Manuscript accepted on :31-08-2021
Published online on: 29-09-2021
Plagiarism Check: Yes
Reviewed by: Dr. Flavio Palmieri

Second Review by: Dr. Hind Shakir

Final Approval by: Dr. Ian James Martin
Shruti Chopra1, Ajit Varma1, Seema Jain2, Sangeeta Jain3, Devendra Choudhary1
1Amity Institute of Microbial Technology, Amity University, Noida, U.P.,India
2Department of Obstetrics and Gynaecology, Vardhman Clinic, Rohini, Delhi,India
3Joy IVF Clinic, Jain Hospital, Pushpanjali Vikas Marg, Delhi, India
Corresponding Author E-mail: shrutimalhotra227@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2230
Abstract
Objective: To study the effect of sperm chromatin condensation (DNA fragmentation index (DFI)) using aniline blue-eosin (AB-E) staining on pregnancy outcomes in patients facing unexplained infertility undergoing intra- uterine insemination (IUI). Our initial hypothesis states that if DNA fragmentation is high then chances of pregnancy are low/NIL hence these patients should be recommended with advanced ART procedures like IVF and ICSI. Design: Prospective study Setting: Tertiary care infertility centre Method: A total of 185 patients with age less than 40 years, non-smokers and without history of any pathogenic infection in the past 2months facing unexplained infertility i.e., males with normal semen analysis reports and females with normal ovulation and hysterosalpingography (HSG) reports were selected for the study. Patients were undergoing their first or second IUI treatment cycle between the period of June 2016 to December 2016. DNA fragmentation index (DFI) using aniline blue- eosin staining method was studied in semen samples provided on the day of IUI procedure. The patients were separated into 3 groups: low DFI (DFI<= 10%), medium DFI (DFI=11 % - 20%), and high DFI (DFI >= 21%) and clinical pregnancy outcomes of IUI were recorded. Statistical analysis was performed using Pearson correlation co-efficient, ANOVA and Shapiro Wilk Test on the above groups. Main Outcome Measures: DNA fragmentation index (DFI) (%), Clinical pregnancy rate (%) Result: The overall clinical pregnancy rate for the selected patient pool was 21.08% with an average DFI of 8.84% in the pregnant female group and 14.65% in the non-pregnant female group. Sperm DFI % and clinical outcomes in IUI treated patients were statistically significant and negatively correlated with correlation coefficient (r) of -0.1, -0.3 and -0.3 in low DFI%, medium DFI and high DFI% groups respectively. Conclusion: Our study demonstrated that DFI (%) and clinical pregnancy rate (%) are significantly and negatively correlated in patients with normal semen parameters undergoing IUI. The higher the DFI% the chances of clinical pregnancy become very low, therefore, these patients should not be recommended IUI but with advanced ART procedures like IVF and ICSI.
Keywords
Aniline Blue- Eosin; Clinical Pregnancy Rate; DNA Fragmentation Index (DFI); IUI; Sperm Chromatin Compaction
Download this article as:| Copy the following to cite this article: Chopra S, Varma A, Jain S, Jain S, Choudhary D. Effect of Sperm Dna Fragmentation Index on Clinical Outcomes of Intra-Uterine Insemination Patients. Biomed Pharmacol J 2021;14(3) |
| Copy the following to cite this URL: Chopra S, Varma A, Jain S, Jain S, Choudhary D. Effect of Sperm Dna Fragmentation Index on Clinical Outcomes of Intra-Uterine Insemination Patients. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/3umZRKG |
Introduction
Infertility is observed in around 15% of males with a normal semen analysis report 1. This proposes that a simple semen analysis may not reveal sperm DNA integrity and cannot predict the fertilization capability of the sperm.
Several mechanisms suggesting the cause of sperm DNA damage have been reported like abnormal chromatin packaging and oxidative stress 2-5, but the exact causes have not yet been fully understood.
