Manuscript accepted on :18-11-2021
Published online on: 04-12-2021
Plagiarism Check: Yes
Reviewed by: Dr. Sadegh Rajabi

Second Review by: Dr. Ahmar Rauf

Final Approval by: Dr. Ayush Dogra
Tooba Mazhar , Vikas Shrivastava
, Vikas Shrivastava and Rajesh Singh Tomar*
and Rajesh Singh Tomar*
Amity Institute of Biotechnology, Amity University Madhya Pradesh, Gwalior, M.P. India
Corresponding Author E-mail: rstomar@amity.edu
DOI : https://dx.doi.org/10.13005/bpj/2299
Abstract
The rapid hike in the usage of metallic as well as non-metallic nanoparticles demands their increased synthesis. In our study we synthesized bimetallic Zn-Cu nanoparticles using the greener route i.e. dry leaves powder of Citrus limon as it is environment-friendly, cost-effective, has a high surface area to volume ratio and hence superior over monometallic nanoparticles synthesized via physical or chemical approach. UTI is the most common nosocomial infection and the bacteria associated with it usually is E. coli. This pathogen forms biofilm and makes it difficult to treat the disease in less time. Hence, antimicrobial as well as antibiofilm activity of synthesized bimetallic nanoparticles was checked against E. coli. Our experimental procedure involved the preparation of ethanolic plant extract using dry leaf powder followed by synthesis of bimetallic nanoparticles. Particles were then characterized by using biophysical techniques such as FTIR, Powder-XRD and SEM-EDX. Next, we identified bacteria isolated from environment and hospital source and prepared their pure cultures. Lastly, we carried out the antibacterial and antibiofilm activity of synthesized nanoparticles against isolated E. coli. Particles showed the colour change from blue to green upon synthesis and were found to be a triclinic primitive type with an average particle size calculated to be 27.76nm as seen in PXRD. FTIR analysis gave characteristic peaks of functional groups. SEM-EDX confirmed successful doping and grain size of the particle. Bacteria isolated from samples showed pink rods in gram staining indicating gram-negative bacilli. Biochemical findings confirmed E. coli in samples. Characteristic zones of inhibition in range 12-18mm establish good antibacterial properties with MIC of synthesized nanoparticles <0.5mg/ml and crystal violet assay assure antibiofilm properties of Zn-Cu. The result of the study can be advantageous to develop an understanding of the development of nano-based medicine for biofilm-producing pathogens.
Keywords
Antibiofilm; Biofilm; Bimetallic Nanoparticles; Biological Synthesis; E. coli
Download this article as:| Copy the following to cite this article: Mazhar T, Shrivastava V, Tomar S. R. Biogenic Synthesis of Bi-Metallic (Zn-Cu) Nanoparticles by Leaf Extract of Citrus Limon and Evaluation of its Antibiofilm Activity Against E. Coli. Biomed Pharmacol J 2021;14(4) |
| Copy the following to cite this URL: Mazhar T, Shrivastava V, Tomar S. R. Biogenic Synthesis of Bi-Metallic (Zn-Cu) Nanoparticles by Leaf Extract of Citrus Limon and Evaluation of its Antibiofilm Activity Against E. Coli. Biomed Pharmacol J 2021;14(4). Available from: https://bit.ly/2ZYmkmQ |
Introduction
Synthesis and characteristics of nanoparticles (NPs) are broadly contemplated over the past few years due to their novel characteristics, yet in addition due to their potential applications in hardware, optoelectronics, data stockpiling, catalysis, biosensors, and surface-enhanced Raman spectroscopy (SERS)1. To increase the effectiveness of the two metals their alloying is done so that the resulting substance has better mechanical usefulness than their starting material. Hence, bimetallic nanoparticles are synthesized that show better traits than their respective monometallic counterparts2. The properties of bimetallic nanoparticles can be altered by manipulating their size, morphology, synthesis and atomic ordering. Albeit the composition of bimetallic nanoparticles comprises of two evident metals yet there can be numerous structures that are possible3. According to structure, they can be classified as mixed or segregated structures, but in consonance, with the atomic arrangement, they can be designated as alloyed, intermetallic, sub-clustered and core-shell4. Bimetallic nanoparticles synthesis can be achieved via several metals like Au, Ag, Pt, Zn, Cu, Ti etc. Zinc and copper metals play numerous