to the CXCR4 receptor has been developed (Sung et al., 2018) which was coupled to polymeric biodegradable nanoparticles to create a carrier that allowed the simultaneous delivery of sorafenib and the MEK inhibitor selumetinib to HSC (Sung et al., 2018).
This combination therapy displayed significant antifibrotic effects in a mouse model. The CXCR4 receptor was also used for the targeting of pirfenidone to HSC (Ullah et al., 2019). In this latter case however, the nanoparticles (liposomes) were not functionalized with receptor recognizing peptides but with the CXCR4 antagonist AMD3100 (plerixafor). The effect of pirfenidone, one of the few FDA-approved drugs against fibrosis, combined with blocking of the chemokine receptor CXCR4 may lead to synergistic effects and is another example of a successful combination therapy”.
Vascular endothelial growth factor (VEGF) is a critical signaling protein that aids in the development of new blood vessels. VEGF participate in the mechanism that returns the blood supply to cells and tissues after being poorly oxygenated blood (Cameron et al., 2020).
It had been proven that in rat liver fibrosis models, treatment with drugs that neutralize VEGF produced by hepatocytes, can markedly alleviate liver fibrosis (Siddiqui et al., 2020). Liu et al., stated that “AMD3100-conjugated liposomes successfully delivered therapeutic VEGF siRNAs to activate CXCR4-overexpressed HSCs. The nano formulations interestingly, downregulated the expression of VEGF, decreased the mean vessel density, and normalized the hepatic vascular structure in the mice livers suffering from CCl4-induced liver fibrosis. In addition, AMD3100 conjugated liposomes suppressed the proliferation and activation of HSCs showing antifibrotic effects”.
Targeting mannose 6-phosphate (M6P)/insulin-like growth factor-II receptor
“Other than peptides and a receptor antagonist, sugar moieties have been used as homing devices as well” (Figure 8).
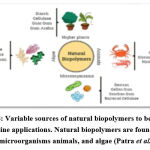 |
Figure 8: Variable sources of natural biopolymers to be used in nanomedicine applications. |
In fibrotic liver, mannose 6-phosphate (M6P)/insulin-like growth factor-II receptor is overexpressed in HSCs rendering it as an attractive targeting issue. Pairing of M6P-modified albumin to hesperidin-loaded liposomes showed marked improvement in the efficacy of chemical drugs and attenuated liver fibrosis (Morsy et al., 2018).
Targeting phosphatidylserine (PS)
Wang et al. concluded that: “Scavenger receptors expressed on liver endothelial cells and Kupffer cells have also been targeted using nano formulations. For instance, phosphatidylserine (PS), which acts as a specific recognition signal for the phagocytosis of apoptotic cells, can target macrophages. Wang et al. showed that PS-modified lipid carriers containing curcumin (Cur– mNLCs) exhibited enhanced retention time, bioavailability, and delivery efficiency of payload, as well as reduced liver damage and fibrosis in vivo” (Wang et al., 2018).
Targeting Integrins
Integrins are main receptor that clings the extracellular matrix proteins as laminins, collagens as well as fibronectin. Type VI collagen is dispersed in HSCs and they express integrins. Hence, targeting collagen VI integrins plays an important role in alleviating hepatic fibrosis (Beljaars et al., 2000). Du et al., 2007 suggested that: “RGD-coupled liposomes have efficiently delivered the encapsulated interferon (IFN)α-1 to the fibrotic liver in rat, achieving a considerable anti-fibrotic activity” (Du et al., 2007). Other studies followed the same path to alleviate different fibrotic tissues (Chung et al., 2016).
Targeting galactosyl receptor
The galactosyl receptor that is expressed on the hepatocytes plays a role in the internalization of molecular asialoglycoproteins and small particles. Knowing that, liposomes decorated with “p-aminophenyl a-D-galactopyranoside”, which binds to galactosyl receptor, are used as transporters to target HSCs with quercetin.
Such “quercetin-loaded galactosylated liposomes” were accumulated 1.7 fold more in the rat liver than non-galactosylated liposomal quercetin and 3.4-fold more than the free quercetin leading to considerable hepatoprotection as it markedly prevented the depletion of native antioxidant levels and it inhibited the collagen production that is responsible for fibrogenesis as well (Mandal et al., 2007). A recent study too used Galactosyl Nanoparticles to Target HSCs Hepatocellular Carcinoma (Liao et al., 2021).
Nanomedicine in Liver Fibrosis Theranostics
“Theranostics”, a hybrid word of “therapeutics” and “diagnostics”, is accomplished by integrating diagnostic and therapeutic functions into a single nanoplatform. Theranostics has been presented as a new and revolutionary therapeutic perception in several types of diseases as liver fibrosis (Gou et al., 2018).
This new path allows concurrent diagnosis and treatment response by using tailored medicine with high specificity and accuracy.
One of the hallmarks of activated HSC is their increased expression of integrin αvβ3 on their surface (Schuppan et al., 2018). In one study, Zhang et al., 2019 stated that: “Hepatitis B core protein nanocages coated with RGD-targeting ligands overloaded with querecitin (RGD–HBc/QR) showed selectivity to activated HSCs by targeting integrin αvβ3 which efficiently repressed the proliferation and activation of HSCs (Zhang et al., 2019) and that by encapsulating a quercetin–gadolinium complex and/or labeling it with near infrared (NIR) fluorescent probes (Cy5.5), the resulted nano formulations (RGD–HBc/QGd) showed great potential as (Magnetic resonance imaging) MRI contrast agents and NIR fluorescent agents for liver fibrosis diagnosis in vivo”.
Another study reported that “relaxin-conjugated PEGylated superparamagnetic iron oxide NPs” (RLX-SPIONs) exhibited specific binding and uptake in TGF-β-activated HSCs, as well as strongly lessened cirrhosis and showed improved contrast in MRI (Nagorniewicz et al., 2019). Micelles attached with inorganic agents were also established for theranostics to alleviate liver fibrosis (Liu et al., 2021).
