Manuscript accepted on :21-06-2021
Published online on: 29-09-2021
Plagiarism Check: Yes
Reviewed by: Dr. Shereen Fathi

Second Review by: Dr. Sirvan Zarei

Final Approval by: Dr. Kishore Kumar Jella
Pacôme Kouadio N’Go1.2*, Lazare Tehoua1, Omar Touhami Ahmed Ahami2, Fatima-Zahra Azzaoui2, Youssef Aboussaleh2
1Peleforo Gon COULIBALY University,Training and Research Unit of Biological Sciences, P.O.Box 1328, Korhogo, Ivory Coast
2Clinical and Cognitive Neurosciences Group, Biology and Health Lab, Ibn Tofail University, P.O.Box 133, Kenitra, Morocco
Corresponding Author E-mail: pacngo.neuro@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2220
Abstract
Chrysophyllum perpulchrum is an endemic medicinal plant used in ivorian tranditional pharmacopeiaeas antipyretic to heal malaria fever. Since three flavonoid compounds have been isolated, catechin and two procyanid in dimers, we are proposed to testthe neuroprotective effectiveness effects using a rat model of Alzheimer Disease (AD). Adult Wistar rats were used as model.Sham-operated rats as controlwere injected by intracerebroventricular route (i.c.v) with1% ammonia(Group1), Aβ rats were microinjected with 10µg/side (i.c.v route, (Group 2)).From 14th day post-surgery required for neuro inflammation and oxidative stress induction,some Aβ-injected rats were daily treated with the extract (300 mg/kg bw, oral route, (Group 3)) for 21 days,sham-operated rats were treated only with plant extract (300 mg/kg bw, oral route, (Group 4)). Rats were then submitted to memory tests with Y maze, object recognition test and Morris water maze. Some oxidative stress markershave been assessed.AD-like rats exhibited significant recognition memory as well as learning and spatial memory deficits.The treatment of AD like-rats with methanolic extract of Chrysophyllum perpulchrum alleviated cognitive disorders by improving the memory recognition index and spatial learning strategy to find the hidden platform. Furthermore,Chrysophyllum perpulchrum extract prevented significantly Aβ-induced lipid proxidation through a decrease of malondialdehyde (MDA) level in the hippocampus and the prefrontal cortex, and also helped to increase the non protein-thiol (NP-SH) antioxidant level.These findings suggest the neuroprotective actions of Chrysophyllum perpulchrum extract on AD-like rats. However,further pharmacological studies are needed to test ability of isolated compounds from Chrysophyllum perpulchrum to counteract full Aβ physiopathology mechanisms before promising to be a drug candidate for AD treatment.
Keywords
Alzheimer disease; brain; Chrysophyllum perpulchrum; oxidative stress; recognition memory; spatial learning
Download this article as:| Copy the following to cite this article: N’Go P. K, Tehoua L, Ahami O. T. A, Azzaoui F. Z, Aboussaleh Y. Cognitive Disorders and Oxidative Stress Status Attenuated by Chrysophyllum Perpulchrum Extract in Alzheimer- like Rat Model of Intracerebroventricular Aβ1-40 injection. Biomed Pharmacol J 2021;14(3). |
| Copy the following to cite this URL: N’Go P. K, Tehoua L, Ahami O. T. A, Azzaoui F. Z, Aboussaleh Y. Cognitive Disorders and Oxidative Stress Status Attenuated by Chrysophyllum Perpulchrum Extract in Alzheimer- like Rat Model of Intracerebroventricular Aβ1-40 injection. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/2Y7xMuN |
Introduction
Alzheimer Disease (AD) is the common form of dementia disorders which is mostly prevalent among ageing population through world. AD is a complex multi factorial neurodegenerative disease characterized by extracellular β-amyloid (Aβ) senile plaques deposition and intracellular neurofibrillary tangles of hyperphosphorylated Tau protein in selective brain areas as hippocampus and cerebral cortex,1 causingmemory loss, progressive cognitive and mood disorders. According to one review of worldwide data, AD represents more than 60% of all dementia in developing countries including those of Sub-Saharan Africa (SSA),2 and the global dementia in people over 60 years is approximately 1.6% in Africa compared to 5.9% and 6.4% in western Europe and north America respectively.3 However, SSA countries show some rapid demographic and economic