Manuscript accepted on :27-09-2021
Published online on: 24-11-2021
Plagiarism Check: Yes
Reviewed by: Dr. Jyotsna Jain

Second Review by: Dr. Sumayah Faruq Kasim

Final Approval by: Dr. Fai Poon
Iurii Kuchyn1* , Dmytro Sazhyn2
, Dmytro Sazhyn2 and Gennadiy Patlazhan3
and Gennadiy Patlazhan3
1Bogomolets National Medical University, Kyiv, Ukraine.
2Faculty of Surgery, Anesthesiology and Intensive Care, Bogomolets National Medical University, Kyiv, Ukraine.
3Patlazhan Сlinic, Odesa, Ukraine.
Corresponding Author E-mail: iuriikuchyn@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2308
Abstract
The aim is to learn the features of aesthetic and reduction surgical interventions on the mammary glands in Ukraine. Materials and methods. The study was conducted by analyzing the inpatient ambulatory cards of 320 patients. Anesthesia was provided by propofol (n=130), sevoflurane (n=140) and combined use of sevoflurane and nalbuphine (n=50). The results of the study. It was found that usage of combined inhalation analgesia of sevoflurane with opioids was characterized by 41.9% less recovery time. It was found that 8 hours after surgery, the individual assessment of pain was lower in the group of combined analgesia with opioids relative to intravenous anesthesia with propofol (87.5%, p<0.05) and inhalation anesthesia with sevoflurane (71, 3%, p<0.05). After 24 hours all patients reported about pain below 1.0 point, however, in groups where sevoflurane and nalbuphine were used, the level of pain self-esteem was 2.61 and 3 times lower than after intravenous propofol. It was found that within 1 hour after surgery, the average cognitive score on the Montreal scale decreased in the group of intravenous propofol by 5.0% (p<0.05) and by 1.7% under inhalation anesthesia with sevoflurane. Under combined anesthesia the cognitive score remained at 12.0 points. The frequency of postoperative nausea was the highest level in the group of inhalation anesthesia - 16.7%. The addition of nalbuphine to sevoflurane significantly reduced the risk of postoperative nausea (χ2=7.250; p=0.007). Conclusions. Combined anesthesia with opioids is a highly effective anesthetic choice for aesthetic and reconstructive interventions on the mammary glands.
Keywords
Aesthetic Interventions; Combined Anesthesia; Mammary Glands; Malbuphine; Propofol; Sevoflurane
Download this article as:| Copy the following to cite this article: Kuchyn I, Sazhyn D, Patlazhan G. Analysis the Safety and Efficacy at Different Types of Anesthesiological Support During Aesthetic Interventions on the Breast Glands in Ukraine. Biomed Pharmacol J 2021;14(4). |
| Copy the following to cite this URL: Kuchyn I, Sazhyn D, Patlazhan G. Analysis the Safety and Efficacy at Different Types of Anesthesiological Support During Aesthetic Interventions on the Breast Glands in Ukraine. Biomed Pharmacol J 2021;14(4). Available from: https://bit.ly/30PdW9K |
Introduction
In Ukraine and around the world, mammoplasty is popular with women. Undoubted advantages of the method are the preservation of most anatomical structures and skin, as well as better psychological status of patients after intervention1. The disadvantages of one-step intervention include the large extent of surgery2, prolongation of the duration of anesthesia, which can cause the switch-on the stress adaptive mechanisms and more complications3.
In addition, despite considerable advances in global anesthesiology in recent years, the effectiveness of perioperative analgesia during reconstructive and aesthetic breast surgery needs to be optimized. Urgent issues are to determine the path of safe analgesia for the development of side effects from various organ systems, in particular, possible cognitive consequences4 and harm in the quality of life, changes in central hemodynamics5.
The method of anesthesia and the drugs used in the plastic surgery of the breast have a number of features compared with the anesthesia in the general surgery. In particular, in low-flow inhalation anesthesia with the use of sevoflurane during plastic surgery, there are some contradictions: on the one hand, some researchers emphasize its better protective effect in ischemic-reperfusion injury6,7 than in injectable propofol8,9.
The most important side effects of general anesthesia that patients experience after breast plastic surgery are postoperative pain, nausea and vomiting10. Untreated or insufficiently managed postoperative pain can have systemic consequences, negatively affecting the quality of life of patients.