Therefore, assessment of sperm chromatin abnormalities is important in treating male infertility as shown in various studies 1,6. Sperm DNA assessment not only affect natural conception but also assisted reproductive technology (ART) success rates.
Intrauterine insemination (IUI) is a common procedure performed for treatment of moderate male factor and unexplained infertility. IUI is a very cost-effective and non-invasive procedure in which the semen washing step removes prostaglandins, immotile sperms, leukocytes and immature germ cells, thereby decreasing free oxygen radical formation and improving the quality of sperm for fertilization.
Elevated levels of sperm DNA fragmentation in men with poor semen parameters have been observed in previous studies 7-8, however few studies show that men with normal semen parameters undergoing their IUI procedure cycles have abnormal sperm chromatin integrity 9.
Assessment of sperm DNA can be done by various methods like, flow cytometric-based sperm chromatin structure assay (SCSA) and terminal deoxynucleotidyl transferase mediated deoxyuridine triphosphate nick end labeling (TUNEL) assay which provide better diagnostic results than the standard semen analysis. Also there are simple, sensitive and inexpensive cytochemical assays which don’t need specialized laboratory setup 10.
In this study we evaluated sperm chromatin compaction or maturation by aniline blue staining, which detects sperm chromatin defects related to their nucleoprotein content 11-12. Lysine rich histones that are retained in the immature sperms are bound by the acidic aniline blue in the sperm head making it appear dark blue whereas, arginine and cysteine rich protamines of mature sperm are not stained by Aniline blue 12.
This method is simpler and cost effective which could be utilized by all ART laboratories for reporting sperm DNA damage in semen analysis to improve treatment outcome in ART.
The aim of the present research was to study the effect of DNA fragmentation index (DFI) for sperm chromatin compaction using Aniline blue-Eosin staining on clinical outcomes in patients undergoing IUI.
Materials and Method
Patient Selection
A total of 185 patients undergoing their first or second IUI cycle were studied between the period of June 2015 to Dec 2016. All patients selected in the study were less than 40 years old having unexplained infertility where the couples facing infertility have all standard investigation tests for ovulation, hysterosalpingography (HSG) and conventional semen parameters are reported as normal. Patients with history of any pathogenic infection in past 2 months and smokers were ruled out.
DNA fragmentation index (DFI) using aniline blue- eosin staining method was studied in semen samples provided on the day of IUI procedure.
The semen samples were washed and prepared, and the female partner underwent IUI procedure and clinical pregnancy outcomes were recorded.
Based on sperm DFI results, the patients were divided into the following 3 groups: low DFI (DFI<= 10%), medium DFI (DFI=11 % – 20%), and high DFI (DFI >= 21%) and clinical outcomes were compared among the 3 groups. This study was carried at a tertiary care infertility centre and permission to conduct the experiments was taken from the hospital ethical committee, also informed written consents were obtained from each patient.
Semen Analysis
Patients provided semen samples for analysis by masturbation after an abstinence of 3 to 6 days. After 30 minutes of liquefaction at room temperature, every sample is analysed for conventional semen parameters (13) and DFI using Aniline blue- eosin staining.
In this technique, semen samples in stained with Aniline Blue- eosin as previously described (14-16). 10 μL of raw semen sample was smeared on a slide and subsequently air dried for each patient. Slides were fixed at room temperature in 4% formalin for 5 minutes, then rinsed with water and air dried. 5% aqueous aniline blue solution (HIMEDIA) (pH 3.5) was used to stain the slides for 5 minutes followed by rinse with water and air drying. Slides were then counter stained for 1 minute in 0.5% eosin (Merck), again rinsed and air dried. A modification of Aniline Blue staining method i.e. a counter stain eosin when used after aniline blue enhances the staining 15, 17.
