Manuscript accepted on :05-11-2021
Published online on: 17-11-2021
Plagiarism Check: Yes
Reviewed by: Dr. Arsalan Zaidi

Second Review by: Dr. Varsha Galani

Final Approval by: Ian James Martin
Michael Ofori1* , Cynthia Amaning Danquah1
, Cynthia Amaning Danquah1 , Selase Ativui1
, Selase Ativui1 , Peace Doe2
, Peace Doe2  and Williams Adu Asamoah1
and Williams Adu Asamoah1
1Department of Pharmacology, Faculty of Pharmacy and Pharmaceutical Sciences, College of Health Sciences, Kwame Nkrumah University of Science and Technology, PMB, Kumasi Ghana.
2Department of Pharmaceutical Science, School of Pharmacy, Central University, Ghana
Corresponding Author E-mail: mofori28@st.knust.edu.gh
DOI : https://dx.doi.org/10.13005/bpj/2289
Abstract
Drug resistant tuberculosis remains one of the major challenges associated with treatment and management of tuberculosis (TB) in the public health system and in clinical settings. In 2020, the World Health Organization (WHO) estimated that about 186,772 people died from drug-resistant tuberculosis out of the 500000 reported cases and this is alarming. There is a pressing need from every angle in drug discovery to develop novel compounds that could possess diverse mechanisms of action to tackle drug-resistant tuberculosis. The Crinum asiaticum bulbs extract are used ethno medicinally to treat upper respiratory tract infections and as well as wound healing agent. The aim of this work is to investigate the in-vitro anti-tuberculosis effect of Crinum asiaticum bulbs extracts and to assess the inhibitory properties against bacteria efflux pumps expression and biofilm formation. The results obtained showed that the Crinum asiaticum bulbs extracts (CAE) were effective in inhibiting Mycobacterium smegmatis (NCTC 8159) and Mycobacterium aurum (NCTC 10437) with minimum inhibitory concentration (MIC) of 125 μg/ml and 250 μg/ml against M. smegmatis and M. aurum respectively. The CAE markedly inhibited the efflux pumps of both M. smegmatis and M. aurum from expressing with the chloroform extract producing the greatest inhibition. The CAE (ethanol, methanol, chloroform and hexane) significantly (***ρ˂0.005) inhibited M. smegmatis’ and M. aurum’s biofilm formation in-vitro. Among the various extracts of Crinum asiaticum, the chloroform extract exhibited the greatest inhibition against M. smegmatis and M. aurum biofilm formation with significance levels of ***ρ˂0.005 and ***ρ˂0.005. In conclusion the CAE has anti-tuberculosis effect and could tackle drug resistant TB as exhibited through the anti-efflux and anti-biofilm forming properties of the extract against the selected Mycobacterium species.
Keywords
Biofilm; Crinum asiaticum; Efflux pumps; HT-SPOTi; Mycobacterium
Download this article as:| Copy the following to cite this article: Ofori M, Danquah C. A, Ativui S, Doe P, Asamoah W. A. In-Vitro Anti-Tuberculosis, Anti-Efflux Pumps and Anti-Biofilm Effects of Crinum Asiaticum Bulbs. Biomed Pharmacol J 2021;14(4). |
| Copy the following to cite this URL: Ofori M, Danquah C. A, Ativui S, Doe P, Asamoah W. A. In-Vitro Anti-Tuberculosis, Anti-Efflux Pumps and Anti-Biofilm Effects of Crinum Asiaticum Bulbs. Biomed Pharmacol J 2021;14(4). Available from: https://bit.ly/3HuvlVG |
Introduction
Tuberculosis (TB) is a communicable disease caused by Mycobacterium tuberculosis and a major cause of ill health 1. It is ranked among the top ten (10) cause of death worldwide and the number one cause of death from a single infectious agent 2. The insurgence of multidrug and extensively drug-resistant tuberculosis (MDR/XDR-TB) is a major problem in the treatment and management of tuberculosis 3. There was a collaborative effort to curb this by the repurposing of standard drugs to tackle drug resistant tuberculosis 4,5. Efforts made by most research institutions toward the development of new antibiotics had proven futile due to emerging resistance that had crippled down antimicrobial research 6. World Health Organization (WHO) 2020 global TB report estimated 500,000 cases of multi-drug resistant TB (MDR-TB) of which 186,772 MDR-TB deaths were confirmed 7. Among new TB cases, 3.5 % are either resistant to the most effective drug that is rifampicin or attaining to multi-drug resistant, if care not taking then there would be an increasing trend in the number of resistant TB cases around the globe 8.
Efflux pump plays a significant role in the evolution of resistance in Mycobacterium tuberculosis serving as transporter for several structural substances and noxious compounds that include antibiotics 9–11. This reduces the intracellular concentration of the pharmacologically active agents and contributes to drug-resistant TB 12. Agents that inhibit Mycobacterium tuberculosis efflux pumps could serve as an adjunct to enhance the sensitivity of established anti-tubercular drugs 13.