roles in biology. Talking about the human body, zinc is involved in the catalysis of near about 200 enzymes5. It also aids immune defence, division of cells, synthesis of protein and DNA along wound healing. It’s a metal with great properties of growth be it from the womb till puberty. Zinc also holds marvellous antibacterial properties that too in minute concentrations6. Similarly, copper holds many functions in the human biological system. It allows the proper functioning of valuable enzymes and establishes the strength of connective and epithelial tissue throughout the body. Free radicals forming in the body cause health issues. Copper get rid of them thereby preventing damage and thereby acting as an antioxidant as well as pro-oxidant. Copper plays a role in the normal functioning of the thyroid gland and haemoglobin production7. Nanoparticle synthesis could be physical, chemical or biological. But, due to long procedures involve in physical methods and loads of toxicants in chemical methods greener route is promoted for synthesizing nanoparticle8. Green nanoparticle synthesis eliminates the release of harmful by-products thereby regulating the environmental remediation process and thus proving itself to be environment friendly9. Green synthesis can be achieved using either plant as well as microbe species such as leaves, seed, roots, fruit or bacteria, algae, fungi respectively. Since green synthesis does not involve any additional reducers, therefore, it is cost-effective, doesn’t require high temperature or pressure, therefore, energy-saving yet can be used for nanoparticle synthesis on a large scale and as discussed earlier it is environment friendly as well11. So, these are the perks of the biological method of nanoparticle manufacturing over physical and chemical methods. Among all plants available for nanoparticle synthesis medicinal plants are favoured over others as they naturally have antibacterial properties, one such plant is Lemon. Citrus limon is one of the predominant crops grown12. Belonging to the family Rutaceae it has good alkaloid content, therefore showing great antibacterial and anticancer activities in extracts of its different parts be it leaves, peel, roots, stem etc.13. Some species of bacteria are difficult to treat because of the biofilm they form to protect themselves14. Biofilms were first demonstrated by great scientist Antonie van Lee-wenhoek. All sorts of bacteria either gram-positive or negative forms biofilm on several surfaces such as medical implants, teeth surface, water bodies as well as living tissues etc.15. Several bacterial species together produce an extracellular polymeric matrix and gets embedded within it. This represents a bacterial biofilm, which may contain single species or can be multiple one’s16. The matrix of biofilm is an internal environment of biofilm that initiates the adhesion of bacteria and also stabilizes the three-dimensional structure of biofilm. Enzymes are secreted in response to supplements available, which decides the composition of the extracellular matrix and also the architecture of biofilm. Urinary catheters readily acquire biofilms on their outer and inner surface which results in urinary tract infections17. The main bacteria involved in causing biofilm-associated UTI is E. coli along with E. faecalis, P. aeruginosa, P. mirabilis and several other gram-negative bacteria18. Antibiotics have helped fight biofilm-associated UTI but they take a longer duration therefore, a different approach using nanoparticles in treating infections associated with biofilms have proved to be more effective19. Silver nanoparticles have already given positive results in this aspect20. But, the use of bimetallic nanoparticles against bacterial biofilms is still vaguely discussed. Hence, we are here to check the efficacy of zinc doped copper nanoparticles synthesized from lemon leaf extract in disrupting biofilms formed by E. coli.
Materials and Methods
Preparation of Plant Extract
The Citrus limon plant material was collected from the botanical garden of Jiwaji University, Gwalior. Leaves of the plant were thoroughly washed using water and then finely ground using mortar and pastel. 8 grams of crushed leaves were dissolved in 50% ethanol. After 24 hours’ extract was filtered using Whatman filter paper and stored in the refrigerator to be used for nanoparticle synthesis21.