Slide examinations were carried under bright field microscope at X 1000 magnification using oil immersion. Immature sperms stain dark blue, whereas the eosin counter stain, stained the mature sperms red pink. The percentage of abnormal sperm chromatin condensation or DNA fragmentation index (DFI) was recorded as the ratio of the number of dark-blue sperm to total number of sperm cells observed and multiplied by 100. A minimum of 200 sperm cells were observed for every slide.
IUI Treatment plan
In the selected pool of patients, the female partners underwent ovulation induction using Clomiphene citrate. An injection of human chorionic gonadotropin (hCG) 10000 IU was given to the female partner when at least 1-2 follicles reached the size of 18 mm in diameter. IUI procedure was scheduled 36 hrs after the injection was given. The male partner provided a fresh semen sample on the day of IUI treatment and the sample was prepared by density gradient centrifugation (DGC) method (13) using sperm grade solutions of 40% and 80% (Vitrolife, Sweden). 1 ml of the prepared semen sample was then deposited using an IUI catheter inside the uterine cavity of the female partner. Serum β-hCG was assessed 15 days after the treatment to detect positive pregnancy (>50mIU/ml). Ultrasound examination was done after 6 weeks to confirm clinical pregnancy.
Statistical Analysis
Statistical analysis was performed using Pearson correlation co-efficient, ANOVA and Shapiro Wilk Test on the above groups.
Results
The 185 patients selected for the study had an average male age of 32.19 years and average female age of 30.30 years. The overall pregnancy rate of this group was 21.08 %.
The pregnant group had an average DFI of 8.84% and the non-pregnant group had 14.65% average DFI.
The patients were divided according to their DFI results into 3 groups: Low DFI % (<= 10%), Medium DFI % (11% – 20%), High DFI % (>=21%), with an average female age of 29.83yrs, 30.61yrs & 31.22yrs respectively; and an average male age of 31.81yrs, 32.33yrs & 33.31 yrs respectively which were not statistically significant
In this study, a negative correlation was observed between percentage of sperm DFI and clinical outcomes among the three groups i.e. low DFI gave high pregnancy results.
Table 1 shows that DFI and clinical outcomes in IUI treated patients are significantly and negatively correlated with correlation co-efficient (r) of -0.1, -0.3 and -0.3 in low DFI%, medium DFI and high DFI% groups respectively.
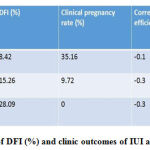 |
Table 1: Correlation of DFI (%) and clinic outcomes of IUI among different groups |
A normality test (Shapiro Wilk test) was performed i.e. a test to compare our distribution of data with normal distribution, we found that the p value was >0.05 hence we do not reject the null hypothesis that the data follow a normal distribution as shown in graph 1 and 2.
Graph 1 and 2: Plotting DNA Fragmentation % with normal distribution curve
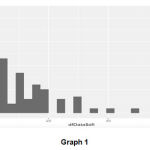 |
Graph 1 |
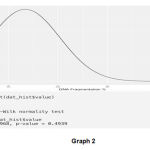 |
Graph 2 |
As we can clearly see that our dataset is positively skewed i.e. most people have a lesser DNA fragmentation % in our dataset. Hence to do further analysis we need to do log transformation of our values as provided in the below graph.
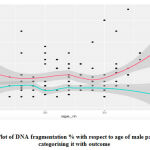 |
Graph 3: Plot of DNA fragmentation % with respect to age of male patients and categorising it with outcome. |
Hence we can say that age of male patients is not a predictor of DFI % and clinical outcomes of IUI.
Discussion
Sperm DNA contributes to 50% of the embryo’s genetic material and any damage to the sperm DNA affects conception rates naturally as well as in ART procedures.Unexplained infertility in males show higher incidence of sperm DNA damage (9), recently assessment of sperm DFI is being carried along with routine tests (18-19), also its importance has been stated in American Urology Association 20.
Sperm chromatin abnormalities could occur at the time of spermatogenesis which takes place within several structures of the male reproductive system. Sperm chromatin is a compact structure, which protects the male genetic material against damages during its passage from testis to the female fallopian tubes 21.