transitions, and that is accompanied by an improvement of lifestyle quality. It could project to an increase of the life expectancy and the incidence of age-related neurodegenerative diseases as AD during upcoming years. Although entire mechanisms of the Aβ neurotoxicity are still unclear, it has been considerably demonstrated that the oxidative stress status plays a critical role in the physiopathogenesis of AD leading to neuronal death.4 Thus, a big challenge is to find out drugs with free radicals scavenging activity for helping to prevent cognitive and behavioral deficits related to oxidative stress.Today, the most drugs classes approved for transiently slow down AD progression are from synthetic chemicals,5 with possibleexisting of side effects on health. However, medicines from natural products seem to be an alternative safety therapy approaches for AD treatment. From this perspective, several studies using cell culture or animal’s model have been conducted with promise to find news drugs from medicinal plants targeting Aβ peptide and its subsequent pathophysiological effects.6 Almost of all valuable researches testing pharmacological properties of medicinal plants extract against AD have used species originated from Asia.Paradoxically, African forests with a abundant and diversity of traditional use plants, scarce studies have so far focused the potential effects of their natural products as anti-AD. In this setting, weplan to promote the effectiveness of african tropical area endemic specie as Chrysophyllum perpulchrum (Sapotaceae) by using of anAD-like rat model. It is used as antipyretic for cure malaria fever in Ivory coast traditional pharmacopeia, and a provider of antioxidant source.7
Here, we tested whether methanolic bark extract of Chrysophyllum perpulchrum could have neuroprotective effects on memory deficits due to oxidative stress in a rat model of intracerebroventricular (i.c.v) Aβ 1-40injection-induced AD.
Materials and Methods
Animals
The experiment was carried out using adult Wistar ratslocally obtained at the animals breeding house of Ibn Tofail University (Kenitra, Morocco). Animals were maintained for acclimation under controlled reference room at 22-25°C with a good relative humidity (50-60%), submitted to a 12 H light/ dark cycle and have free access to standard food (ALF SAHEL Company of Casablanca) and tap water. All experimental protocols were carried out according to NIH guide for the care and use of laboratory animals and approved by local Ibn Tofail university local ethic committee.
Experimental Design
Chemicals of plant material
Bioactive compounds from Chrysophyllum perpulchrum termed Catechin (P1) and two dimeric procyanidins (P2 and P3)(Fig.1) were chromatographically isolated and tested for free radicals scavenging activity using2,2-diphényl-1-picrylhydrazyl,their activity is similar to quercetin. 7,8The processes of methanolic, total phenolic contents or isolation compounds extractionhave been described elsewhere.7 Acute toxicity study estimated lethal dose 50 of Chrysophyllum perpulchrum to 1250 mg/kg of body weight (bw).8
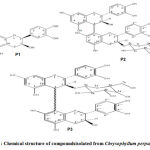 |
Figure 1: Chemical structure of compoundsisolated from Chrysophyllum perpulchrum. |
Aβ preparation and treatments
Synthetic Aβ1-40 peptide (Sigma Aldrich, St. Louis, USA) were prepared as stock solution at concentration 1mg/ml in 1% ammonia, and aliquots were stored at – 20°C. Before use, Aβ1-40 solution was aggregated by incubation at 37°C for 4 days.9 For the stereotactic surgery, animals were prior anesthetized with chloral (300 mg/Kg, i.p, in 7% solution) and were bilaterally i.c.v injectedwith Aβat the lateral ventricle level ( coordinates Antero-poosterior= -0.8, Lateral = ± 1.4, Dorso-ventral = -3.4)with a 10-µl Hamilton microsyringe. Animals were randomly divided into four experimental groupsof 6-7 animals each:(1)Shamgroup (10µl of 1% ammonia by i.c.v route), (2)Aβgroup (10µg/side of Aβ 1-40, i.cv route), (3)Aβ + CPrats (10µg/sidei.c.v route and treated with 300 mg/kg of methanolic bark extract of Chrysophyllum perpulchrum), (4)Sham + CP (Sham operated rat and treated 300 mg/kg of methanolic bark extract of Chrysophyllum perpulchrum).