“Next to small chemical entities with antifibrotic effects, that have been coupled and incorporated in many of the above reviewed drug carriers, biological products like antisense oligonucleotides, siRNA, miRNA, and cytokines can be powerful tools to modify fibrosis. The therapeutic applications of siRNA have become clear in recent years, and many different miRNA and siRNA molecules (Omar et al., 2018) have been tested in experimental animal models of fibrosis. Delivery is the key issue for siRNA and miRNA molecules. Although cytokines play a key role in vivo in regulating fibrogenesis and are active in the picomolar range, their pleiotropic activities have strongly limited their therapeutical use. This may be solved using targeting strategies (Bansal et al., 2014)”.
Natural Products used in Nanomedicine as Antifibrotic Agents
Scientific communities are now focusing on the studies related to natural nano bioactive compounds to produce pioneering efficient active ingredients with minor side effects than synthetic prevailing molecules.
Application of nanotechnology systems using natural components in the medical field has been widely considered in the last few years (Ramana et al., 2014; Shah et al., 2020; Dehghani Tafti et al., 2021). There is evidence that the association of nono systems with natural compounds may help to reduce the progress of drug resistance and hence these systems play an important role in developing formulations that are target specific, potent and of little side effects for the treatment of liver fibrosis. It is to be renowned that natural components can either be used as the polymer which is the drug carrier (Figure 9) or as the therapeutic component.
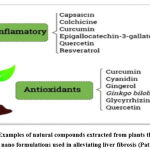 |
Figure 9: Examples of natural compounds extracted from plants that are being formulated as nano formulations used in alleviating liver fibrosis (Patra et al., 2018). |
Egyptian Experience
Various Egyptian research groups are involved in the advancement of nanocarriers for applications of drug-delivery including the usage of nanocarriers to improve the effectiveness of different pharmaceutical formulations including oral, nasal, parenteral, transdermal.. etc.
Hussein et al., 2017 compared the therapeutic potential of nano-encapsulated and nano-emulsion of a natural constituent, carvacrol, on thioacetamide-induced liver fibrosis in rats. The nano formulations of carvacrol succeeded to ameliorate thioacetamide-induced liver fibrosis (Hussein et al., 2017).
Khattab et al., developed and characterized self-nanoemulsifying formulation of coenzyeme Q10 (CoQ10) to investigate its biochemical and physiological effect on liver cirrhosis in CoQ10 rats and they compared it with CoQ10 powder. The team demonstrated that CoQ10 nano formulation attenuated thioacetamide-induced liver fibrosis mainly through inhibiting collagen production (Khattab et al., 2017).
El-Mezayen et al., 2017 prepared a novel vitamin A-coupled imatinib-loaded liposomes regarding VA-coupling efficiency as well as imatinib entrapment resulting in significant anti-fibrotic effects with reduced cytotoxicity compared to conventional imatinib (El-Mezayen et al., 2017).
El-Mezayen et al., 2018 stated that: “vitamin A stores in hepatic stellate cells were targeted to deliver valsartan to HSCs to treat liver fibrosis and encapsulation of valsartan into vitamin A-coupled liposomes targeted HSCs, caused a significant activation of hepatic nuclear PPAR-γ receptors in HSCs confirming the powerful antifibrotic activity of valsartan liposomes” (El-Mezayen et al., 2018).
Infection by Schistosoma mansoni is an endemic pathogen in Egypt and it is often progressed to severe hepatic disorders as liver fibrosis or necrosis. Two studies have been done depending on the improvement of the efficiency of praziquantel through nanotechnology ideas.
The first team intended to elevate the therapeutic outcome of the main antischistosomal drug worldwide, praziquantel through including it into a novel carrier which is solid lipid nanoparticles (SLNs). Such modulation enhanced praziquantel bioavailability and antischistosomal efficacy and hence alleviated liver fibrosis which is a common result of such case (Radwan et al., 2019).
The second Egyptian team of researchers intended to study the anti-schistosomal activity of curcumin as well as “curcumin loaded gold-nanoparticles (Cur-GNPs)” in the presence of praziquantel. They found that the curcumin in combination with praziquantel altered the biochemical, and immunological damage and hence, alleviated liver fibrosis (Mokbel et al., 2020).
Conclusions and Future Perspectives
This review outlines the nanotechnology strategies that are used to progress novel specific and accurate systems for alleviating liver fibrosis. This field of nanomedicine systems continues to be increasingly growing and developing so as to aid in both the diagnosis and treatment of liver fibrosis.
Variable categories of inorganic and organic NPs have been widely inspected, including “metal oxide NPs, liposomes, metal NPs, protein NPs, polymer NPs and organic–inorganic hybrid NPs”. Each category has its rewards and drawbacks. However inorganic NPs are found to be efficient with comparatively low synthesizing expenses, but their functionality and design elasticity are limited. On the other hand, organic NPs have wide functionalities as well as broad design flexibility but they are unstable and they are of high cost.
Many studies in recent years showed advanced way of using nanoparticles in targeting hepatic fibrotic cells to alleviate liver fibrosis as numerous receptor types that are expressed on HSCs have been targeted by means of the suitable targeting ligands that are conjugated at the surface of liposomes
Recommendations to be considered to advance the medical application of nanomedicine systems in the future:
Developing stimuli-responsive nano systems, that can smartly respond to both endogenous or exogenous stimuli and release the needed therapeutic load at targeting sites.
Exploring advanced targeted therapies is a priority.
Employing clever nanomedicine structures that syndicate multiple functionalities, including prolonged blood retention, targeted delivery, responsiveness to stimuli, improved tissue penetration and disease progressive monitoring.
Regular assessment of long-term toxicity, pharmacokinetics of the systems and immunogenicity.
Acknowledgment
Authors would like to thank the Pharmacology Department at Medical Research and Clinical Studies Institute (National Research Centre) for giving us the opportunity to conduct all our experimental studies.