Microorganisms that form biofilms are leading cause of nosocomial and recurrent infections, they form a sticky exopolysaccharide which is the main virulence factor causing biofilm-related infections 14. Bacteria embedded in biofilm are more resistant to antimicrobials than planktonic bacteria, making treatment difficult 15,16. Study of biofilms in Mycobacterium smegmatis and Mycobacterium aurum as potential surrogate model for Mycobacterium tuberculosis aid in understanding biofilm formation in other pathogenic Mycobacteria like Mycobacterium tuberculosis 17. The global TB emergency has been further exacerbated by extensively drug-resistant (XDR) TB strains that are resistant to our best antibiotics and very difficult to treat, this stimulates an urgent need for the development of new drugs for the treatment of Mycobacterial infections 18. Agents possessing anti-Mycobacterium effect with anti-efflux and anti-biofilm forming activities are important in the tackling of multi-drug and extensively-drug resistant tuberculosis. These agents may increase the concentration of anti-tuberculosis drugs within Mycobacterium cell and therefore increase the pharmacologic and therapeutic effects 19,20.
Crinum asiaticum is a perennial bulbous plant and widely distributed in Malaysia, Papua New Guinea and Mauritius 21. The ethno medicinal uses of the plant vary from country to country 22,23. In Ghana, the plant is prevalent in the southern Ashanti, traditionally used to treat and manage upper respiratory tract infections, inflammatory disorders and skin infections caused by bacteria and fungi. The plant contains several alkaloids with distinct pharmacological activities 24. Antinociceptive and antioxidant effects of the plant had been reported 25,26. Analgesic and anti-inflammatory properties of the bulbs of Crinum asiaticum had also been reported27,28.
Materials and Method
Materials
Rotary evaporator (Buchi Labotechnik Rotavap R-210), autoclave (SANOclav), centrifuge (Joshansen, Germany), Middle Brook 7H10 agar (Difco), Middle brook 7H9 broth (Difco), 96-well half skirted PCR plates, 96-well micro titre plates (Star lab, UK), Crinum asiaticum bulbs, Tryptase soy broth (Himedia laboratory), fluorimeter (Joshansen, Germany), Spectrophotometer (Joshansen, Germany).
Drugs and chemicals
N-hexane (Surechem), Methanol (Surechem), Chloroform (Prolabo), Ethanol (LabChem), Ethidium bromide solution (Sigma-Aldrich Inc), OADC, 0.5% (V/V) Glycerol, 0.2% (V/V) Tween 80, verapamil (Ernest Chemist Ltd, Ghana), 80 % glucose w/v (Sigma G7528).
Mycobacterial species
Mycobacterium smegmatis (NCTC 8159) and Mycobacterium aurum (NCTC 10437) strains obtained from public health, England and stored in the cell culture laboratory in the Department of Pharmacology, Kwame Nkrumah University of Science and Technology (KNUST).
Plant materials collection and preparation
The bulbs of Crinum asiaticum were harvested at the forecourt of the Department of Horticulture, KNUST with GPS code 6.6790397, -1.5660286 and was authenticated by Mr Clifford Asare in the Department of Herbal Medicine, KNUST with herbarium specimen code KNUST/HM2020/B004. The bulbs were washed in clean water, chopped and blended fresh, extraction was made using different organic solvents with varied polarities (70 % ethanol, 99.8 % methanol, 99.8 % chloroform and 95 % hexane). Cold maceration conducted for 72 hours with constant stirring. Filtration was done and filtrates were concentrated using rotary evaporator (Buchi Labotechnik Rotavap R-210). The concentrated extracts were stored in containers, sealed, labeled and refrigerated at 4 ˚C.
Qualitative phytochemical screening on the bulbs of Crinum asiaticum extract
The bulbs of Crinum asiaticum extract (CAE) was screened for the presence of secondary metabolites such as flavonoids, tannins, triterpenoids, glycosides, sterols, coumarins, saponins, alkaloids. Experimental procedures were based on different methods prescribed.
Test for alkaloids
Four drops of 2 % H2SO4 were added to 5 ml of CAE extract, filtered and three drops of Dragendoff’s reagent added to the 1 ml of filtrate. Orange red precipitation formed suggested the presence of Alkaloids 29.
Test for tannins
This test was based on what was reported by Maxson and Rooney. Three drops of FeCl2 were added to the 1 ml filtrate of CAE, formation of dark green colour indicated the presence of tannins30.
Test for saponins
On 1 ml of CAE filtrate, 2 ml of distilled water was added and shaken vigorously. The mixture was allowed to stand for 10 minutes and the formation of foam on the surface of the mixture persisted more than 10 minutes indicated the presence of saponin 31.
Test for flavonoids
One milliliter of CAE filtrate was added to 2 ml of dilute NaOH. A golden yellow colour indicated the presence of flavonoids 32.
Test for glycosides
An amount of 5 ml of the filtrate of CAE was mixed with 25 ml of dilute H2SO4, the mixture was boiled for 15 minutes, cooled and neutralized with 10 % NaOH. Five milliliters of Fehling’s solution was added to the mixture. Brick red precipitate indicated the presence of glycosides 33.
Test for triterpenoids
A mixture of 5 ml of extract and 2 ml of chloroform was formed, four drops of concentrated sulphuric acid was carefully added. Reddish brown layer at the junction indicated the presence of triterpenoids 33.