First of all, anesthesia protection provides maximum safety of the operated patient. It is also necessary to minimize the depressant effects of general anesthesia. Finally, it is very important to ensure the comfort of anesthesia care for a patient who acquires the status of a client in cosmetic surgery. In this case, the “client is always right”, he is an active participant in the transformation of his own body and he assesses the outcome of interventions with her.
Today there is a wide choice of anesthesia methods for aesthetic interventions on the breast. Local anesthesia and combined general anesthesia to the greatest extent realizes the selectivity of the action of different drugs on the nociceptive systems, and provides the ability to “fine-tune” the action of the component that needs to be strengthened or weakened at a given time. Inhaled anesthetics are the first choice for at least two reasons. The first is the ability to quickly achieve the required concentration in the body and, if necessary, as quickly as possible to reduce it, which reduces the induction and recovery periods. Secondly there is the ease and accuracy of control over this process (measurement of alveolar concentration of inhaled anesthetic).
The purpose of this study was to study the features of aesthetic and reduction surgery on the mammary glands in the Odessa region of Ukraine, to learn the principles of anesthesia and the effectiveness of combined anesthesia.
Material and research methods
The study was conducted by analyzing inpatient cards of 320 patients admitted for reconstructive and aesthetic surgery on the mammary glands in clinics in Odessa during 2018-2020. Inclusion criteria: obtaining informed consent of patients to conduct the study; planned surgery; age from 21 to 65 years; the risk of anesthesia for ASA I-III; lack of cognitive impairment; absence of chronic diseases in the stage of exacerbation and decompensation; absence of hearing and vision disorders. The mean age of patients was 36.8±10.8 years. Mean height (163.0±6.9 cm) and weight (68.2±10.4, kg) were harmonious and were characterized by a normal body mass index (BMI) – 23.4±3.8 kg / m². The most common comorbidities were varicose veins of the lower extremities (14.2%) and anemia (11.7%).
In the majority of patients who applied for reconstructive interventions, the initial signs of fatty involution were observed (219 people, 68.3%), in 56 patients – complete fatty involution of the mammary glands (17.5%), in 45 people. Mammary glands were represented by glandular tissue (14.2%).
For statistical analysis results we used Statistica for Windows Version 10.0 (Stat Soft inc., USA). Parameters are presented in the form M±m, where M is the Mean, m is standard deviation. In the analysis of categorical group data, the criterion Pearson χ2 with Yates correction was used. The assessment of the probability of the therapeutic effect was performed taking into account the absolute (AE) and relative (RE) efficacy, as well as the odds ratio (OR), with the calculation of confidence intervals and the reliability criterion for RR and OR. At the case of p<0.05, differences were statistically significant.
Results of the research
Surgical interventions observed in the retrospective study were represented by 58 reduction interventions (11.2%) and 462 primary breast augmentation (88.8%) both individually (n=428, 82.3%) and in combination with mastopexy. (n=26, 5.0%) and correction of inverted nipples (n=8, 1.5%), a total of 584 implants were done. The mean follow-up for all patients was 25.1 months (range 6–60 months). The coverage of follow-up after 5 years for all patients was 92%. Among 320 patients in 200 cases reconstructive interventions were performed on both breasts (62.0%), in 120 women – on one breast (38.0%). Thus, a total of 520 surgical interventions were analyzed.
The average duration of reconstructive intervention with a unilateral flap was 387.12±97.5 min or 6.45±1.7 hours. Bilateral reconstructive interventions lasted 567.27±110.2 min or 9.45±1.9 h (Tab. 1).
Table 1: Characteristics of reconstructive interventions in retrospective study
| Parameters | Index |
| Age, years | 36,7±6,92 |
| Duration of intervention at a 1-sided flap, min. | 387,12±97,5 |
| Duration of intervention at a 2-sided flap, min. | 567,27±110,2 |
| Duration of surgical revision at 1-sided intervention, min. | 417,19±84,5 |
| Duration of surgical revision at 2-sided intervention, min. | 605,27±90,4 |
No significant difference was found between cases with the need for an surgical revision and without it. Neither in the case of unilateral (387.12±97.5 min vs. 417.19±84.5 min, p=0.257), nor in bilateral interventions (567.27±110.2 min vs. 605.27±90.4 min), p=0.219) was not found a significantly larger number of revisions with a longer anesthesia time.