Conflict of interest
Authors declared that there is no conflict of interest
Funding sources
No funding sources
References
- Adhikari A, Polley N, Darbar S, Bagchi D, Pal SK (2016). Citrate functionalized Mn3O4 in nanotherapy of hepatic fibrosis by oral administration. Future science OA 2(4):
CrossRef - Aggarwal N, Sachin, Nabi B, Aggarwal S, Baboota S, Ali J (2021). Nano-based drug delivery system: a smart alternative towards eradication of viral sanctuaries in management of NeuroAIDS. Drug delivery and translational research.
CrossRef - Algandaby MM, Al-Sawahli MM, Ahmed OAA, Fahmy UA, Abdallah HM, Hattori M, et al. (2016). Curcumin-Zein Nanospheres Improve Liver Targeting and Antifibrotic Activity of Curcumin in Carbon Tetrachloride-Induced Mice Liver Fibrosis. Journal of biomedical nanotechnology 12(9): 1746-1757.
CrossRef - Anselmo AC, Mitragotri S (2015). A Review of Clinical Translation of Inorganic Nanoparticles. The AAPS journal 17(5): 1041-1054.
CrossRef - Azzam M, El Safy S, Abdelgelil SA, Weiskirchen R, Asimakopoulou A, de Lorenzi F, et al. (2020). Targeting Activated Hepatic Stellate Cells Using Collagen-Binding Chitosan Nanoparticles for siRNA Delivery to Fibrotic Livers. Pharmaceutics 12(6).
CrossRef - Bai K, Hong B, He J, Huang W (2020a). Antioxidant Capacity and Hepatoprotective Role of Chitosan-Stabilized Selenium Nanoparticles in Concanavalin A-Induced Liver Injury in Mice. Nutrients 12(3).
CrossRef - Bai X, Su G, Zhai S (2020b). Recent Advances in Nanomedicine for the Diagnosis and Therapy of Liver Fibrosis. Nanomaterials 10(10).
CrossRef - Bansal R, Prakash J, De Ruiter M, Poelstra K (2014). Interferon gamma peptidomimetic targeted to hepatic stellate cells ameliorates acute and chronic liver fibrosis in vivo. Journal of controlled release : official journal of the Controlled Release Society 179: 18-24.
CrossRef - Beljaars L, Molema G, Schuppan D, Geerts A, De Bleser PJ, Weert B, et al. (2000). Successful targeting to rat hepatic stellate cells using albumin modified with cyclic peptides that recognize the collagen type VI receptor. The Journal of biological chemistry 275(17): 12743-12751.
CrossRef - Bondi ML, Fontana G, Carlisi B, Giammona G (2003). Preparation and characterization of solid lipid nanoparticles containing cloricromene. Drug delivery 10(4): 245-250.
CrossRef - Bottger R, Pauli G, Chao PH, Al Fayez N, Hohenwarter L, Li SD (2020). Lipid-based nanoparticle technologies for liver targeting. Advanced drug delivery reviews 154-155: 79-101.
CrossRef - Breitkopf K, Godoy P, Ciuclan L, Singer MV, Dooley S (2006). TGF-beta/Smad signaling in the injured liver. Zeitschrift fur Gastroenterologie 44(1): 57-66.
CrossRef - Cameron AC, Welsh P, Neves KB, Newby DE, Touyz RM, Lang NN (2020). Acute vascular effects of vascular endothelial growth factor inhibition in the forearm arterial circulation. Journal of hypertension 38(2): 257-265.
CrossRef - Chan YT, Wang N, Tan HY, Li S, Feng Y (2020). Targeting Hepatic Stellate Cells for the Treatment of Liver Fibrosis by Natural Products: Is It the Dawning of a New Era? Frontiers in pharmacology 11:
CrossRef - Chen L, Chen R, Kemper S, Charrier A, Brigstock DR (2015). Suppression of fibrogenic signaling in hepatic stellate cells by Twist1-dependent microRNA-214 expression: Role of exosomes in horizontal transfer of Twist1. American journal of physiology. Gastrointestinal and liver physiology 309(6): G491-499.
CrossRef - Choi Y, Yoo JH, Lee JH, Lee Y, Bae MK, Kim YD, et al. (2020). Connective tissue growth factor (CTGF) regulates the fusion of osteoclast precursors by inhibiting Bcl6 in periodontitis. International journal of medical sciences 17(5): 647-656.
CrossRef - Chung C, Gorelick FS (2016). Targeting alphav Integrins in Pancreatic Fibrosis: Progress in Resolving the Scar. Cellular and molecular gastroenterology and hepatology 2(4): 405-406.
CrossRef - Datta P, Bang S, Yue Z, Beach T, Stilgenbauer M, Wang H, et al. (2020). Engineering liposomal nanoparticles of cholesterol-tethered amphiphilic Pt(iv) prodrugs with prolonged circulation time in blood. Dalton transactions 49(24): 8107-8113.
CrossRef - de Alwis NM, Day CP (2008). Non-alcoholic fatty liver disease: the mist gradually clears. Journal of hepatology 48 Suppl 1: S104-112.
CrossRef - de Carvalho TG, Garcia VB, de Araujo AA, da Silva Gasparotto LH, Silva H, Guerra GCB, et al. (2018). Spherical neutral gold nanoparticles improve anti-inflammatory response, oxidative stress and fibrosis in alcohol-methamphetamine-induced liver injury in rats. International journal of pharmaceutics 548(1): 1-14.
CrossRef - de Castro Bras LE, Frangogiannis NG (2020). Extracellular matrix-derived peptides in tissue remodeling and fibrosis. Matrix biology : journal of the International Society for Matrix Biology 91-92: 176-187.
CrossRef - Degre D, Lemmers A, Gustot T, Ouziel R, Trepo E, Demetter P, et al. (2012). Hepatic expression of CCL2 in alcoholic liver disease is associated with disease severity and neutrophil infiltrates. Clinical and experimental immunology 169(3): 302-310.