In-vitro anti-Mycobacterial screening of Crinum asiaticum bulbs extracts (CAE).
Screening of anti-Mycobacterial property of the bulbs of Crinum asiaticum extracts (methanol, ethanol, chloroform and hexane extract) against Mycobacterium smegmatis and Mycobacterium aurum was investigated using high–throughput spot culture growth inhibition assay (HT-SPOTi) technique 34.
Middle-brook 7H10 (MB7H10) agar with 0.5% glycerol was autoclaved. Oleic albumin dextrose and catalase (OADC 10 % v/v) was added to the agar as supplement. The agar was placed in water bath at 55° to 60°C to avoid solidification.
Two-folds serial dilution of the extracts were carried out in 0.3 % dimethyl sulfoxide (DMSO) using PCR half-skirted 96-well plate to give a wider range of concentration.
Mycobacterial strains (Mycobacterium smegmatis (NCTC 8159) and Mycobacterium aurum (NCTC 10437) were inoculated in 10 ml MB7H10 contained in falcon tubes and incubated at 37 ˚C for 18-24 hours. After the incubated period, microbial growth on the agar were washed with 0.9 % (w/v) normal saline solution. Serial dilution began when 100 µL of the washed bacterial was added into a falcon tube containing 10 ml sterile normal saline, followed with a transfer of 1 ml of the bacterial suspension form the previous tube into a new falcon tube containing 9 ml of normal saline, 1 ml of the suspension was further transferred into the last falcon tube containing 19 ml of normal saline to mark the end of the serial dilution method (content in the last falcon tube was used for the SPOTI). Two microliters of the various Crinum asiaticum bulbs extracts (CAE) taken from PCR half-skirted plate was dispensed in 96-well plate accordingly as reported 34. In each well of the plate containing the already dispensed CAE, 200 µL of the prepared agar was added followed with the addition of 2 µL of the bacterial suspension (content in the last falcon tube) with the aid of a multi-channel pipette onto the agar.
Plate was covered, sealed with paraffin then wrapped with aluminum foil. Plate incubated at 37˚C for 18-24 h. After incubation, plate was observed and the minimum inhibitory (MIC) concentration determined.
In-vitro determination of efflux pumps inhibitory activity of Crinum asiaticum bulb extracts against Mycobacterium smegmatis and Mycobacterium aurum.
The efflux-pump inhibitory assay is simple and utilizes ethidium bromide (EtBr) which is a known substrate for efflux pumps and the method to determine the efflux pump inhibitory effects of agents has been reported 35. This is an improved and optimized method as compared to previously reported methods 36,37. Verapamil a known efflux pump inhibitor (EPI) was used as standard drug in this experiment.
Mycobacterium smegmatis (NCTC 8159) and Mycobacterium aurum (NCTC 10437) were cultured in MB7H9 broth supplemented with albumin, dextrose and catalase (ADC). Optical density (OD) of the bacterial suspension was determined to be 0.8. Five milliliters (5 ml) of the bacterial suspension was added to 5 ml of MB7H9 and the OD was adjusted to 0.4. This was achieved by the addition of broth or bacterial suspension in sterile falcon tube. Bacterial suspension with OD 0.4 was centrifuged at 3000 rmp for 10 min. Supernatant was discarded and pellets re-suspended in sterile 10 ml of phosphate buffer saline (PBS) by vortexing.
The minimum inhibitory concentration of the extracts determined earlier was halved in order to work with sub minimum concentrations per ml of the extracts. The concentration of verapamil used was 125 mg/L 35. Into a well-labelled eppendorf tube, 500 µL of buffered bacterial suspension (test group) was added and 500 µL of PBS was dispensed into an eppendorf tube to serve as the (blank group). Two microliters of the sub-MIC of drug samples were added into each eppendorf tube (test and blank) followed with 2.5 µL of 80 % (w/v) glucose. Contents in the eppendorf tubes were thoroughly mixed by vortexing. (100 µL) aliquot was transferred into a sterile 96-well plate.
Lastly, 5 µL EtBr with concentration of 50 mg/L was added to each well and was immediately inserted into a fluorimeter with excitation wavelength of 530nm and emission wavelength of 600nm. The relative fluorescence values were recorded, analyzed and plotted as the relative fluorescence against time.
In-vitro determination of biofilm inhibitory effect of Crinum asiaticum bulbs extracts against Mycobacterium smegmatis (NCTC 8159), Mycobacterium aurum (NCTC 10437)
This method for the determination of anti-biofilm effect of natural agents has been reported previously 14. The use of micro titer plate for biofilm formation and the screening of anti-biofilm activities of agents can be quantitatively determined 35,38,39.