Sperm chromatin compaction involves the replacement of histones by protamines which help in the final condensation and dense packaging of the sperm DNA into unique tightly coiled “doughnut-loop” subunits 22-23.
The chromatin condensation process may sometimes lead to breaks and nicks in the sperm DNA, a failure to repair these breaks results in DNA damage 24.
In this study, the aniline blue -eosin staining evaluates the degree of sperm chromatin compaction 25-26, where the lysine rich histones that are retained in the immature sperms are bound by the acidic aniline blue in the sperm head making it appear dark blue, as compared to the arginine and cysteine rich protamines of the mature sperm DNA which are stained pink with eosin counter stain, thereby enhancing the immature sperm heads for better observation 14,15,17.
In our study we used the density gradient centrifugation method for sperm preparation which has shown lower sperm deformity and DFI in comparison to unprocessed semen in previous studies 27.
Our study though based on a smaller patient group, demonstrated that higher the DFI% the chances of clinical pregnancy become very low, therefore, these patients should not be recommended IUI but with advanced ART procedures like IVF and ICSI. High DFI % group of >=21% did not report any pregnancy whereas low DFI % group reported a pregnancy rate of 35.16% making DFI a good predictor for IUI outcomes.
Also, on doing normality distribution test we clearly observed that our data set was positively skewed i.e. most people had a lesser DNA fragmentation % in our data set. Hence to do further analysis we dida log transformation of our values which showed that age of male patients is not a predictor of DFI % and clinical outcomes of IUI. Therefore, more studies should be carried out with a vast patient pool of different age groups at multiple centres so as to have a bell-shaped normal distribution curve.
Previous studies by Duran et.al.,(28) reported that higher the DNA fragmentation levels, lower the pregnancy rates in IUI cycles where DFI was significantly higher in failed cycles and no pregnancy was achieved with sperm DFI > 12% by TUNEL method. Yang et.al., 29
in their study observed a significantly low pregnancy rate in IUI cyles where male samples had a DFI > 25% compared to those where DFI<25%. In another study, Bungum et.al., 30 lower biochemical pregnancy rates, clinical pregnancy rates and delivery rates were observed in IUI patients with >=30% DFI. Hui et.al.,31 reported that couples with elevated DFI levels should choose ICSI treatment instead of IUI and DFI should be used as a routine screening marker. In a meta-analysis, it was observed that the conception rate in females was 7.3 times higher where the male partner had a normal DFI than those with higher DFI (32-33). In our study, the male age and female age were statistically significant in all the 3 groups of low DFI, medium DFI and high DFI, indicating that age is not a predictor of DFI, also IUI outcomes were independent of age of male patients.
Conclusion
DNA fragmentation index (DFI) based on sperm chromatin compaction/condensation is a very important parameter for the assessment of male fertility and for predicting reproductive outcome. Our study demonstrated that DFI (%) and clinical pregnancy rate (%) are significantly and negatively correlated in patients with normal semen parameters undergoing IUI. The higher the DFI% the chances of clinical pregnancy become very low, therefore, these patients should not be recommended IUI but with advanced ART procedures like IVF and ICSI. Further assessment of sperm DNA status and different types of damages is required to establish their overall importance in natural as well as ART conceptions. We suggest this test for analyzing sperm DNA fragmentation be a part of the routine semen analysis for patients suffering infertility, as it is simple method to establish in an ART laboratory and also is cost-effective and would not put financial pressure on patients who are already socially, psychologically and economically disturbed.
Acknowledgement
We would like to thank the technical staff at Joy IVF and Amity University for their support.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Agarwal A, Allamaneni SS. Sperm DNA damage assessment: a test whose time has come. Fertil Steril 2005; 84:850-3.
CrossRef - Aitken RJ, De Iulis GN. Origins and consequences of DNA damage in male germ cells. Reprod Biomed Online 2007; 14(6):727–733.