Synthesis and Characterisation of bimetallic nanoparticles
1ml of 1:9 diluted lemon leaf extract was added to the mixture of 0.01M ZnNO3 and 0.1M CuSO4.5H2O and kept on a magnetic stirrer for 4 hours at 25°C at the speed of 400 rpm. Once the particles are reduced, they were dried on a Petri plate in a hot air oven. The next step is scrapping of particles and collecting their powder in micro centrifugation tubes for washing with ethanol. After 5-6 wash at 8000 rpm for 5 minutes’ particles are dried on a dry bath and after this step, they are ready to be utilised for further experiments22. Synthesized particles were sent to SAIF-STIC for characterization using PXRD, FTIR and SEM-EDX.
Sample Collection and Bacterial Isolation
Environmental samples were collected using sewage water from different locations and clinical isolates were gathered from Birla Institute of Medical Research, Gwalior. Bacterial isolation was achieved by using the spread plate technique on nutrient agar media23.
Biochemical identification of bacteria
Bacteria isolated were identified to be E.coli using Bergey’s Manual Method involving gram staining and biochemical methods like IMViC test, lactose fermentation tests and hydrogen sulphide production test24.
Pure culture formation
Pure cultures of isolated bacteria will be formed by streak plate method/pour plate method25. Different agar mediums were used for streaking i.e. Mac Conkey agar, Nutrient Agar as well as Eosin Methylene Blue Agar.
Antimicrobial activity of Zn-Cu Bimetallic Nanoparticles (BMNPs)
Well, the diffusion method was opted to determine the antibacterial activity of synthesized nanoparticles26. Nutrient agar plates were prepared, and wells were punched in a medium. Next 100 μl of bacteria from broth culture was spread over a medium using an L-shaped spreader. Lastly, 50 μl of 10mg/ml of particles were added in wells along with monometallic counterparts.
Antibiofilm activity of Zn-Cu BMNPs
Formation of biofilm by the bacteria and anti-biofilm activity of synthesized Zn-Cu particles was determined by crystal violet assay using 96 well microtiter plate. 200μl of bacterial broth is added in wells and kept in an incubator for 48 hours. After the defined period bacterial culture broth is replaced with fresh broth in some wells and particles, antibiotics and monometallic nanoparticles are added in other wells. The microtiter plate is again incubated for 24 hours. After 24 hours’ broth is discarded, and wells are air-dried for 15 minutes then washed with 0.9% NaCl twice and then stained with 0.4% (w/v) crystal violet dye for 30 minutes. After this excess stain is removed by washing wells with distilled water and after that 33% glacial acetic acid is added to wells and O.D. is taken on a microtiter plate reader at 630nm. Biofilm formed was determined by using several formulae27.
Results
Change of colour
0.01M zinc acetate which is the transparent solution in water and 0.1M copper sulphate which is the blue solution in water, when mixed in water appear blue. Lemon leaf extract addition turns the solution green in colour resulting in zinc-copper bimetallic nanoparticles. Particles dried on Petri plate are easily scrapable due to the hygroscopic nature of copper sulphate. Figure 1 shows particle synthesis on a magnetic stirrer and dried nanoparticles on a Petri plate.
 |
Figure 1: (a) Synthesis of zinc doped copper nanoparticles on a magnetic stirrer (b) Dried nanoparticles |
P-XRD
P-XRD pattern of Zn-Cu bimetallic nanoparticles show diffraction peaks at 2θ values 25.95o, 28.62o, 31.82o, 35.57o, 40.84o, 56.40o and 58.49o corresponding to hkl values (003), (210), (100), (002), (012), (021) and (202) respectively as seen in Figure 2, which is in harmony with JCPDS file no. 001-1136 indicating effective incorporation of Zn+ ions in CuO lattice thereby indicating triclinic primitive structure28. The average crystal size of nanoparticles calculated by the Scherrer formula was found to be 27.76nm.