Among 520 flaps in 30 cases (5.8%) there was a need for revision operation. As reasons for the revision, we noted 14 venous thrombi (46.7%), 10 arterial thrombi (33.3%), 6 hematomas (20.0%). The flaps used for breast reconstruction in 41.1% (214 flaps) were primary, 47.1% (245 flaps) were secondary and 11.8% (61 flaps) were tertiary. For obvious reasons, the duration of hospital stay was longer in the case of revision interventions (6.79±2.79 days vs. 8.90±2.11 days, p<0.0001). These patients required rehabilitation after additional anesthesia, surgery in general, and possible complications. The average time to resolve the question of revision was 20.73 hours±2.12, the shortest was 15 minutes, and the longest was 5.74 days.
Patients received smooth (n=527, 90.2%) and textured (n=57, 9.8%) silicone implants. The average implant size was 253.98 ml (range 150–304 ml). The location of implants was subglandular (n=327, 56.0%), sub fascial (n=110, 18.8%), subspectral (n=89, 15.2%), subaxillary (n=58, 9.9%). Patients were implanted through a transaxillary incision (n=325, 55.6%) and the rest through an inframammary fold (n=259, 44.4%).
The following results were obtained by analyzing the methods of anesthesia in performing reconstructive interventions on the mammary glands. In general, when performing reduction and aesthetic interventions, combined methods of anesthesia were used – total intravenous anesthesia based on propofol (n=130, 40.6%), low-flow inhalation anesthesia with sevoflurane (n=140, 43.8%). Nalbuphine regimens (n=50, 15.6%) were used as combined analgesia.
The time of awakening of patients after surgery and cessation of anesthetic in the group of sevoflurane with the addition of nalbuphine was an average of 7.8±0.9 minutes (p <0,05), regardless of the duration of manipulation and body weight. In the propofol group, the recovery time was, on average, 16.0±2.4 minutes and depended on the duration of surgery, the time of surgery, body weight and total dose, Fig. 1.
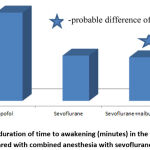 |
Figure 1: The duration of time to awakening (minutes) in the postoperative period compared with combined anesthesia with sevoflurane and opioids. |
At the same time, no significant difference in the time of awakening in comparison with the isolated introduction of sevoflurane (8.1±0.5) was found. Thus, the total awakening time under combined inhalation anesthesia was 2.05 times less than with intravenous propofol, Fig. 1.
The first standing up in the group of intravenous anesthesia was noted after 23.0±3.3 hours, with combined inhalation anesthesia – after 16.2±3.4 hours. With isolated inhalation anesthesia the time was 16.8±3.7 hours. That is, the use of combined inhalation analgesia of sevoflurane with opioids was characterized by 41.9% less recovery time, Fig. 2.
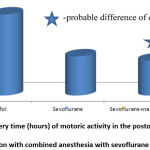 |
Figure 2: Recovery time (hours) of motoric activity in the postoperative period in comparison with combined anesthesia with sevoflurane and opioids. |
It is possible that this pattern was the result of more adequate analgesia in the first day after surgery, Fig. 3.
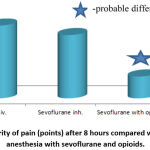 |
Figure 3: Severity of pain (points) after 8 hours compared with combined anesthesia with sevoflurane and opioids. |
In particular, 8 hours after surgery, the individual assessment of pain by VAS was lower in the group of combined analgesia with opioids (by scores) relative to intravenous anesthesia with propofol (87.5%, p <0.05) and inhalation anesthesia with sevoflurane (71.3 %, p <0.05), fig. 3.
It should be added that the severity of pain after 8 hours was directly correlated with other clinical indicators – systolic blood pressure (r=0.55, p <0.05, Fig. 4), diastolic blood pressure (r=0.40, p <0.05, Fig. 5) and heart rate (r=0.37, p <0.05, Fig. 6).