Mycobacterium smegmatis (NCTC 8159) and Mycobacterium aurum (NCTC 10437) were inoculated in 3-5 ml sterile trypticase soy broth (TSB) and incubated for 24 h at 37˚C. Mycobacterial cultures after incubation was diluted (1:100) immediately in a sterile TSB. Hundred microliters (100 µL) of diluted bacterial suspension was dispensed into designated wells in 96-well plate. Two wells each for extract, one for standard drug (ciprofloxacin), one as negative control (Mycobacterium cells only) and two for blank (broth + extract only). Hundred microliters (100 µL) of 5 % extract was dispensed into the wells contained the bacterial suspension, plate was covered with cling film, wrapped in aluminum foil and incubated at 37˚C for 24 h. Contents were aspirated out from the wells after incubation and plate was thoroughly washed with PBS. Designated wells were stained with 125 µL 0.1% crystal violet (w/v) solution for 10 min, thereafter plate was washed with distilled water to remove stains and plate dried at room temperature. (200 µL) of 95 % alcohol was dispensed into the wells and plate was incubated for 15 minutes at room temperature, 125 µL of content in each well was transferred (ethanol/crystal violet stains) into a separate 96-well plate in triplicate.
The optical density was measured at wavelength 630 nm, results analyzed as total biofilm formed and percentage biofilms inhibition.
Percentage Biofilm inhibition;-
Where, control is Mycobacterium cells only
Blank is growth medium and drug only
Results and discussion
Phytochemical screening
Table 1: Phytochemical screening of Crinum asiaticum bulbs
| Phyto-constituents | Presence |
| Glycosides | + |
| Tannins | + |
| Alkaloids | + |
| Saponins | + |
| Triterpenoid | + |
|
Flavonoids |
+ |
Anti-Mycobacterial screening of Crinum asiaticum bulbs extracts
The extracts were screened against Mycobacterium smegmatis and it was established that methanolic, ethanolic, chloroformic, and hexane extracts exhibited minimum inhibitory concentrations (MIC) of 125, 250, 500, and 500 µg/ml respectively.
Table 2: Minimum inhibitory concentrations (MIC) of Crinum asiaticum bulb extracts. MIC (µg/ml)
| Bacteria strains | Ethanolic extract | Chloroformic extract | Methanolic extract | Hexane extract | Isoniazid |
| Mycobacterium smegmatis | 250 | 250 | 125 | 500 | 62.5 |
| Mycobacterium aurum | 500 | 250 | 500 | 250 | 62.5 |
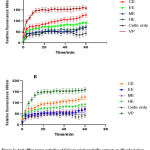
Efflux pump inhibitory effects of Crinum asiaticum bulbs extracts on Mycobacterium smegmatis and Mycobacterium aurum
The principle behind this assay is that a known substrate of efflux pumps called ethidium bromide (EtBr) fluoresces when in living microorganism but does not show any fluorescence outside the cells. The amount of fluorescence produced depends on the accumulation of EtBr in the cells of microorganisms 40. The fluorescence produced was measured with fluorimeter and was plotted against time.
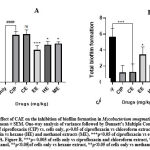
The effect of Crinum asiaticum bulb extracts on Mycobacterium smegmatis biofilm formation.
There were significant inhibition on biofilm formation by the extracts of Crinum asiaticum bulbs (CAE) with the chloroformic extract showing the greatest inhibition. Effects of extracts were analyzed and represented as a percentage of inhibition (figure 2A) and the total biofilm formed (figure 2B) after anti biofilm screening of CAE.
The effect of Crinum asiaticum bulb extracts on Mycobacterium aurum biofilm formation.
The various extracts of Crinum asiaticum bulbs (CAE) showed significant inhibitory effects on M. aurum biofilm formation. A and B showed quantitative representation on how well CAE were able to inhibit the bacteria from forming biofilm. Figure 3A showed the percentage inhibition of biofilm exhibited by the various extracts, figure 3B showed the amount of biofilm formed after treatment with the individual extracts.
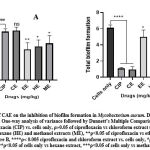 |
Figure 3: Effect of CAE on the inhibition of biofilm formation in Mycobacterium aurum. Data was expressed as mean ± SEM. One-way analysis of variance followed by Dunnett’s Multiple Comparison Test. |
Management of tuberculosis is threatened with the insurgence of multi-drug resistance and extensively drug resistance tuberculosis in medicine and public health 41–43. Anti–tuberculosis agents on martek are not functioning effectively due to the emergence of resistance in our health care systems; this had led to long treatment duration resulting to patient non-compliance and had contributed to sub-lethal therapeutic dose 44. There are higher demands for new anti-microbial agents that could be used to tackle drug resistance in tuberculosis management 45,46, therefore the need to discover and develop new compounds with novel mechanism of action effective in the management of tuberculosis.
Plants possess wider range of secondary metabolites and the presence of these metabolites represent the medicinal properties of plants 47. The Crinum asiaticum bulbs extracts (CAE) contains glycosides, tannins, alkaloids, triterpenoids, saponins and flavonoids (Table 1). The bioactivities exhibited by the Crinum asiaticum bulbs extracts could be due to the listed secondary metabolites. Bioactivities of CAE were tested against Mycobacterium tuberculosis surrogates model species; thus Mycobacterium smegmatis and Mycobacterium aurum since they are fast growing, non pathogenic and are used in high through put screening for tuberculosis drug discovery 48–50. Moreover, these strains share most genes with M. tuberculosis 51 making them ideal for this work.