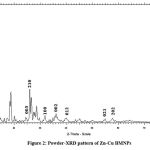 |
Figure 2: Powder-XRD pattern of Zn-Cu BMNPs |
FTIR
FTIR analysis of biosynthesized Zn-Cu BMNPs show characteristic peaks in Figure 3. Table 1 describes functional groups corresponding to peaks. Defined peaks indicate vibrational N-H stretching and bending of a primary aliphatic amine group, O-H stretch vibration of alcohol, C=C stretching of conjugated and cyclic alkene, S=O stretching of sulfonate and sulfone groups, C-O stretching of vinyl ether, strong C-Br stretching of halo compound and several others.
 |
Figure 3: FTIR analysis of synthesized Zn-Cu BMNPs. |
Table 1: Vibrational frequencies of functional groups obtained by FTIR
| S. no. | Frequency (cm-1) | Possible functional groups |
| 1. | 3369.27 cm−1 | N-H stretch of aliphatic primary amine |
| 2. | 3150.06 cm−1 | O-H stretch of alcohol |
| 3. | 1628.48 cm−1 | C=C stretching of conjugated alkene |
| 4. | 1515.99 cm−1 | N-H amine bending
C=C stretching of cyclic alkene |
| 5. | 1195.18 cm−1 | S=O stretching of sulfonate |
| 6. | 1111.30 cm−1 | S=O stretching of sulfone |
| 7. | 1013.76 cm−1 | C-O stretching of vinyl ether |
| 8. | 863.02 cm−1
804.79 cm−1 |
C-H bending 1,2,4-trisubstituted |
| 9. | 672.19 cm−1
623.47 cm−1 |
C-Br stretching of halo compound |
SEM-EDX
Figure 4 and Figure 5 shows SEM and EDX results of synthesized nanoparticles respectively. Scanning electron microscope along with energy dispersive X-ray analyser tells about the grain size of the particle along with elemental constitution to know the successful doping and formation of bimetallic nanoparticles29. SEM image of particles shows the uniform size of particles at a scale of 1micrometer which is slightly larger than 100nm as it is only a section of particle. EDX shows weight% of Zn, Cu and O along with some amount of S which may be due to functional groups in plant leaf extract30.
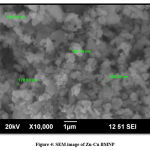 |
Figure 4: SEM image of Zn-Cu BMNP |
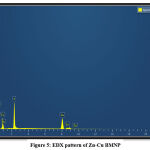 |
Figure 5: EDX pattern of Zn-Cu BMNP |
Bacterial identification
Gram staining
Figure 6 shows gram staining image of isolated bacteria which clearly shows pink colonies that are rod-shaped and few spherical. This indicates our bacteria are gram-negative coccobacilli i.e. can be E. coli confirmed by biochemical tests. E is for Environmentally isolated bacteria and C is Clinically isolated bacteria.
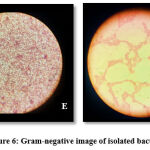 |
Figure 6: Gram-negative image of isolated bacteria. E-Environmentally isolated bacteria, C-Clinically isolated bacteria |
Lactose Fermentation Test
Figure 7 shows Mac Conkey broth inoculated with test bacteria turned from purple to yellow and bubbles were seen in Durham indicates acid and gas production by the bacteria which is positive for E. coli31.
 |
Figure 7: Lactose Fermentation test for isolated bacteria |
IMViC Test
Specially performed for coliform bacteria IMViC is an abbreviation for four tests i.e. Indole test, Methyl red test, Voges-Proskauer test and Citrate utilisation test. In the indole test addition of Kovac’s reagent to tryptone water, previously inoculated with the test organism, forms a cherry red colour ring and indicates a positive test. MR-VP broth is used for methyl red and voges-proskauer test. The addition of methyl red to MR-VP broth with test organism turns red and indicates positive methyl red test while the addition of alpha naphthol and potassium hydroxide turning MR-VP broth yellow indicates positive Voges-Proskauer test. In the citrate utilisation test, Simmons citrate agar turns blue after 24 hours with streaked bacteria. In E.coli indole and methyl red come positive and Voges-Proskauer and citrate utilisation comes negative32. Figure 8 shows IMViC results are indicating E. coli.