CrossRef - Tesarik J, Mendoza TR, Mendoza C. Sperm nuclear DNA damage: update on the mechanism, diagnosis and treatment. Reprod Biomed Online. 2006; 12(6):715–721.
CrossRef - Ozmen B, Koutlaki N, Youssry M, Diedrich K, Al Hasani S. DNA damage of human spermatozoa in assisted reproduction: origins, diagnosis, impacts, and safety. Reprod Biomed Online. 2007;14(3):384–395.
CrossRef - Hekmatdoost A, Lakpour N, & Sadeghi Sperm Chromatin Integrity: Etiologies and Mechanisms of Abnormality, Assays, Clinical Importance, Preventing and Repairing Damage. Avicenna J Med Biotechnol. 2009 Oct-Dec; 1(3): 147–160.
- Bungum M, Bungum L, Giwercman A. Sperm chromatin structure assay (SCSA): a tool in diagnosis and treatment of infertility. Asian J Androl 2011; 13:69-75.
CrossRef - Moskovtsev SI, Willis J, White J, Mullen JB. Sperm DNA damage: correlation to severity of semen abnormalities. Urology. 2009;74(4): 789–93.
CrossRef - Zini A, Fischer MA, Sharir S, Shayegan B, Phang D, Jarvi K. Prevalence of abnormal sperm DNA denaturation in fertile and infertile men. Urology. 2002;60(6):1069–72
CrossRef - Alkhayal A, Gabriel M, Zeidan K et.al. Sperm DNA and chromatin integrity in semen samples used for intrauterine insemination. J Assist Reprod Genet 2013; 30:1519–1524
CrossRef - Talebi AR, Vahidi S, Aflatoonian A, Ghasemi N, Ghasemzadeh J, Firoozabadi RD, et al. Cytochemical evaluation of sperm chromatin and DNA integrity in couples with unexplained recurrent spontaneous abortions. Andrologia 2012;44 Suppl 1:462-70.
CrossRef - Auger, M. Mesbah, C. Huber, and J. P. Dadoune. Aniline blue staining as a marker of sperm chromatin defects associated with different semen characteristics discriminates between proven fertile and suspected infertile men. International Journal of Andrology 1990; vol. 13, no. 6, pp. 452–462.
CrossRef - Hammadeh M, Zeginiadov T, Rosenbaum P, Georg T, Schmidt W, Strehler E. Predictive value of sperm chromatin condensation (aniline blue staining) in the assessment of male fertility. Arch Androl.2001;46(2):99–104.
CrossRef - World Health Organization. WHO laboratory manual for the examination and processing of human semen [Internet]. 5th ed.Geneva: World Health Organization; c2013 [cited 2013 Mar 4].Available from: http://www.who.int/reproductivehealth/ publications/ infertility/9789241547789/en/index.html.
- Wong A, Chuan SS, Patton WC, Jacobson JD, Corselli J, Chan PJ. Addition of eosin to the aniline blue assay to enhance detection of immature sperm histones. Fertil Steril 2008; 90:1999-2002.
CrossRef - Park YS, Kim MK, Lee SH, Cho JW, Song IO, Seo JT. Efficacy of testicular sperm chromatin condensation assay using aniline blueeosin staining in the IVF-ET cycle. Clin Exp Reprod Med 2011; 38:142-7.
CrossRef - S. Kim, M. J. Kang, S. A. Kim et al.. The utility of sperm DNA damage assay using toluidine blue and aniline blue staining in routine semen analysis. Clinical and Experimental Reproductive Medicine 2013; vol. 40, no. 1, pp. 23–28.
CrossRef - Patil P., Bambulkar S., Ajgaonkar S., Patil R., Patil A. and Nikam V. DNA fragmentation index (DFI) of human semen bt modified Aniline blue method. Cibtech Journal of Bio-Protocols ISSN: 2319–3840 (Online) 2013; Vol. 2 (3) September-December, pp.1-5.