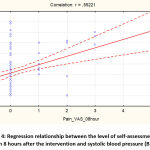 |
Figure 4: Regression relationship between the level of self-assessment of pain 8 hours after the intervention and systolic blood pressure (BP). |
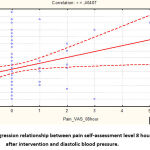 |
Figure 5: Regression relationship between pain self-assessment level 8 hours after intervention and diastolic blood pressure. |
 |
Figure 6: Regression relationship between pain self-esteem 8 hours after the intervention and heart rate. |
As you can see, there is a direct correlation between the analog scale of self-assessment of pain and the level of postoperative blood pressure:
SBP=120,69+3,9833*PainVAS_8h.
That is, a higher level of individual pain assessment was characterized by an increase in systolic blood pressure in the early postoperative period. A similar pattern was obtained for diastolic blood pressure:
DBP=70,688+1,6921*PainVAS_8h.
The intensity of pain in the postoperative period after 12 hours is shown in Fig. 7. It was found that the individual assessment of pain in the group of combined anesthesia after 12 hours was 1.27±0.06 points, which is 39.4% less than in isolated anesthesia with sevoflurane and 65.4% less than in intravenous anesthesia with propofol.
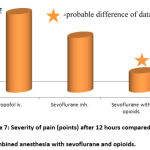 |
Figure 7: Severity of pain (points) after 12 hours compared with combined anesthesia with sevoflurane and opioids. |
It should also be noted that 24 hours after surgery, all patients reported low pain intensity – below 1.0 point, however, in the groups where sevoflurane was used by inhalation (0.23±0.02 points) and in combine scheme( 0,23±0.02 points), the level of self-esteem pain was in 2.61 and 3 times lower than after intravenous propofol, Fig. 8.
 |
Figure 8: Severity of pain (points) after 24 hours compared with combined anesthesia with sevoflurane and opioids. |
The influence of general anesthesia on the state of higher mental functions is not a resolved problem of modern anesthesiology. The study of the epidemiology of early and persistent postoperative cognitive dysfunction was devoted to the international multicenter randomized study Interpocional study of post-operative cognitive dys-function in middle age – ISPOCD 2 [1998-2001]. It shows that cognitive impairment after non-cardiac surgery under general anesthesia is about 19.2% of cases.
Subsequently, within 1 hour after surgery, the average cognitive score on the Montreal scale decreased to 11.4±0.06 in the group of intravenous propofol (5.0%, p <0.05) and up to 11.8±0.02 under inhalation anesthesia with sevoflurane (1.7%). At the same time, under combined anesthesia with sevoflurane and opioids, the cognitive score on the Montreal scale remained constant – 12.0 points.
These results are probably related to two important aspects that can be achieved with combined anesthesia: first, an adequate level of analgesia during surgery and the duration of postoperative analgesia are important; in addition, as already mentioned, combined anesthesia has a shorter time of induction of anesthesia and time of awakening, compared to one-component anesthesia.
And the use of inhalation anesthesia with sevoflurane provides control of anesthesia.
The fact that the controllability of anesthesia is due to the fact that 3 hours after the intervention in the groups of inhalation anesthesia – both single-component and combined, there is a complete restoration of cognitive functions, in contrast to intravenous anesthesia with propofol, Fig. 9.
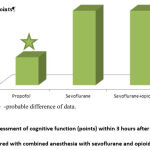 |
Figure 9: Assessment of cognitive function (points) within 3 hours after surgery compared with combined anesthesia with sevoflurane and opioids. |
In addition, the issue of postoperative nausea and vomiting is an urgent problem, despite the many studies on this topic. At present, inhalation anesthetics have firmly taken a position in anesthesiology practice due to the undoubted advantages, primarily, the controllability of anesthesia.
Therefore, one of the tasks of our study was to assess the frequency of postoperative nausea in the group of combined anesthesia and the risk of its occurrence depending on the method of analgesia. General data on the frequency of postoperative nausea are given in Table 2.
Table 2: The general frequency of postoperative nausea with different methods of anesthesia.
| Nausea | Abs. | % |
| Intravenous anesthesia with propofol | 9 | 6,9 |
| Inhalation anesthesia with sevoflurane | 23 | 16,4 |
| Combined inhalation anesthesia with sevoflurane and opioid analgesics | 3 | 6,0 |
As you can see, the frequency of postoperative nausea was the highest in the group of inhalation anesthesia – 16.7%. In the groups of intravenous anesthesia and combined anesthesia, the frequency of postoperative nausea was 6.9 and 6.0%, respectively, table. II.