CrossRef - Dehghani Tafti A, Mirjalili BBF, Bamoniri A, Salehi N (2021). Rapid four-component synthesis of dihydropyrano[2,3-c]pyrazoles using nano-eggshell/Ti(IV) as a highly compatible natural based catalyst. BMC chemistry 15(1):
CrossRef - Deng Y, Li J, Zhou M, Liang Z, Zhao L (2021). c-Myc affects hedgehog pathway via KCNQ1OT1/RAC1: A new mechanism for regulating HSC proliferation and epithelial-mesenchymal transition. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver.
CrossRef - Du SL, Pan H, Lu WY, Wang J, Wu J, Wang JY (2007). Cyclic Arg-Gly-Asp peptide-labeled liposomes for targeting drug therapy of hepatic fibrosis in rats. The Journal of pharmacology and experimental therapeutics 322(2): 560-568.
CrossRef - Duong HT, Dong Z, Su L, Boyer C, George J, Davis TP, et al. (2015). The use of nanoparticles to deliver nitric oxide to hepatic stellate cells for treating liver fibrosis and portal hypertension. Small 11(19): 2291-2304.
CrossRef - El-Mezayen NS, El-Hadidy WF, El-Refaie WM, Shalaby TI, Khattab MM, El-Khatib AS (2017). Hepatic stellate cell-targeted imatinib nanomedicine versus conventional imatinib: A novel strategy with potent efficacy in experimental liver fibrosis. Journal of controlled release : official journal of the Controlled Release Society 266: 226-237.
CrossRef - El-Mezayen NS, El-Hadidy WF, El-Refaie WM, Shalaby TI, Khattab MM, El-Khatib AS (2018). Oral vitamin-A-coupled valsartan nanomedicine: High hepatic stellate cell receptors accessibility and prolonged enterohepatic residence. Journal of controlled release : official journal of the Controlled Release Society 283: 32-44.
CrossRef - El-Ratel IT, Tag El-Din TEH, Bedier MM (2020). Beneficial effects of curcumin as a native or nanoparticles form on productive efficiency, liver and kidney functions, antioxidative status and immunity of heat-stressed growing rabbits. Journal of animal physiology and animal nutrition 104(6): 1778-1787.
CrossRef - Fan QQ, Zhang CL, Qiao JB, Cui PF, Xing L, Oh YK, et al. (2020). Extracellular matrix-penetrating nanodrill micelles for liver fibrosis therapy. Biomaterials 230:
CrossRef - Giannitrapani L, Soresi M, Bondi ML, Montalto G, Cervello M (2014). Nanotechnology applications for the therapy of liver fibrosis. World journal of gastroenterology 20(23): 7242-7251.
CrossRef - Gou Y, Miao D, Zhou M, Wang L, Zhou H, Su G (2018). Bio-Inspired Protein-Based Nanoformulations for Cancer Theranostics. Frontiers in pharmacology 9:
CrossRef - Greenbaum LE, Wells RG (2011). The role of stem cells in liver repair and fibrosis. The international journal of biochemistry & cell biology 43(2): 222-229.
CrossRef - Gui T, Sun Y, Shimokado A, Muragaki Y (2012). The Roles of Mitogen-Activated Protein Kinase Pathways in TGF-beta-Induced Epithelial-Mesenchymal Transition. Journal of signal transduction 2012:
CrossRef - Guo HH, Feng CL, Zhang WX, Luo ZG, Zhang HJ, Zhang TT, et al. (2019). Liver-target nanotechnology facilitates berberine to ameliorate cardio-metabolic diseases. Nature communications 10(1):
CrossRef - Han X, Wu Y, Yang Q, Cao G (2020). Peroxisome proliferator-activated receptors in the pathogenesis and therapies of liver fibrosis. Pharmacology & therapeutics 222:
CrossRef - Hanafusa H, Ninomiya-Tsuji J, Masuyama N, Nishita M, Fujisawa J, Shibuya H, et al. (1999). Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta-induced gene expression. The Journal of biological chemistry 274(38): 27161-27167.
CrossRef - Hassan R, Tammam SN, Safy SE, Abdel-Halim M, Asimakopoulou A, Weiskirchen R, et al. (2019). Prevention of hepatic stellate cell activation using JQ1- and atorvastatin-loaded chitosan nanoparticles as a promising approach in therapy of liver fibrosis. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V 134: 96-106.
CrossRef - Hawkins MJ, Soon-Shiong P, Desai N (2008). Protein nanoparticles as drug carriers in clinical medicine. Advanced drug delivery reviews 60(8): 876-885.
CrossRef - He Q, Zhang J, Chen F, Guo L, Zhu Z, Shi J (2010). An anti-ROS/hepatic fibrosis drug delivery system based on salvianolic acid B loaded mesoporous silica nanoparticles. Biomaterials 31(30): 7785-7796.
CrossRef - He W, Turkeshi A, Li X, Zhang H (2020). Progress in systemic co-delivery of microRNAs and chemotherapeutics for cancer treatment by using lipid-based nanoparticles. Therapeutic delivery 11(9): 591-603.
CrossRef` - Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, Brenner DA (1999). The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo. Journal of hepatology 30(1): 77-87.
CrossRef - Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, et al. (2013). Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nature medicine 19(12): 1617-1624.
CrossRef - Hong F, Ji J, Ze X, Zhou Y, Ze Y (2020). Liver Inflammation and Fibrosis Induced by Long-Term Exposure to Nano Titanium Dioxide (TiO(2)) Nanoparticles in Mice and Its Molecular Mechanism. Journal of biomedical nanotechnology 16(5): 616-625.
CrossRef - Huang G, Brigstock DR (2012). Regulation of hepatic stellate cells by connective tissue growth factor. Frontiers in bioscience 17: 2495-2507.
CrossRef - Hussein J, El-Banna M, Mahmoud KF, Morsy S, Abdel Latif Y, Medhat D, et al. (2017). The therapeutic effect of nano-encapsulated and nano-emulsion forms of carvacrol on experimental liver fibrosis. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 90: 880-887.