The in-vitro anti-tubercular effect of Crinum asiaticum bulbs extracts (CAE) were tested against Mycobacterium smegmatis and Mycobacterium aurum, the MIC recorded (Table 2) showed marked inhibition of the extracts against growth of the selected microorganisms. On M. smegmatis, methanolic extract produced an MIC as low as 125 µg/ml whiles ethanolic and chloroformic extracts on the other hand produced MIC of 250 µg/ml. Minimum inhibitory concentrations recorded when CAE tested against Mycobacterium aurum gave significant effects with chloroform and hexane extracts producing greater inhibitory effects of MIC 250 µg/ml. The MIC’s recorded demonstrate some level of prospect in terms of the anti-tuberculosis potential of the bulbs of Crinum asiaticum.
Drug extrusion by multi-drug efflux pump serves as an important factor among the major mechanisms that lead to multi-drug-resistant and extensively drug resistant tuberculosis 52,53. Mycobacterium efflux pumps inhibitors improve tuberculosis therapy54 by inhibiting the expression of the bacteria efflux pumps which could enhance the therapeutic effects of already established anti-tuberculous drugs, by reducing resistance associated with TB therapy thus serving as an adjunct therapy 54–56. The Ethidium bromide (EtBr) is a substrate for efflux pumps, the accumulation of the EtBr intracellularly suggests how effective an agent inhibits the efflux pumps therefore increased fluorescence level 57. In recent years, there had been increased interest in the discovery and development of compounds that could inhibit microbial efflux pumps 55. In the efflux pump inhibitory assay, the various Crinum asiaticum bulb extracts gave marked inhibitory effect on M. smegmatis and M. aurum efflux pumps activation with the chloroform extract showing the greatest inhibition compared to the others (Figure 1). The in-vitro inhibitory effects of CAE suggests is a good lead for the development of bacterial efflux pump inhibitors.
Biofilm formation is one of the phenomenon that contribute to bacteria resistance to antibiotics 58. They are formed on surfaces of objects severely interrupting treatment 59. Several evidences suggested that natural products from plant possess antimicrobial and anti biofilm effects that had provided novel techniques to tackle multi-drug resistance 60,61. The Crinum asiaticum bulbs extracts produced significant biofilm inhibitory effects (***ρ ˂ 0.05) on M. smegmatis and M. aurum (Figure 2 and 3). The chloroform extract performed better in inhibiting the bacteria biofilm. It reduced M. smegmatis (Figure 2A and B) biofilm significantly (***ρ ˂ 0.05) with a percentage inhibition of 77.6 ± 4.445 %. The hexane and methanol extract also did better in the inhibition of M. smegmatis biofilm (**ρ ˂ 0.05) with percentage biofilm inhibition of 60.45 ± 3.562 % and 62.32 ± 2.132 % respectively. The ethanol extract performed poorly in inhibiting M. smegmatis biofilm (*ρ ˂ 0.05) with a percentage biofilm inhibition of 52.14 ± 5.261 %. On M. aurum biofilm formation (Figure 3A and B), chloroform, ethanol, methanol and hexane extracts significantly (***ρ ˂ 0.005) inhibited the bacterium biofilm formation with their respective percentage biofilm of 84.7 ± 1.324, 52.43 ± 1.249, 63.08 ± 2.178, 57.62 ± 1.938. The chloroform extract produced the greatest inhibition (***ρ ˂ 0.005) compared to other extracts. This suggests the anti-biofilm activity of CAE against M. smegmatis and M. aurum, it could be used to develop lead anti-biofilm compounds. The Crinum asiaticum bulbs extracts exhibited anti-Mycobacterial activity, efflux pump inhibitory effect and anti-biofilm forming effect. A drug which is a good efflux pump inhibitor and biofilm inhibitor could be working by different mechanisms which will be difficult for resistance to develop 62–64, therefore Crinum asiaticum bulb could be a suitable candidate.
Conclusion
This study demonstrates that Crinum asiaticum bulbs extracts possess anti-tuberculosis activity in vitro and this was exhibited through its susceptibility against Mycobacterium smegmatis and Mycobacterium aurum, efflux pump and biofilm inhibition. This could serve as lead in drug discovery to develop compounds with varying mechanisms that could tackle multi drug resistant tuberculosis, also it could be used as adjunct to enhance the susceptibility of standard anti-tubercular drugs.
Acknowledgement
The authors would like to thank the technical staff at the Cell Culture Laboratory and the drug discovery team in the Department of Pharmacology, Kwame Nkrumah University of Science and Technology (KNUST) for their immense contribution towards this work.
Conflict of Interest
This is to certify that the authors have no conflict of interest
Funding Sources
There is no funding source.
References
- World Health Organization report. Global tuberculosis report. J Chem Inf Model. 2020;53(9):1689-1699.
- Ghebreyesus T. Global tuberculosis report 2019. World Heal Organ. 2019;92(4):1-297. apps.who.int/bookorders.