Assessing the data obtained in terms of absolute risk (absolute risk – AR), as well as the odds ratio (OR), we received the following results – Table 3.
Table 3: Absolute risk (AR) and the Odds ratio (OR) of the developing of postoperative nausea
| Groups | AR,% | OR | Cumulative odds ratio | Test for departure from linear trend |
| Intravenous anesthesia with propofol | 7,0 | 1,0 | 1.295 [0.670-2.505] | χ2=7.250
p=0.007 |
| Inhalation anesthesia with sevoflurane | 16,0 | 2,64 | ||
| Combined inhalation anesthesia with sevoflurane and opioid analgesics | 6,0 | 0,86 |
The increase in the absolute risk of postoperative nausea was 2.64 times with inhalation anesthesia with sevoflurane, compared with intravenous. However, the addition of nalbuphine to sevoflurane significantly reduced the risk of postoperative nausea, Cumulative odds ratio – 1.295 [0.670-2.505], in the analysis of the linear trend – χ2=7.250 (p=0.007).
The results of the assessment of the occurrence of the expected event in the studied groups in a prospective study by the Kaplan-Meyer method were interesting. At the same time, the event determined the probability of nausea, the time interval was selected as an hourly observation during the first 2 days of the postoperative period, Fig. 10. As can be seen, in the first 20 hours of observation there was no significant difference between the groups, however, a slight divergence of the curve was observed at the end of the 1st day of the postoperative period in the group of isolated anesthesia with sevoflurane.
 |
Figure 10: Kaplan-Meyer curve in anesthesia groups to assess the likelihood of nausea in the early postoperative period. |
In general, no case of postoperative vomiting was recorded with the use of combined anesthesia, in contrast to intravenous anesthesia (2.3%, 3 cases) and inhalation anesthesia (6.4%, 9 cases), Table 4.
Table 4: The total frequency of postoperative vomiting using different methods of anesthesia
| Vomiting | Abs. | % |
| Intravenous anesthesia with propofol | 3 | 2,3 |
| Inhalation anesthesia with sevoflurane | 9 | 6,4 |
| Combined inhalation anesthesia with sevoflurane and opioid analgesics | 0 | 0,0 |
The frequency of postoperative complications with different methods of anesthesia is shown in Table 5.
Table 5: Frequency of postoperative complications using different methods of anesthesia
| Complications | Abs. | % |
| Intravenous anesthesia with propofol | 4 | 3,1 |
| Inhalation anesthesia with sevoflurane | 5 | 3,6 |
| Combined inhalation anesthesia with sevoflurane and opioid analgesics | 1 | 2,0 |
All complications were related to the occurrence of Baker’s contracture and required re-examination.
Discussion
Most studies comparing the efficacy of propofol and sevoflurane for general anesthesia found no significant differences in the time of awakening and extubation of patients after general surgery11. In our study, the time of awakening of patients after surgery and cessation of anesthetic in the group of sevoflurane with the addition of nalbuphine was an average of 7.8±0.9 minutes (p <0.05), regardless of the duration of manipulation and body weight. In the propofol group, the recovery time was, on average, 16.0±2.4 minutes and depended on the duration of surgery, the time of surgery, body weight and total dose. At the same time, some researchers note that when nalbuphine was added to the total intravenous anesthesia regimen, in contrast, the recovery rate after anesthesia was higher than with sevoflurane inhalation (8 and 12 minutes, respectively)12. According to our own study, the use of combined inhalation analgesia of sevoflurane with opioids was characterized by 41.9% less recovery time.
According to a systematic review of the consequences of aesthetic operations on the mammary glands in 8361 patients, postoperative complications in the form of pain were 7.51% of women, impaired sensitivity – about 16% of patients13. In our study, within 8 hours after surgery, individual assessment of pain by VAS was lower in the group of combined analgesia with opioids (in point scores) relative to intravenous anesthesia with propofol (87.5%, p <0.05) and inhalation anesthesia with sevoflurane (71, 3%, p <0.05). The main mechanism of postoperative pain in reconstructive and aesthetic augmentative mammoplasty is the stretching of the major pectoris muscle under the action of the implant transmitted by the pectoral nerves14. In own study, it was found that the intensity of pain after 8 hours was directly correlated with other clinical indicators – systolic blood pressure (r=0.55, p <0.05), diastolic blood pressure (r=0.40, p <0, 05) and heart rate (r=0.37, p <0.05).