CrossRef - Immordino ML, Dosio F, Cattel L (2006). Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. International journal of nanomedicine 1(3): 297-315
- Jia Z, Gong Y, Pi Y, Liu X, Gao L, Kang L, et al. (2018). pPB Peptide-Mediated siRNA-Loaded Stable Nucleic Acid Lipid Nanoparticles on Targeting Therapy of Hepatic Fibrosis. Molecular pharmaceutics 15(1): 53-62.
CrossRef - Jimenez-Rosado M, Perez-Puyana V, Sanchez-Cid P, Guerrero A, Romero A (2021). Incorporation of ZnO Nanoparticles into Soy Protein-Based Bioplastics to Improve Their Functional Properties. Polymers 13(4).
CrossRef - Jimenez Calvente C, Sehgal A, Popov Y, Kim YO, Zevallos V, Sahin U, et al. (2015). Specific hepatic delivery of procollagen alpha1(I) small interfering RNA in lipid-like nanoparticles resolves liver fibrosis. Hepatology 62(4): 1285-1297.
CrossRef - Kantari-Mimoun C, Castells M, Klose R, Meinecke AK, Lemberger UJ, Rautou PE, et al. (2015). Resolution of liver fibrosis requires myeloid cell-driven sinusoidal angiogenesis. Hepatology 61(6): 2042-2055.
CrossRef - Kaps L, Leber N, Klefenz A, Choteschovsky N, Zentel R, Nuhn L, et al. (2020). In Vivo siRNA Delivery to Immunosuppressive Liver Macrophages by alpha-Mannosyl-Functionalized Cationic Nanohydrogel Particles. Cells 9(8).
CrossRef - Ketabat F, Pundir M, Mohabatpour F, Lobanova L, Koutsopoulos S, Hadjiiski L, et al. (2019). Controlled Drug Delivery Systems for Oral Cancer Treatment-Current Status and Future Perspectives. Pharmaceutics 11(7).
CrossRef - Khattab A, Hassanin L, Zaki N (2017). Self-Nanoemulsifying Drug Delivery System of Coenzyme (Q10) with Improved Dissolution, Bioavailability, and Protective Efficiency on Liver Fibrosis. AAPS PharmSciTech 18(5): 1657-1672.
CrossRef - Klein S, Frohn F, Magdaleno F, Reker-Smit C, Schierwagen R, Schierwagen I, et al. (2019). Rho-kinase inhibitor coupled to peptide-modified albumin carrier reduces portal pressure and increases renal perfusion in cirrhotic rats. Scientific reports 9(1):
CrossRef - Kostallari E, Hirsova P, Prasnicka A, Verma VK, Yaqoob U, Wongjarupong N, et al. (2018). Hepatic stellate cell-derived platelet-derived growth factor receptor-alpha-enriched extracellular vesicles promote liver fibrosis in mice through SHP2. Hepatology 68(1): 333-348.
CrossRef - Krishnan G, Subramaniyan J, Chengalvarayan Subramani P, Muralidharan B, Thiruvengadam D (2017). Hesperetin conjugated PEGylated gold nanoparticles exploring the potential role in anti-inflammation and anti-proliferation during diethylnitrosamine-induced hepatocarcinogenesis in rats. Asian journal of pharmaceutical sciences 12(5): 442-455.
CrossRef - Krithika R, Jyothilakshmi V, Prashantha K, Verma RJ (2015). Mechanism of protective effect of phyllanthin against carbon tetrachloride-induced hepatotoxicity and experimental liver fibrosis in mice. Toxicology mechanisms and methods 25(9): 708-717.
CrossRef - Kurniawan DW, Booijink R, Pater L, Wols I, Vrynas A, Storm G, et al. (2020). Fibroblast growth factor 2 conjugated superparamagnetic iron oxide nanoparticles (FGF2-SPIONs) ameliorate hepatic stellate cells activation in vitro and acute liver injury in vivo. Journal of controlled release : official journal of the Controlled Release Society 328: 640-652.
CrossRef - Lan X, Zhao J, Zhang Y, Chen Y, Liu Y, Xu F (2020). Oxymatrine exerts organ- and tissue-protective effects by regulating inflammation, oxidative stress, apoptosis, and fibrosis: From bench to bedside. Pharmacological research 151:
CrossRef - Leber N, Kaps L, Aslam M, Schupp J, Brose A, Schaffel D, et al. (2017). SiRNA-mediated in vivo gene knockdown by acid-degradable cationic nanohydrogel particles. Journal of controlled release : official journal of the Controlled Release Society 248: 10-23.
CrossRef - Lee YA, Wallace MC, Friedman SL (2015). Pathobiology of liver fibrosis: a translational success story. Gut 64(5): 830-841.
CrossRef - Li L, Li H, Zhang Z, Zheng J, Shi Y, Liu J, et al. (2018). Recombinant truncated TGFbeta receptor II attenuates carbon tetrachlorideinduced epithelialmesenchymal transition and liver fibrosis in rats. Molecular medicine reports 17(1): 315-321.
CrossRef - Li Q, Ding Y, Guo X, Luo S, Zhuang H, Zhou J, et al. (2019a). Chemically modified liposomes carrying TRAIL target activated hepatic stellate cells and ameliorate hepatic fibrosis in vitro and in vivo. Journal of cellular and molecular medicine 23(3): 1951-1962.
CrossRef - Li R, Li Z, Feng Y, Yang H, Shi Q, Tao Z, et al. (2020). PDGFRbeta-targeted TRAIL specifically induces apoptosis of activated hepatic stellate cells and ameliorates liver fibrosis. Apoptosis : an international journal on programmed cell death 25(1-2): 105-119.
CrossRef - Li S, Meng Lin M, Toprak MS, Kim DK, Muhammed M (2010). Nanocomposites of polymer and inorganic nanoparticles for optical and magnetic applications. Nano reviews 1.
CrossRef - Li Y, Pu S, Liu Q, Li R, Zhang J, Wu T, et al. (2019b). An integrin-based nanoparticle that targets activated hepatic stellate cells and alleviates liver fibrosis. Journal of controlled release : official journal of the Controlled Release Society 303: 77-90.