- Oleszczuk K, Augustinsson L, Bayat N, Giwercman A., Bungum M. Prevalence of high DNA fragmentation index in male partners of unexplained infertile couples. Andrology 2013;1:357-60.
CrossRef - Panner Selvam MK, Agarwal A. A systematic review on sperm DNA fragmentation in male factor infertility: laboratory assessment. Arab Journal of Urology 2018;16:65-76.
CrossRef - Jarow J, Sigman M, Kolettis PN et al. The optimal evaluation of the infertile male: best practice statement reviewed and validity confirmed 2011. American Urological Association.
- Love CC, Kenney RM. Scrotal heat stress induces altered sperm chromatin structure associated with a decrease in protamine disulfide bonding in the stallion. Biol Reprod. 1999;60(3):615–620.
CrossRef - D’Occhio MJ, Hengstberger KJ, Johnston SD. Biology of sperm chromatin structure and relationship to male fertility and embryonic survival. Animal Reprod Sci. 2007;101(1-2):1–17.
CrossRef - Balhorn R, Cosman M, Thornton K, Krishnan VV, Corzett M, Bench E, Kramer C, et al. Protamine mediated condensation of DNA in mammalian sperm. In: Gagnon C, editor. The male gamete: from basic knowledge to clinical applications. Illinois: Cache River Press 1999; pp. 55–70.
- Aoki VW, Liu L, Carrell DT. Identification and evaluation of a novel sperm protamine abnormality in a population of infertile males. Hum Reprod 2005; 20:1298-1306.
CrossRef - Agarwal A, Erenpreiss J, Sharma R. Sperm chromatin assessment. In: Gardner DK, Weissman A, Howles CM, Shoham Z, editors. Textbook of assisted reproductive technologies. 3rd ed. London: Informa Healthcare 2009; p. 67-84.
CrossRef - Sellami A, Nozha C, Soumaya BZ, Hanen S, Sahbi K, Tarek R and Leila K. Assessment of Chromatin Maturity in Human Spermatozoa: Useful Aniline Assay for Routine Diagnosis of Male Infertility. Hindawi Publishing Corporation. Advances in Urology 2013; Article ID 578631, 8 pages
CrossRef - Xue X, Wang WS, Shi JZ, Zhang SL, Zhao WQ, Shi WH et al. Efficacy of swim-up versus density gradient centrifugation in improving sperm deformity rate and DNA fragmentation index in semen samples from teratozoospermic patients. J Assist Reprod Genet 2014;31:1161-6.
CrossRef - Duran EH, Morshedi M, Taylor S et al. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod 2002;17:3122-8. 10.
CrossRef - Yang XY, Zhang Y, Sun XP, Cui YG, Qian XQ, Mao YD, Liu JY. Sperm chromatin structure assay predicts the outcome of intrauterine insemination. National Journal of Andrology 2011;17:977-83
- Bungum M, Humaidan P, Axmon A et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod 2007;22:174-9.
CrossRef - Hui LU, Yong LIU, Zi-jue ZHU, Xiao-rong CAO, Yu-fang XIAO, Yong ZHU, Wen-bo SHI, Can SUN, Feng YAN, Zheng LI. Sperm DNA Fragmentation may Influence IUI Outcome but could be Treated by ICSI: Evidence from Human Sperm Bank. Journal of Reproduction and Contraception 2014; Volume 25, Issue 3, Pages 165-176.
- Bungum M, Humaidan P, Spano M, Jepson K, Bungum L, Giwercman A, et al.. The predictive value of sperm chromatin structure assay(SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod 2004; 19:1401-8.
CrossRef - Evenson D, Wixon R. Meta-analysis of sperm DNA fragmentation using the sperm chromatin structure assay. Reprod Biomed Online 2006; 12:466-72.
CrossRef