A great number of authors prefer multimodal anesthesia for mamal gland plastic surgery, which involves a combination of general anesthesia with opioids, NSAIDs, or local injections of anesthetics into the surgical wound to achieve the best analgesia in the perioperative period12,15. We found that the individual assessment of pain in the group of combined anesthesia after 12 hours was 1.27±0.06 points, which is 39.4% less than in isolated anesthesia with sevoflurane and 65.4% less than in intravenous anesthesia with propofol
Although opioid analgesics have been an integral part of the surgical analgesic regimen for many years, modern researchers emphasize the limited use of these drugs due to the large number of adverse reactions in the postoperative period. J. Frauenknecht found that intraoperative administration of opioids did not reduce the level of postoperative pain and length of hospital stay of patients after general surgery16. Conversely, patients who did not receive narcotic analgesics in the perioperative period required less or no need for postoperative opioid administration, making recovery easier without tremor, nausea, or pain17. It should also be noted that 24 hours after surgery, all patients reported about low pain severity, below 1.0 point, however, in the groups where sevoflurane was used by inhalation (0.23±0.02 points) and combined with opioids (0.20±0.02 points), and the level of pain self-esteem was 2.61 and 3 times lower than after intravenous propofol.
An urgent problem of modern surgery and anesthesiology is the presence of potential risk of cognitive impairment after surgery and anesthesia, especially in elderly and debilitated patients, which can significantly impair their quality of life and increase mortality18. In own work within 1 hour after surgery, the average cognitive score on the Montreal scale decreased to 11.4±0.06 in the group of intravenous propofol (5.0%, p <0.05) and to 11.8±0, 02 under inhalation anesthesia with sevoflurane (1.7%). At the same time, under combined anesthesia with sevoflurane and opioids, the cognitive score on the Montreal scale remained constant – 12.0 points.
According to another meta-analysis, MMSE values at 6 h, 1 day, 3 days and 7 days after intravenous anesthesia with propofol were significantly lower than those with sevoflurane anesthesia. This may be because sevoflurane has a shorter duration of action and is eliminated more quickly than propofol18. In a study of day hospital patients, S. Parida and A. S. Badhe (2014) concluded that recovery of cognitive function after surgery under sevoflurane anesthesia was slightly faster than with propofol anesthesia, but it should be noted that nitric oxide was used in both groups19. In addition, some researchers have described the neuroprotective properties of sevoflurane in animal models, linking this to the regulation of hippocampal receptor expression20,21. In contrast, a much larger number of researchers claim that propofol reduces the incidence of postoperative cognitive dysfunction, while sevoflurane, in contrast, has a more harmfull effect on the mental state of patients after surgery22,23.
Conclusions
The total awakening time under combined inhalation anesthesia was 2.05 times less than with intravenous propofol. The use of combined inhalation analgesia of sevoflurane with opioids was characterized by 41.9% diminishing of recovery time.
After 8 hours of intervention, the individual assessment of pain by VAS was lower in the group of combined analgesia with opioids (in point score) relative to intravenous anesthesia with propofol (87.5%, p <0.05) and inhalation anesthesia with sevoflurane (71.3%, p <0.05). The severity of pain after 8 hours was directly correlated with other clinical indicators – systolic blood pressure (r=0.55, p <0.05), diastolic blood pressure (r=0.40, p <0.05) and heart rate (r=0.37, p <0.05).
After 12 hours, the individual assessment of pain in the group of combined anesthesia was 1.27±0.06 points, which is 39.4% less than in isolated anesthesia with sevoflurane and 65.4% less than in intravenous anesthesia with propofol.
Twenty-four hours after surgery, all patients reported about diminishing of pain severity – below 1.0, however, in the groups where sevoflurane was used by inhalation and in combination with opioids, the level of pain self-esteem was 2.61 and 3 times lower than after intravenous administration of propofol.