- Seung KJ, Keshavjee S, Rich ML. Multidrug-Resistant Tuberculosis and Extensively Drug-Resistant Tuberculosis. Cold Spring Harb Perspect Med. 2015;5(9):a017863-a017863. doi:10.1101/cshperspect.a017863
CrossRef - Maitra A, Bates SDS, Shaik M, et al. Repurposing drugs for treatment of tuberculosis: A role for non-steroidal anti-inflammatory drugs. Br Med Bull. 2016;118(1):138-148. doi:10.1093/bmb/ldw019
CrossRef - Maitra A, Evangelopoulos D, Chrzastek A, et al. Carprofen elicits pleiotropic mechanisms of bactericidal action with the potential to reverse antimicrobial drug resistance in tuberculosis. J Antimicrob Chemother. 2020;75(11):3194-3201. doi:10.1093/jac/dkaa307
CrossRef - Scaini R, Dias A, Rosa V, et al. Journal of Molecular Graphics and Modelling Molecular modelling and competitive inhibition of a Mycobacterium tuberculosis multidrug-resistance ef fl ux pump. 2019;87:98-108. doi:10.1016/j.jmgm.2018.11.016
CrossRef - Tiberi S, Vjecha MJ, Zumla A, Galvin J, Migliori GB, Zumla A. Accelerating development of new shorter TB treatment regimens in anticipation of a resurgence of multi-drug resistant TB due to the COVID-19 pandemic. Int J Infect Dis. 2021;(xxxx):2-5. doi:10.1016/j.ijid.2021.02.067
CrossRef - Chapman E, Bhakta S. Whole-Cell Assays for Discovering Novel Efflux Inhibitors for Use as Antibiotic Adjuvants. 2019:34-37. doi:10.32474/CTBM.2019.01.000109
CrossRef - Rossi E De, Aínsa JA, Riccardi G. Role of mycobacterial efflux transporters in drug resistance: an unresolved question. FEMS Microbiol Rev. 2006;30(1):36-52. doi:10.1111/j.1574-6976.2005.00002.x
CrossRef - Amaral L, Martins A, Spengler G, Molnar J. Efflux pumps of Gram-negative bacteria: What they do, how they do it, with what and how to deal with them. Front Pharmacol. 2014;4 JAN(January):1-11. doi:10.3389/fphar.2013.00168
CrossRef - Balganesh M, Dinesh N, Sharma S, Kuruppath S, Nair AV, Sharma U. Efflux pumps of Mycobacterium tuberculosis play a significant role in antituberculosis activity of potential drug candidates. Antimicrob Agents Chemother. 2012;56(5):2643-2651. doi:10.1128/AAC.06003-11
CrossRef - Maier V, Kunert O, Bucar F. Putative Mycobacterial E ffl ux Inhibitors from the Seeds of Aframomum melegueta. 2012.
- Balganesh M, Dinesh N, Sharma S, Kuruppath S, Nair AV, Sharma U. Efflux Pumps of Mycobacterium tuberculosis Play a Significant Role in Antituberculosis Activity of Potential Drug Candidates. Antimicrob Agents Chemother. 2012:2643-2651. doi:10.1128/AAC.06003-11
CrossRef - Kırmusaoğlu S. The Methods for Detection of Biofilm and Screening Antibiofilm Activity of Agents. 2019.
CrossRef - Gebreyohannes G, Nyerere A, Bii C, Sbhatu DB. Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon. 2019;5(8):e02192-e02192. doi:10.1016/j.heliyon.2019.e02192
CrossRef - Abebe GM. The Role of Bacterial Biofilm in Antibiotic Resistance and Food Contamination. Chaves Lopez C, ed. Int J Microbiol. 2020;2020:1705814. doi:10.1155/2020/1705814
CrossRef - Abidi SH, Ahmed K, Sherwani SK. Original Research Article Detection of Mycobacterium Smegmatis Biofilm and its Control by Natural Agents Mycobacterium smegmatis culture. 2014;3(4):801-812.
- Haydel SE. Extensively drug-resistant tuberculosis: A sign of the times and an impetus for antimicrobial discovery. Pharmaceuticals. 2010;3(7):2268-2290. doi:10.3390/ph3072268
CrossRef - Di Perri G, Bonora S. Which agents should we use for the treatment of multidrug-resistant Mycobacterium tuberculosis? J Antimicrob Chemother. 2004;54(3):593-602. doi:10.1093/jac/dkh377
CrossRef - Angula KT, Legoabe LJ, Beteck RM. Chemical classes presenting novel antituberculosis agents currently in different phases of drug development: A 2010–2020 review. Pharmaceuticals. 2021;14(5). doi:10.3390/ph14050461
CrossRef - Mahomoodally MF, Sadeer NB, Suroowan S, Jugreet S, Lobine D, Rengasamy KRR. Ethnomedicinal, phytochemistry, toxicity and pharmacological benefits of poison bulb – Crinum asiaticum L. South African J Bot. 2021;136:16-29. doi:10.1016/j.sajb.2020.06.004
CrossRef - Haque M, Jahan S, Rahmatullah M. Ethnomedicinal Uses of Crinum asiaticum : a Review. World J Pharm Pharm Sci. 2014;3(9):119-128.