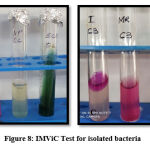 |
Figure 8: IMViC Test for isolated bacteria |
Hydrogen Sulphide Production Test
SIM agar is used to make a stab and then inoculated with the test organism. The blackening of the medium indicates a positive test. But our test bacteria didn’t cause any blackening as seen in Figure 9. Hence, indicating the presence of E. coli33.
 |
Figure 9: Hydrogen sulphide production test for isolated bacteria |
Pure culture formation
Three different media were used to streak pure colonies of isolated E.coli. In Figure 10, pink colonies on Mac Conkey agar, White colonies on Nutrient agar and Green metallic sheen colonies on EMB agar indicates E.coli along with biochemical confirmation34.
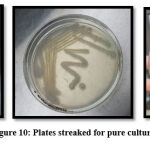 |
Figure 10: Plates streaked for pure culture |
Antimicrobial Activity assay
Using the well diffusion method antimicrobial activity of zinc doped copper oxide bimetallic nanoparticles was calculated35. Culture taken from Luria Bertani broth had colony-forming units as 1.31*108 CFU/ml and 1.72*108 CFU/ml for clinical and environmental samples respectively which is in accordance with 0.5M McFarland standard. The culture broth was diluted up to 105 CFU/ml for activity assay. 100μl of 105 CFU/ml culture was pipetted on nutrient agar plated and well diffusion assay is done with particle alone and also a synergistic effect of the particle with antibiotic was checked. Comparison of bimetallic Zn-Cu was also tested against monometallic counterparts. Figure 11 and Table 2 shows the inhibition zones achieved. Monometallic zinc oxide and copper oxide nanoparticles were unable to kill the bacteria at this conc. thereby showing no zone of inhibition. Stock for all particles was 10mg/ml. 50μl of particles are added in wells thereby making final conc. 0.5mg/ml. Thus, MIC of synthesized nanoparticles was found to be <0.5mg/ml. The Antibiotic used was gentamicin sulfate.
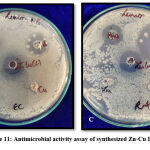 |
Figure 11: Antimicrobial activity assay of synthesized Zn-Cu BMNP |
Table 2: Zones of inhibition (mm)
|
Samples |
Zn-Cu BMNP |
Zn-Cu BMNP+A |
A |
ZnO MMNP |
CuO MMNP |
| E | 18mm | 12mm | 16mm | Nil | Nil |
| C | 17mm | 16mm | 32mm | Nil | Nil |
BMNP-Bimetallic Nanoparticle
A – Antibiotic (Gentamicin Sulfate)
MMNP-Monometallic Nanoparticle
Antibiofilm assay
Crystal violet assay is used to determine biofilm formation by the bacteria and also antibiofilm activity of synthesized nanoparticles against 2 isolates E from environment and C from the clinical (nosocomial) source. Table 3 indicates biofilm formed by the bacteria and its disruption in presence of Zn-Cu BMNP from lemon leaf extract. Here S indicates strong biofilm formation and N indicates negative or no biofilm formed due to the presence of a particle. The formula used is in reference with measurement of biofilm formation by clinical isolates of Escherichia coli is method-dependent36.
Table 3: Biofilm-Antibiofilm activity assay.
| Samples | E | C | ||
| Formula | Biofilm | Antibiofilm | Biofilm | Antibiofilm |
| BF=AB-CW | 0.89 | 0.0374 | 0.84 | 0.003 |
| S | N | S | N | |
| BF=AB/CW | 10.4 | 1.39 | 9.86 | 1.03 |
| S | N | S | N | |
| SBF=AB-CW/G | 0.544 | 0.0521 | 0.5346 | 0.0065 |
| W | N | W | N | |
Discussion
In this study, we have synthesized zinc doped copper oxide bimetallic nanoparticles and characterized them via biophysical techniques such as P-XRD, FTIR and SEM-EDX. The result of the study indicates that the synthesized nanoparticles are triclinic and has an average particle size of 27.76nm. These particles have shown significant antibacterial and antibiofilm activity against E. coli with MIC of bimetallic nanoparticles <0.5mg/ml. The results of the study are more significant than previous studies carried out by Malka, E in 2013 and Khalid. A in 2021 with reference to their particle size and antibacterial activity37, 38. The result of the anti-biofilm activity of zinc doped copper oxide bimetallic nanoparticles is highly significant as compared with previous study carried out by Ashajyothi. C. in 2016 which was limited to use of monometallic copper and zinc oxide nanoparticles39.