CrossRef - Liao W, Tang Y, Hu Z, Wang C, Chen Y, Zhang Y, et al. (2021). Preparation of Galactosyl Nanoparticles and Their Targeting Efficiency to Hepatocellular Carcinoma. Journal of nanoscience and nanotechnology 21(2): 987-994.
CrossRef - Lin SW, Shyong YJ, Kuo PC, Tsai JC (2021). Topical application of sebacoyl dinalbuphine ester-loaded nanostructured lipid carriers alleviate pruritus in scratching mouse model. International journal of pharmaceutics:
CrossRef - Liu C, Chen X, Yang L, Kisseleva T, Brenner DA, Seki E (2014). Transcriptional repression of the transforming growth factor beta (TGF-beta) Pseudoreceptor BMP and activin membrane-bound inhibitor (BAMBI) by Nuclear Factor kappaB (NF-kappaB) p50 enhances TGF-beta signaling in hepatic stellate cells. The Journal of biological chemistry 289(10): 7082-7091.
CrossRef - Liu Y, Cavallaro PM, Kim BM, Liu T, Wang H, Kuhn F, et al. (2021). A role for intestinal alkaline phosphatase in preventing liver fibrosis. Theranostics 11(1): 14-26.
CrossRef - Luo J, Zhang P, Zhao T, Jia M, Yin P, Li W, et al. (2019). Golgi Apparatus-Targeted Chondroitin-Modified Nanomicelles Suppress Hepatic Stellate Cell Activation for the Management of Liver Fibrosis. ACS nano 13(4): 3910-3923.
CrossRef - Mandal AK, Das S, Basu MK, Chakrabarti RN, Das N (2007). Hepatoprotective activity of liposomal flavonoid against arsenite-induced liver fibrosis. The Journal of pharmacology and experimental therapeutics 320(3): 994-1001.
CrossRef - Melgert BN, Olinga P, Jack VK, Molema G, Meijer DK, Poelstra K (2000). Dexamethasone coupled to albumin is selectively taken up by rat nonparenchymal liver cells and attenuates LPS-induced activation of hepatic cells. Journal of hepatology 32(4): 603-611.
CrossRef - Miao H, Zhang Y, Huang Z, Lu B, Ji L (2019). Lonicera japonica Attenuates Carbon Tetrachloride-Induced Liver Fibrosis in Mice: Molecular Mechanisms of Action. The American journal of Chinese medicine 47(2): 351-367.
CrossRef - Mieli-Vergani G, Vergani D, Czaja AJ, Manns MP, Krawitt EL, Vierling JM, et al. (2018). Autoimmune hepatitis. Nature reviews. Disease primers 4:
CrossRef - Moghimi SM, Hunter AC, Murray JC (2001). Long-circulating and target-specific nanoparticles: theory to practice. Pharmacological reviews 53(2): 283-318.
- Mohamed AA, Khater SI, Hamed Arisha A, Metwally MMM, Mostafa-Hedeab G, El-Shetry ES (2021). Chitosan-stabilized selenium nanoparticles alleviate cardio-hepatic damage in type 2 diabetes mellitus model via regulation of caspase, Bax/Bcl-2, and Fas/FasL-pathway. Gene 768:
CrossRef - Mohammadpour R, Cheney DL, Grunberger JW, Yazdimamaghani M, Jedrzkiewicz J, Isaacson KJ, et al. (2020). One-year chronic toxicity evaluation of single dose intravenously administered silica nanoparticles in mice and their Ex vivo human hemocompatibility. Journal of controlled release : official journal of the Controlled Release Society 324: 471-481.
CrossRef - Mohammed ES, El-Beih NM, El-Hussieny EA, El-Ahwany E, Hassan M, Zoheiry M (2021). Effects of free and nanoparticulate curcumin on chemically induced liver carcinoma in an animal model. Archives of medical science : AMS 17(1): 218-227.
CrossRef - Mokbel KEM, Baiuomy IR, Sabry AEA, Mohammed MM, El-Dardiry MA (2020). In vivo assessment of the antischistosomal activity of curcumin loaded nanoparticles versus praziquantel in the treatment of Schistosoma mansoni. Scientific reports 10(1):
CrossRef - Morsy MA, Nair AB (2018). Prevention of rat liver fibrosis by selective targeting of hepatic stellate cells using hesperidin carriers. International journal of pharmaceutics 552(1-2): 241-250.
CrossRef - Muller RH, Mader K, Gohla S (2000). Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V 50(1): 161-177.
CrossRef - Nagorniewicz B, Mardhian DF, Booijink R, Storm G, Prakash J, Bansal R (2019). Engineered Relaxin as theranostic nanomedicine to diagnose and ameliorate liver cirrhosis. Nanomedicine : nanotechnology, biology, and medicine 17: 106-118.
CrossRef - Nie X, Chen Z, Pang L, Wang L, Jiang H, Chen Y, et al. (2020). Oral Nano Drug Delivery Systems for the Treatment of Type 2 Diabetes Mellitus: An Available Administration Strategy for Antidiabetic Phytocompounds. International journal of nanomedicine 15: 10215-10240.
CrossRef - Omar R, Yang J, Alrushaid S, Burczynski FJ, Minuk GY, Gong Y (2018). Inhibition of BMP4 and Alpha Smooth Muscle Actin Expression in LX-2 Hepatic Stellate Cells by BMP4-siRNA Lipid Based Nanoparticle. Journal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques 21(1): 119-134.
CrossRef - Omenetti A, Choi S, Michelotti G, Diehl AM (2011). Hedgehog signaling in the liver. Journal of hepatology 54(2): 366-373.
CrossRef - Oro D, Yudina T, Fernandez-Varo G, Casals E, Reichenbach V, Casals G, et al. (2016). Cerium oxide nanoparticles reduce steatosis, portal hypertension and display anti-inflammatory properties in rats with liver fibrosis. Journal of hepatology 64(3): 691-698.
CrossRef - Pardeshi C, Rajput P, Belgamwar V, Tekade A, Patil G, Chaudhary K, et al. (2012). Solid lipid based nanocarriers: an overview. Acta pharmaceutica 62(4): 433-472.