Within 1 hour after surgery, the mean cognitive score on the Montreal scale decreased in the group of intravenous propofol by 5.0% (p <0.05) and by 1.7% under inhalation anesthesia with sevoflurane. Under combined anesthesia with sevoflurane and opioids, the cognitive score on the Montreal scale remained stable at 12.0 points.
The frequency of postoperative nausea was the highest in the group of inhalation anesthesia – 16.7%. The increase in the absolute risk of postoperative nausea was 2.64 times with inhalation anesthesia with sevoflurane, compared with intravenous. The addition of nalbuphine to sevoflurane significantly reduced the risk of postoperative nausea – χ2=7.250 (p=0.007).
Acknowledgement
We would like to express our special thanks of gratitude to Bogomolets National Medical University in particular the members of the Faculty of Surgery, Anesthesiology and Intensive Care as well as the personnel of the Patlazhan Сlinic in Odesa, which also helped us in doing research.
Secondly, we would also like to thank all our colleagues for help in finalizing this project within the limited period.
Conflict of interest
There is no conflict of interest.
Funding Sources
There is no funding Source
References
- Sherwin A., Buggy D. J. Anaesthesia for breast surgery. BJA Educ, 2018; 18(11): 342-348. https://doi.org/10.1016/j.bjae.2018.08.002.
CrossRef - Lundström S., Twycross R., Mihalyo M., Wilcock A. Propofol. Pain Symptom Manag, 2010. 40(3): 466–470. https://doi.org/10.1016/j. jpainsymman.2010.07.001
CrossRef - Rowland J. H., Desmond K. A., Meyerowitz B. E. Role of breast reconstructive surgery in physical and emotional outcomes among breast cancer survivors. Natio. Cancer Instute, 2000; 92(17): 1422-1429. https://doi.org/10.1093/jnci/92.17.1422
CrossRef - Zheng J-W., Meng B., Li X-Y., Lu B., Wu G-R., Chen J-P.NF-κB/P65 signaling pathway: a potential therapeutic target in postoperative cognitive dysfunction after sevoflurane anesthesia. Eur Rev Med Pharmacol Sci, 2017; 21(2): 394–407. https://doi.org/10.17219/acem/134740
CrossRef - Qin G., Luo H., Yin X., Wang L., Zhang J., Cao Y., Zhang Z., Ye, Z., Wang E. Effects of Sevoflurane on Hemodynamics and Inducible Nitric Oxide Synthase/Soluble Guanylate Cyclase Signaling Pathway in a Rat Model of Pulmonary Arterial Hypertension. Anesth Analg, 2017; 125(1):184–189. https://doi.org/10.1213/ANE.0000000000001937.
CrossRef - Claroni C., Torregiani G., Covotta M., Sofra M., Scotto Di Uccio A., Marcelli M., Naccarato A., Forastiere E. Protective effect of sevoflurane preconditioning on ischemia-reperfusion injury in patients undergoing reconstructive plastic surgery with microsurgical flap, a randomized controlled trial. BMC Anesthesiol, 2016; 16(1): 66. https://doi.org/10.1186/s12871-016-0230-1.
CrossRef - Lim J., Oh C., Yoon T-G., Lee J.Y., Lee S-H., Yoo Y-B., Yang J-H., Kim S-H. The effect of propofol and sevoflurane on cancer cell, natural killer cell, and cytotoxic T lymphocyte function in patients undergoing breast cancer surgery: an in vitro analysis. BMC Cancer, 2018; 18(1): 159. https://doi.org/10.1186/s12885-018-4064-8.
CrossRef - Chen H-P., Hsu Y-H., Hua K-C., Lin C-C., Lo Y-F., Yu H-P. Comparison of sevoflurane versus propofol under auditory evoked potential monitoring in female patients undergoing breast surgery. Biomed J, 2013; 36(3) :125–131. https://doi.org/10.4103/2319-4170.113228
CrossRef - Erb T., von Ungern-Sternberg B., Moll J., Frei F. Impact of high concentrations of sevoflurane on laryngeal reflex responses. Paediatr Anaesth, 2017; 27(3): 282–289. https://doi.org/10.1111/pan.13062
CrossRef - Sharma S., Chang D. W., Koutz C. Incidence of hematoma associated with ketorolac after TRAM flap breast reconstruction. Plast Reconstr Surg, 2001; 107(2): 352–355. https://doi.org/10.1097/00006534-200102000-00009
CrossRef - Kocaturk O., Keles S. Recovery characteristics of total intravenous anesthesia with propofol versus sevoflurane anesthesia: a prospective randomized clinical trial. J Pain Res, 2018; 11: 1289–1295. https://doi.org/10.2147/JPR.S164106.