The possible reason for achieving less particle size of Zn-Cu BMNP may be due to the use of biogenic synthesis method at room temperature. Moreover, the synthesized particle has given the significant result against E. coli with reference to antibacterial and antibiofilm activity. It may be due to the use of fusion of two metals i.e. zinc and copper and phytochemicals of Citrus limon used for reduction and as capping agents while synthesis of nanoparticles.
Conclusion
Zinc-Copper Bimetallic Particles synthesized from Citrus limon leaf extract were found to be in nano range with average particle size 27.76nm as characterised by P-XRD, FTIR, SEM-EDX. Sample collected from both environmental and clinical sources found to have E. coli as confirmed by gram staining as well as biochemical tests. Biofilm assay of pure culture showed positive results thereby indicating biofilm formed by the bacteria is remarkable. The antimicrobial activity of nanoparticles synthesized against E. coli forming biofilm, was significant as compared to antibiotics giving zones of inhibition 12-18nm. Antibiofilm activity of synthesized nanoparticles was found to be quite effective.
Acknowledgement
We wish to express our sincere acknowledgement to Dr Ashok Kumar Chauhan, President, RBEF parent organization of Amity University Madhya Pradesh (AUMP), Dr Aseem Chauhan, Additional President, RBEF and chairman of AUMP, Gwalior, Lt. Gen. V.K. Sharma, AVSM (Retd.), Vice-Chancellor of AUMP, Gwalior for providing necessary facilities, their valuable support and encouragement throughout the work. We are thankful to Prof (Dr) Rajesh Singh Tomar, Director, Amity Institute of Biotechnology & Dean (Academics), Amity University Madhya Pradesh, Gwalior.
Conflict of interest
There is no conflict of interest.
Funding Source
There is no funding source.
References
- Srinoi P, Chen, Y. T., Vittur V, Marquez, M. D and Lee T. R. Bimetallic nanoparticles: enhanced magnetic and optical properties for emerging biological applications in Applied Sciences, 2018,8(7), 1106.
CrossRef - Shah A, Latif-ur-Rahman, Qureshi R, & Zia-ur-Rehman. Synthesis, characterization and applications of bimetallic (Au-Ag, Au-Pt, Au-Ru) alloy nanoparticles in Reviews on advanced materials science, 2012, 30(2), 133-149.
- Huynh K. H, Pham X. H, Kim J, Lee S. H, Chang H, Rho W. Y and Jun B. H. Synthesis, Properties, and Biological Applications of Metallic Alloy Nanoparticles in International Journal of Molecular Sciences, 2020, 21(14), 5174.
CrossRef - Zaleska-Medynska A, Marchelek M, Diak M and Grabowska, E. Noble metal-based bimetallic nanoparticles: the effect of the structure on the optical, catalytic and photocatalytic properties in Advances in colloid and interface science, 2016, 229, 80-107.
CrossRef - Classen H. G, Gröber U, Löw D, Schmidt J and Stracke H. Zinc deficiency. Symptoms, causes, diagnosis and therapy in Medizinische Monatsschrift fur Pharmazeuten, 2011, 34(3), 87-95.
- Price C. T, Langford J. R and Liporace, F. A. Essential nutrients for bone health and a review of their availability in the average North American diet in The open orthopaedics journal, 2012, 6, 143.
CrossRef - Davis C. D. Low dietary copper increases fecal free radical production, fecal water alkaline phosphatase activity and cytotoxicity in healthy men in The Journal of nutrition, 2003, 133(2), 522-527.
CrossRef - Singh A, Gautam P. K, Verma A, Singh V, Shivapriya P. M, Shivalkar, S, Sahu A.K and Samanta S. K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review in Biotechnology Reports, 2020, 25, e00427.
CrossRef - Rónavári A, Igaz N, Adamecz D. I, Szerencsés B, Molnar C, Kónya Z., Pfeiffer I and Kiricsi, M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications in Molecules, 2021, 26(4), 844.