CrossRef - Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, et al. (2018). Nano based drug delivery systems: recent developments and future prospects. Journal of nanobiotechnology 16(1):
CrossRef - Peng F, Tee JK, Setyawati MI, Ding X, Yeo HLA, Tan YL, et al. (2018). Inorganic Nanomaterials as Highly Efficient Inhibitors of Cellular Hepatic Fibrosis. ACS applied materials & interfaces 10(38): 31938-31946.
CrossRef - Petros RA, DeSimone JM (2010). Strategies in the design of nanoparticles for therapeutic applications. Nature reviews. Drug discovery 9(8): 615-627.
CrossRef - Poilil Surendran S, George Thomas R, Moon MJ, Jeong YY (2017). Nanoparticles for the treatment of liver fibrosis. International journal of nanomedicine 12: 6997-7006.
CrossRef - Pradere JP, Kluwe J, De Minicis S, Jiao JJ, Gwak GY, Dapito DH, et al. (2013). Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology 58(4): 1461-1473.
CrossRef - Pucek A, Tokarek B, Waglewska E, Bazylinska U (2020). Recent Advances in the Structural Design of Photosensitive Agent Formulations Using “Soft” Colloidal Nanocarriers. Pharmaceutics 12(6).
CrossRef - Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E, et al. (2009). Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Critical reviews in therapeutic drug carrier systems 26(6): 523-580.
CrossRef - Qiao JB, Fan QQ, Xing L, Cui PF, He YJ, Zhu JC, et al. (2018). Vitamin A-decorated biocompatible micelles for chemogene therapy of liver fibrosis. Journal of controlled release : official journal of the Controlled Release Society 283: 113-125.
CrossRef - Radwan A, El-Lakkany NM, William S, El-Feky GS, Al-Shorbagy MY, Saleh S, et al. (2019). A novel praziquantel solid lipid nanoparticle formulation shows enhanced bioavailability and antischistosomal efficacy against murine S. mansoni infection. Parasites & vectors 12(1):
CrossRef - Ramana KV, Singhal SS, Reddy AB (2014). Therapeutic potential of natural pharmacological agents in the treatment of human diseases. BioMed research international 2014:
CrossRef - Rani V, Verma Y, Rana K, Rana SVS (2018). Zinc oxide nanoparticles inhibit dimethylnitrosamine induced liver injury in rat. Chemico-biological interactions 295: 84-92.
CrossRef - Reddy LH, Couvreur P (2011). Nanotechnology for therapy and imaging of liver diseases. Journal of hepatology 55(6): 1461-1466.
CrossRef - Reebye V, Huang KW, Lin V, Jarvis S, Cutilas P, Dorman S, et al. (2018). Gene activation of CEBPA using saRNA: preclinical studies of the first in human saRNA drug candidate for liver cancer. Oncogene 37(24): 3216-3228.
CrossRef - Schuppan D, Ashfaq-Khan M, Yang AT, Kim YO (2018). Liver fibrosis: Direct antifibrotic agents and targeted therapies. Matrix biology : journal of the International Society for Matrix Biology 68-69: 435-451.
CrossRef - Schuster-Gaul S, Geisler LJ, McGeough MD, Johnson CD, Zagorska A, Li L, et al. (2020). ASK1 inhibition reduces cell death and hepatic fibrosis in an Nlrp3 mutant liver injury model. JCI insight 5(2).
CrossRef - Senoo H, Yoshikawa K, Morii M, Miura M, Imai K, Mezaki Y (2010). Hepatic stellate cell (vitamin A-storing cell) and its relative–past, present and future. Cell biology international 34(12): 1247-1272.
CrossRef - Shah BM, Palakurthi SS, Khare T, Khare S, Palakurthi S (2020). Natural proteins and polysaccharides in the development of micro/nano delivery systems for the treatment of inflammatory bowel disease. International journal of biological macromolecules 165(Pt A): 722-737.
CrossRef - Sheng RF, Jin KP, Yang L, Wang HQ, Liu H, Ji Y, et al. (2018). Histogram Analysis of Diffusion Kurtosis Magnetic Resonance Imaging for Diagnosis of Hepatic Fibrosis. Korean journal of radiology 19(5): 916-922.
CrossRef - Siddiqui H, Rawal P, Bihari C, Arora N, Kaur S (2020). Vascular Endothelial Growth Factor Promotes Proliferation of Epithelial Cell Adhesion Molecule-Positive Cells in Nonalcoholic Steatohepatitis. Journal of clinical and experimental hepatology 10(4): 275-283.
CrossRef - Silva CO, Pinho JO, Lopes JM, Almeida AJ, Gaspar MM, Reis C (2019). Current Trends in Cancer Nanotheranostics: Metallic, Polymeric, and Lipid-Based Systems. Pharmaceutics 11(1).
CrossRef - Sung YC, Liu YC, Chao PH, Chang CC, Jin PR, Lin TT, et al. (2018). Combined delivery of sorafenib and a MEK inhibitor using CXCR4-targeted nanoparticles reduces hepatic fibrosis and prevents tumor development. Theranostics 8(4): 894-905.
CrossRef - Tao XM, Li D, Zhang C, Wen GH, Wu C, Xu YY, et al. (2021). Salvianolic acid B protects against acute and chronic liver injury by inhibiting Smad2C/L phosphorylation. Experimental and therapeutic medicine 21(4):
CrossRef - Tee JK, Peng F, Ho HK (2019). Effects of inorganic nanoparticles on liver fibrosis: Optimizing a double-edged sword for therapeutics. Biochemical pharmacology 160: 24-33.
CrossRef - Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA (2002). Myofibroblasts and mechano-regulation of connective tissue remodelling. Nature reviews. Molecular cell biology 3(5): 349-363.
CrossRef - Tomita K, Teratani T, Suzuki T, Shimizu M, Sato H, Narimatsu K, et al. (2014). Acyl-CoA:cholesterol acyltransferase 1 mediates liver fibrosis by regulating free cholesterol accumulation in hepatic stellate cells. Journal of hepatology 61(1): 98-106.