CrossRef - Çaparlar C. Ö., Özhan M. O., Süzer M. A. Fast-track anesthesia in patients undergoing outpatient laparoscopic cholecystectomy: comparison of sevoflurane with total intravenous anesthesia. Clin. Anesth, 2017; 37: 25–30. https://doi.org/10.1016/j.jclinane.2016.10.036
CrossRef - Heimes A. S., Stewen K., Hasenburg A. Psychosocial Aspects of Immediate versus Delayed Breast Reconstruction. Breast Care (Basel), 2017; 12(6): 374–377. https://doi.org/10.1159/000485234
CrossRef - Filip C. I., Jecan C. R., Raducu L. Immediate Versus Delayed Breast Reconstruction for Postmastectomy Patients. Controversies and Solutions. Chirurgia (Bucur), 2017; 112(4): 378–86. https://doi.org/10.21614/chirurgia.112.4.378
CrossRef - Hart A. M., Broecker J. S., Kao L., Losken A. Opioid use following outpatient breast surgery: are physicians part of the problem? Reconstr. Surg, 2018; 142(3): 611–620. https://doi.org/10.1097/PRS.0000000000004636
CrossRef - Frauenknecht J., Kirkham K. R., Jacot-Guillarmod A., Albrecht E. Analgesic impact of intra-operative opioids vs. opioid-free anaesthesia: a systematic review and meta-analysis. Anaesthesia, 2019; 74(5): 651–662. https://doi.org/10.1111/anae.14582
CrossRef - Mulier J. P. Is opioid-free general anesthesia for breast and gynecological surgery a viable option? Curr Opin Anaesthesiol, 2019; 32(3): 257–262. https://doi.org/10.1097/ACO.0000000000000716
CrossRef - Sun H., Zhang G., Ai B., Zhang H., Kong X., Le W-T. A systematic review: comparative analysis of the effects of propofol and sevoflurane on postoperative cognitive function in elderly patients with lung cancer. BMC Cancer, 2019; 19(1): 1248. https://doi.org/10.1186/s12885-019-6426-2
CrossRef - Parida S., Badhe A.S. Comparison of cognitive, ambulatory, and psychomotor recovery profiles after day care anesthesia with propofol and sevoflurane. J Anesth, 2014; 28(6): 833–838. https://doi.org/10.1007/s00540-014-1827-5
CrossRef - Haseneder R., Starker L., Berkmann J., Kellermann K., Jungwirth B., Blobner M., Eder M., Kochs E., Rammes G. Sevoflurane anesthesia improves cognitive performance in mice, but does not influence in vitro long-term potentation in hippocampus CA1 stratum radiatum. PLoS One, 2013; 8(5): e64732. https://doi.org/10.1371/journal.pone.0064732
CrossRef - Xu T., Bo L., Wang J., Zhao Z., Xu Z., Deng X. Risk factors for early postoperative cognitive dysfunction after non-coronary bypass surgery in Chinese population. J Cardiothorac Surg, 2013; 8: 204. https://doi.org/10.1186/1749-8090-8-204
CrossRef - Geng Y-J., Wu Q-H., Zhang R-Q. Effect of propofol, sevoflurane, and isoflurane on postoperative cognitive dysfunction following laparoscopic cholecystectomy in elderly patients: A randomized controlled trial. J Clin Anesth, 2017; 38: 165–171. https://doi.org/10.1016/j.jclinane.2017.02.007
CrossRef - Tian H-T., Duan X-H., Yang Y-F., Wang Y., Bai Q-L., Zhang X. Effects of propofol or sevoflurane anesthesia on the perioperative inflammatory response, pulmonary function and cognitive function in patients receiving lung cancer resection. Eur Rev Med Pharmacol Sci, 2017; 21(23): 5515–5522. https://doi.org/10.26355/eurrev_201712_13943