CrossRef - Singh J, Dutta T, Kim K. H, Rawat M, Samddar P and Kumar P. ‘Green’synthesis of metals and their oxide nanoparticles: applications for environmental remediation in Journal of nanobiotechnology, 2018, 16(1), 1-24.
CrossRef - Shah M, Fawcett D, Sharma S. Tripathy S. K and Poinern G. E. J. Green synthesis of metallic nanoparticles via biological entities in Materials, 8(11), 2015, 7278-7308.
CrossRef - Klimek-Szczykutowicz M, Szopa A and Ekiert, H. Citrus limon (Lemon) phenomenon—a review of the chemistry, pharmacological properties, applications in the modern pharmaceutical, food, and cosmetics industries, and biotechnological studies in Plants, 2020, 9(1), 119.
CrossRef - Kawaii S, Tomono Y, Katase E, Ogawa K, Yano M, Koizumi M, Ito C and Furukawa H. Quantitative study of flavonoids in leaves of Citrus plants in Journal of agricultural and food chemistry, 2000, 48(9), 3865-3871.
CrossRef - Banerjee D, Shivapriya P. M, Gautam P. K, Misra K, Sahoo A. K and Samanta, S. K. A review on basic biology of bacterial biofilm infections and their treatments by nanotechnology-based approaches in Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 2019, 1-17.
CrossRef - Donlan R. M. Biofilms and device-associated infections in Emerging infectious diseases, 2001, 7(2), 277.
CrossRef - Kassinger S. J and Van Hoek M. L. Biofilm architecture: An emerging synthetic biology target in Synthetic and systems biotechnology, 2020, 5(1), 1-10.
CrossRef - Gunardi W. D, Karuniawati A, Umbas R, Bardosono S, Lydia A, Soebandrio A and Safari D. Biofilm-Producing Bacteria and Risk Factors (Gender and Duration of Catheterization) Characterized as Catheter-Associated Biofilm Formation in International Journal of Microbiology, 2021.
CrossRef - Flores-Mireles A. L, Walker J. N, Caparon M and Hultgren S. J. Urinary tract infections: epidemiology, mechanisms of infection and treatment options in Nature reviews microbiology, 2015, 13(5), 269-284.
CrossRef - Vallet-Regí M, González B and Izquierdo-Barba I. Nanomaterials as promising alternative in the infection treatment in International journal of molecular sciences, 2019, 20(15), 3806.
CrossRef - Siddique M. H, Aslam B, Imran M., Ashraf A, Nadeem H, Hayat S, Khurshid M, Afzal M, Malik I.R, Shahzad M, Qureshi U, Khan Z.U.H and Muzammil, S. Effect of Silver Nanoparticles on Biofilm Formation and EPS Production of Multidrug-Resistant Klebsiella pneumonia in BioMed research international, 2020.
CrossRef - Alkhulaifi M. M, Alshehri J. H, Alwehaibi M. A, Awad M. A, Al-Enazi N. M, Aldosari N. S, Hatamleh A.A Abdel-Raouf N. Green synthesis of silver nanoparticles using Citrus limon peels and evaluation of their antibacterial and cytotoxic properties in Saudi Journal of Biological Sciences, 2020, 27(12), 3434-3441.
CrossRef - Mourdikoudis S, Pallares R. M and Thanh N. T. Characterization techniques for nanoparticles: comparison and complementarity upon studying nanoparticle properties in Nanoscale, 2018, 10(27), 12871-12934.
CrossRef - Karah N, Rafei R, Elamin W, Ghazy A, Abbara A, Hamze M and Uhlin B. E. Guideline for Urine Culture and Biochemical Identification of Bacterial Urinary Pathogens in Low-Resource Settings in Diagnostics, 2020, 10(10), 832.
CrossRef - Ruangpan L and Tendencia E. A. Laboratory manual of standardized methods for antimicrobial sensitivity tests for bacteria isolated from aquatic animals and environment. Aquaculture Department, Southeast Asian Fisheries Development Center, 2004.
- Valgas C, Souza S. M. D, Smânia E. F and Smânia Jr, A. Screening methods to determine antibacterial activity of natural products in Brazilian journal of microbiology, 2007, 38(2), 369-380.