CrossRef - Tsuchida T, Friedman SL (2017). Mechanisms of hepatic stellate cell activation. Nature reviews. Gastroenterology & hepatology 14(7): 397-411.
CrossRef - Ullah A, Wang K, Wu P, Oupicky D, Sun M (2019). CXCR4-targeted liposomal mediated co-delivery of pirfenidone and AMD3100 for the treatment of TGFbeta-induced HSC-T6 cells activation. International journal of nanomedicine 14: 2927-2944.
CrossRef - Van Rooyen DM, Gan LT, Yeh MM, Haigh WG, Larter CZ, Ioannou G, et al. (2013). Pharmacological cholesterol lowering reverses fibrotic NASH in obese, diabetic mice with metabolic syndrome. Journal of hepatology 59(1): 144-152.
CrossRef - Vivero-Escoto JL, Vadarevu H, Juneja R, Schrum LW, Benbow JH (2019). Nanoparticle mediated silencing of tenascin C in hepatic stellate cells: effect on inflammatory gene expression and cell migration. Journal of materials chemistry. B 7(46): 7396-7405.
CrossRef - Wang H, Zheng M, Gao J, Wang J, Zhang Q, Fawcett JP, et al. (2020a). Uptake and release profiles of PEGylated liposomal doxorubicin nanoparticles: A comprehensive picture based on separate determination of encapsulated and total drug concentrations in tissues of tumor-bearing mice. Talanta 208:
CrossRef - Wang J, Pan W, Wang Y, Lei W, Feng B, Du C, et al. (2018). Enhanced efficacy of curcumin with phosphatidylserine-decorated nanoparticles in the treatment of hepatic fibrosis. Drug delivery 25(1): 1-11.
CrossRef - Wang M, Zhang M, Fu L, Lin J, Zhou X, Zhou P, et al. (2020b). Liver-targeted delivery of TSG-6 by calcium phosphate nanoparticles for the management of liver fibrosis. Theranostics 10(1): 36-49.
CrossRef - Wang X, Gao Y, Li Y, Huang Y, Zhu Y, Lv W, et al. (2020c). Roseotoxin B alleviates cholestatic liver fibrosis through inhibiting PDGF-B/PDGFR-beta pathway in hepatic stellate cells. Cell death & disease 11(6):
Cro0ssRef - Wong L, Yamasaki G, Johnson RJ, Friedman SL (1994). Induction of beta-platelet-derived growth factor receptor in rat hepatic lipocytes during cellular activation in vivo and in culture. The Journal of clinical investigation 94(4): 1563-1569.
CrossRef - Xu R, Li Y, Yan H, Zhang E, Huang X, Chen Q, et al. (2019). CCL2 promotes macrophages-associated chemoresistance via MCPIP1 dual catalytic activities in multiple myeloma. Cell death & disease 10(10):
CrossRef - Yan J, Huang H, Liu Z, Shen J, Ni J, Han J, et al. (2020). Hedgehog signaling pathway regulates hexavalent chromium-induced liver fibrosis by activation of hepatic stellate cells. Toxicology letters 320: 1-8.
CrossRef - Yang L, Kwon J, Popov Y, Gajdos GB, Ordog T, Brekken RA, et al. (2014). Vascular endothelial growth factor promotes fibrosis resolution and repair in mice. Gastroenterology 146(5): 1339-1350 e1331.
CrossRef - Yang L, Seki E (2012). Toll-like receptors in liver fibrosis: cellular crosstalk and mechanisms. Frontiers in physiology 3:
CrossRef - Yin C, Evason KJ, Asahina K, Stainier DY (2013). Hepatic stellate cells in liver development, regeneration, and cancer. The Journal of clinical investigation 123(5): 1902-1910.
CrossRef - You Y, Zhu F, Li Z, Zhang L, Xie Y, Chinnathambi A, et al. (2021). Phyllanthin prevents diethylnitrosamine (DEN) induced liver carcinogenesis in rats and induces apoptotic cell death in HepG2 cells. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 137:
CrossRef - Younis N, Shaheen MA, Abdallah MH (2016). Silymarin-loaded Eudragit((R)) RS100 nanoparticles improved the ability of silymarin to resolve hepatic fibrosis in bile duct ligated rats. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 81: 93-103.
CrossRef - Zhang Q, Chang X, Wang H, Liu Y, Wang X, Wu M, et al. (2020). TGF-beta1 mediated Smad signaling pathway and EMT in hepatic fibrosis induced by Nano NiO in vivo and in vitro. Environmental toxicology 35(4): 419-429.
CrossRef - Zhang Q, Xu D, Guo Q, Shan W, Yang J, Lin T, et al. (2019). Theranostic Quercetin Nanoparticle for Treatment of Hepatic Fibrosis. Bioconjugate chemistry 30(11): 2939-2946.
CrossRef - Zhang Y, Li Y, Mu T, Tong N, Cheng P (2021a). Hepatic stellate cells specific liposomes with the Toll-like receptor 4 shRNA attenuates liver fibrosis. Journal of cellular and molecular medicine 25(2): 1299-1313.
CrossRef - Zhang Y, Xie F, Yin Y, Zhang Q, Jin H, Wu Y, et al. (2021b). Immunotherapy of Tumor RNA-Loaded Lipid Nanoparticles Against Hepatocellular Carcinoma. International journal of nanomedicine 16: 1553-1564.
CrossRef - Zuo L, Zhu Y, Hu L, Liu Y, Wang Y, Hu Y, et al. (2019). PI3-kinase/Akt pathway-regulated membrane transportation of acid-sensing ion channel 1a/Calcium ion influx/endoplasmic reticulum stress activation on PDGF-induced HSC Activation. Journal of cellular and molecular medicine 23(6): 3940-3950.
CrossRef - WHO, 2018, https://www.youtube.com/watch?v=EYq7dW3ez7M