- Asmawi MZ, Arafat OM, Amirin S, Eldeen IM. In vivo Antinociceptive Activity of Leaf Extract of Crinum asiaticum and Phytochemical Analysis of the Bioactive Fractions. Int J Pharmacol. 2010;7(1):125-129. doi:10.3923/ijp.2011.125.129
CrossRef - Riris ID, Simorangkir M, Silalahi A. Antioxidant, toxicity and antibacterial properties of ompu-ompu (Crinum asiaticum-L) ethanol extract. Rasayan J Chem. 2018;11(3):1229-1235. doi:10.31788/RJC.2018.1133090
CrossRef - Ratnasooriya WD, Deraniyagala SA, Bathige SDNK, Hettiarachchi HDI. Leaf extract of Crinum bulbispermum has antinociceptive activity in rats. J Ethnopharmacol. 2005;97(1):123-128. doi:10.1016/j.jep.2004.10.024
CrossRef - Jeong YJ, Sohn EH, Jung YH, et al. Anti-obesity effect of Crinum asiaticum var. japonicum Baker extract in high-fat diet-induced and monogenic obese mice. Biomed Pharmacother. 2016;82:35-43. doi:10.1016/j.biopha.2016.04.067
CrossRef - Kim YH, Kim KH, Han CS, et al. Anti-inflammatory activity of Crinum asiaticum Linne var. japonicum extract and its application as a cosmeceutical ingredient. J Cosmet Sci. 2008;59(5):419-430.
- Md AR, S M Azad H, Nazim UA, Md SI. Analgesic and anti-inflammatory effects of Crinum asiaticum leaf alcoholic extract in animal models. African J Biotechnol. 2013;12(2):212-218. doi:10.5897/ajb12.1431
CrossRef - Trease, G. E., Evans WC. Pharmacognosy 16th edition. WB Sanders Co Ltd, New York. 2009:42–44. 221–229, 246–249, 304–306, 331–332, 391–393.
CrossRef - Maxson E.D and Rooney L.W. Chem49_719.Pdf. 1972:11.
CrossRef - Ajayi AO, Fadeyi TE. Antimicrobial Activities and Phytochemical Analysis of Moringa oleifera Leaves on Staphylococus aureus and Streptococcus species. Am J Phytomedicine Clin Ther. 2015;10(3):1-11. www.ajpct.org.
CrossRef - Edeoga H.O, Okwu D., Mbaebie BO. Phytochemical Constituents of Some Nigerian Medicinal plants. African J Biotechnol. 1989;53(July):160.
CrossRef - Gupta J, Gupta A. Preliminary phytochemical screening of leaves of Moringa oleifera Lam . Preliminary phytochemical screening of leaves of Moringa oleifera Lam . 2018;(March 2014).
CrossRef - Danquah CA, Maitra A, Gibbons S, Faull J, Bhakta S. HT-SPOTi: A rapid drug susceptibility test (DST) to evaluate antibiotic resistance profiles and novel chemicals for anti-infective drug discovery. Curr Protoc Microbiol. 2016;2016(February):17.8.1-17.8.12. doi:10.1002/9780471729259.mc1708s40
CrossRef - Danquah CA, Kakagianni E, Khondkar P, et al. Analogues of Disulfides from Allium stipitatum Demonstrate Potent Anti-tubercular Activities through Drug Efflux Pump and Biofilm Inhibition. Sci Rep. 2018;8(1):1-7. doi:10.1038/s41598-017-18948-w
CrossRef - Martins M, Santos B, Martins A, et al. An instrument-free method for the demonstration of efflux pump activity of bacteria. In Vivo (Brooklyn). 2006;20(5):657-664.
- Martins M, McCusker MP, Viveiros M, et al. A Simple Method for Assessment of MDR Bacteria for Over-Expressed Efflux Pumps. Open Microbiol J. 2013;7(1):72-82. doi:10.2174/1874285801307010072
CrossRef - Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: From the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95-108. doi:10.1038/nrmicro821
CrossRef - Abidi SH, Ahmed K, Sherwani SK, Bibi N, U.Kazmi S. Detection of Mycobacterium Smegmatis Biofilm and its Control by Natural Agents. Int J Curr Microbiol Appl Sci. 2014;3(4):801-812.
- Rodrigues L, Wagner D, Viveiros M, et al. Thioridazine and chlorpromazine inhibition of ethidium bromide efflux in Mycobacterium avium and Mycobacterium smegmatis. J Antimicrob Chemother. 2008;61(5):1076-1082. doi:10.1093/jac/dkn070
CrossRef - Hmama Z. Management of Drug-Resistant TB. 2013.