CrossRef - Gurunathan S, Han J. W, Kwon D. N and Kim, J. H. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria in Nanoscale research letters, 2014, 9(1), 1-17.
CrossRef - Barapatre A, Aadil K. R and Jha, H. Synergistic antibacterial and antibiofilm activity of silver nanoparticles biosynthesized by lignin-degrading fungus in Bioresources and Bioprocessing, 2016, 3(1), 1-13.
CrossRef - Cretu V, Postica V, Mishra A. K, Hoppe M, Tiginyanu I, Mishra Y. K, Chow L, De Leeuw N. H, Adelung R and Lupan O. Synthesis, characterization and DFT studies of zinc-doped copper oxide nanocrystals for gas sensing applications in Journal of Materials Chemistry A. 2016;4(17):6527-39.
CrossRef - Fernandes, D. M., Silva, R., Hechenleitner, A. W., Radovanovic, E., Melo, M. C., & Pineda, E. G. Synthesis and characterization of ZnO, CuO and a mixed Zn and Cu oxide. Materials Chemistry and Physics, 2009, 115(1), 110-115.
CrossRef - Gnanasangeetha D and SaralaThambavani, D. One pot synthesis of zinc oxide nanoparticles via chemical and green method in Research Journal of Material Sciences, 2013, 2320, 6055.
- Batabyal K, Banerjee A, Pal S, Dey S, Joardar S. N, Samanta, I, Isore D. P and Singh, A. D. Detection, characterization, and antibiogram of extended-spectrum beta-lactamase Escherichia coli isolated from bovine milk samples in West Bengal, India in Veterinary world, 2018, 11(10), 1423.
CrossRef - Hemraj V, Diksha S and Avneet G. A review on commonly used biochemical test for bacteria in Innovare Journal of Life Science, 2013, 1(1), 1-7.
- Karunakaran E, Vernon D, Biggs C. A, Saul A, Crawford D and Jensen H. Enumeration of sulphate-reducing bacteria for assessing potential for hydrogen sulphide production in urban drainage systems in Water Science and Technology, 2016, 73(12), 3087-3094.
CrossRef - Iyadorai T, Mariappan V, Vellasamy K. M, Wanyiri J. W, Roslani A. C, Lee G. K, Sears C and Vadivelu J. Prevalence and association of pks+ Escherichia coli with colorectal cancer in patients at the University Malaya Medical Centre, Malaysia in PloS one, 2020, 15(1), e0228217.
CrossRef - Hamed A. A, Kabary H, Khedr M and Emam A. N. Antibiofilm, antimicrobial and cytotoxic activity of extracellular green-synthesized silver nanoparticles by two marine-derived actinomycete in RSC Advances, 2020, 10(17), 10361-10367.
CrossRef - Naves P, Del Prado G, Huelves L, Gracia M, Ruiz V, Blanco J, Rodríguez-Cerrato V and Soriano, F. Measurement of biofilm formation by clinical isolates of Escherichia coli is method‐dependent. Journal of applied microbiology, 2008, 105(2), 585-590.
CrossRef - Malka E, Perelshtein I, Lipovsky A, Shalom Y, Naparstek L, Perkas N, Patick T, Lubart R, Nitzan Y, Banin E and Gedanken, A. Eradication of multi‐drug resistant bacteria by a novel Zn‐doped CuO nanocomposite in Small, 2013, 9(23), 4069-4076.
CrossRef - Khalid A, Ahmad P, Alharthi A. I, Muhammad S, Khandaker M. U. Rehman, M, Faruque M. R. I, Din I. D, Alotaibi M. A, Alzimami K and Bradley D. A. Structural, optical and antibacterial efficacy of pure and zinc-doped copper oxide against pathogenic bacteria in Nanomaterials, 2021, 11(2), 451.
CrossRef - Ashajyothi C, Harish K. H, Dubey N and Chandrakanth, R. K. Antibiofilm activity of biogenic copper and zinc oxide nanoparticles-antimicrobials collegiate against multiple drug resistant bacteria: a nanoscale approach in Journal of Nanostructure in Chemistry, 2016, 6(4), 329-341.
CrossRef