CrossRef - Augustine J, Jain N. Cross roads in the management of Multi Drug Resistant Tuberculosis (MDR-TB). Curr Med Res Pract. 2014;4(2):78-82. doi:10.1016/j.cmrp.2014.03.001
CrossRef - Otu A, Umoh V, Habib A, Ameh S, Lawson L, Ansa V. Drug resistance among pulmonary tuberculosis patients in Calabar, Nigeria. Pulm Med. 2013;2013. doi:10.1155/2013/235190
CrossRef - Sandhu GK. Tuberculosis: current situation, challenges and overview of its control programs in India. J Glob Infect Dis. 2011;3(2):143-150. doi:10.4103/0974-777X.81691
CrossRef - Hoagland DT, Liu J, Lee RB, Lee RE. New agents for the treatment of drug-resistant Mycobacterium tuberculosis. Adv Drug Deliv Rev. 2016;102:55-72. doi:10.1016/j.addr.2016.04.026
CrossRef - Annunziato G. Strategies to Overcome Antimicrobial Resistance (AMR) Making Use of Non-Essential Target Inhibitors: A Review. Int J Mol Sci. 2019;20(23):5844. doi:10.3390/ijms20235844
CrossRef - Cholarajan A, Cholarajan A, Thirumurugan D. An Introductory Introductory Chapter : Chapter : Secondary Secondary Metabolites Metabolites Durairaj. 2018:3-22. doi:10.5772/intechopen.79766
CrossRef - Gupta A, Bhakta S, Kundu S, Gupta M, Srivastava BS, Srivastava R. Fast-growing, non-infectious and intracellularly surviving drug-resistant Mycobacterium aurum: A model for high-throughput antituberculosis drug screening. J Antimicrob Chemother. 2009;64(4):774-781. doi:10.1093/jac/dkp279
CrossRef - Gupta A, Bhakta S. An integrated surrogate model for screening of drugs against Mycobacterium tuberculosis. J Antimicrob Chemother. 2012;67(6):1380-1391. doi:10.1093/jac/dks056
CrossRef - Namouchi A, Cimino M, Favre-Rochex S, Charles P, Gicquel B. Phenotypic and genomic comparison of Mycobacterium aurum and surrogate model species to Mycobacterium tuberculosis: Implications for drug discovery. BMC Genomics. 2017;18(1):25-28. doi:10.1186/s12864-017-3924-y
CrossRef - Malhotra S, Vedithi SC, Blundell TL. Decoding the similarities and differences among mycobacterial species. PLoS Negl Trop Dis. 2017;11(8):1-18. doi:10.1371/journal.pntd.0005883
CrossRef - Sun J, Deng Z, Yan A. Bacterial multidrug efflux pumps: Mechanisms, physiology and pharmacological exploitations. Biochem Biophys Res Commun. 2014;453(2):254-267. doi:10.1016/j.bbrc.2014.05.090
CrossRef - Amaral L, Martins M, Viveiros M. Enhanced killing of intracellular multidrug-resistant Mycobacterium tuberculosis by compounds that affect the activity of efflux pumps. 2007;(January):1237-1246. doi:10.1093/jac/dkl500
CrossRef - Pule CM, Sampson SL, Warren RM, et al. Efflux pump inhibitors: Targeting mycobacterial efflux systems to enhance TB therapy. J Antimicrob Chemother. 2016;71(1):17-26. doi:10.1093/jac/dkv316
CrossRef - Szumowski JD, Adams KN, Edelstein PH, Ramakrishnan L. Antimicrobial efflux pumps and Mycobacterium tuberculosis drug tolerance: evolutionary considerations. Curr Top Microbiol Immunol. 2013;374:81-108. doi:10.1007/82_2012_300
CrossRef - Rodrigues L, Cravo P, Viveiros M. Efflux pump inhibitors as a promising adjunct therapy against drug resistant tuberculosis: a new strategy to revisit mycobacterial targets and repurpose old drugs. Expert Rev Anti Infect Ther. 2020;18(8):741-757. doi:10.1080/14787210.2020.1760845
CrossRef - Pal S, Misra A, Banerjee S, Dam B. Journal of King Saud University – Science Adaptation of ethidium bromide fluorescence assay to monitor activity of efflux pumps in bacterial pure cultures or mixed population from environmental samples. J King Saud Univ – Sci. 2020;32(1):939-945. doi:10.1016/j.jksus.2019.06.002
CrossRef - Cepas V, López Y, Muñoz E, et al. Relationship between Biofilm Formation and Antimicrobial Resistance in Gram-Negative Bacteria. Microb Drug Resist. 2019;25(1):72-79. doi:10.1089/mdr.2018.0027
CrossRef - Abebe GM. The Role of Bacterial Biofilm in Antibiotic Resistance and Food Contamination. Int J Microbiol. 2020;2020. doi:10.1155/2020/1705814
CrossRef - Lu L, Hu W, Tian Z, et al. Developing natural products as potential anti-biofilm agents. Chinese Med (United Kingdom). 2019;14(1):1-17. doi:10.1186/s13020-019-0232-2
CrossRef - Alam K, Farraj DAA, Mah-e-Fatima S, et al. Anti-biofilm activity of plant derived extracts against infectious pathogen-Pseudomonas aeruginosa PAO1. J Infect Public Health. 2020;13(11):1734-1741. doi:10.1016/j.jiph.2020.07.007
CrossRef - Sharma A, Gupta VK, Pathania R. Efflux pump inhibitors for bacterial pathogens: From bench to bedside. Indian J Med Res. 2019;149(2):129-145. doi:10.4103/ijmr.IJMR_2079_17
CrossRef - Reza A, Mark Sutton J, Rahman KM. Effectiveness of efflux pump inhibitors as biofilm disruptors and resistance breakers in gram- negative (ESKAPEE) bacteria. Antibiotics. 2019;8(4). doi:10.3390/antibiotics8040229
CrossRef - Zimmermann S, Klinger-Strobel M, Bohnert JA, et al. Clinically Approved Drugs Inhibit the Staphylococcus aureus Multidrug NorA Efflux Pump and Reduce Biofilm Formation. Front Microbiol. 2019;10(December):1-13. doi:10.3389/fmicb.2019.02762
CrossRef