The treatments with plant extract was done from 14th day post-surgery, a required period for Aβ to cause neuroinflammation and oxidative stress.10, 11
Cognitive Testing
Y-maze
It was used to evaluate spatial working memory in rodent,12,13after all the treatments. The apparatus was made with in fine-wood with three arms (A, B and C) measuring 40 in length, 10cm in width and 13 cm in height) and painted in different colour patterns. A central platform is formed by tri-angles of 120° between each arm. The procedure consists to give 8 min-session to each rat for exploring freely the maze. The sequence of arms entrieswas monitored with a camera video. An entry was validated when the four paws are within the arms. Alternation is defined as a triad of successive entry in different arms (i.e. ABCACBAACB= 5 alternations). Spontaneous alternation behaviour was calculated with following equation: % alternation= 100 x (number of alternation/ total arm entries – 2).
Recognition Memory Test (NORT)
The NORT is suitable to assess AD-related cognitive impairment.14The object recognition test procedure was conducted as describe by Ennaceur and Delacour.15The apparatus is an Open box with floor measurements 50 cm in length, 50 cm in width, and 40 cm in height walls. In the trial (familiarization session), rats were allowed 5 min to explore freely the box with two identical objects and return in their home cage. After 2 h delay, to evaluate short term recognition Memory (STM), rats were return in open field in which one object was switched by another one different for colour, sharpe and size, and experiment was repeated during 5 min with one novel object and one identical previously explored.To evaluate long -term memory (LTM) 24h later from the familiarization phase, rats were submitted to explore again two objects for 5 min, one identical and another novel one. Objects and box were cleaned with ethanol 70% during intertrial period. The exploration time of each object was recorded the video tracking, and the exploration feature is defined as the directing the nose at a distance less than 1 cm from the object. The ratio of preference of the novel object of each animal was calculated from the exploration frequency of novel object divided the total frequency spent for exploring both objects.
Morris Water Maze (MWM)
The MWM was performed to study spatial learning and memory. The apparatus was slightly modified of Morris Water maze test,16 with circular open lightly grey pool (120 cm in diameter and 40 Cm in depth) filled with tepid water (22°C) at 30 Cm of width. It isdivided into four virtual quadrants (North, South, West, and East) each including a visual cue outside the surface of water on the wall of the maze. In the habituation phase, animals were allowed 60 s to swim freely in the tank in order to define the best position of the platform which remained fixed during the all test sessions. Then, in the training phase, rats were left in the tank facing the wall and then allowed to swim to find the hidden platform submerged to 1 cm to the surface of water. If the animal did not find the platform after 60 s elapsed, it was gently guided to it and stay for 15s before returning in home cage. The testing was completed in fiveconsecutive days including four trials each. The behaviour of rat was recorded by a video camera placed on the ceiling above the maze.
We considered the escape latency parameter as the time spent to find the platform.
Oxidative stress level assay
After behavioural testing, rats were anesthetized with choral with (300mg/kg) and killed by decapitation. Brain regions correspondent to Hippocampus and Prefrontal cortexareas were quickly removed and homogenized in ice-cold 50 mMTris-HCl buffer(pH 7.4). The homogenate was then centrifuged at 3000 rpm for 30 min at 4°C to obtain a supernatant that was used.
Analysis of Non-protein Thiols (NP-SH) level
NP-SH level was assessed according to the method described by Ellman et al.17The supernatant was treated with 10%of trichloroacetic acid to precipitate proteins, and the sample was centrifuged at 2.000 rpm for 10 min. To the supernatant, was added 1 M of potassium buffer (pH7.4) and 1mM of Ellman’s reagent (5,5-dithiobis-2-nitrobenzoic acid). The NP-SHs levels were determined at 412 nm and expressed as µmol/g of tissue.
Determination of Lipid peroxidation level
The assay Malondialdehyde (MDA) level, an important index of lipid peroxidation, was described in the method of Satoh et al.18Briefly, 500 µl of supernatant correspondent to sample of hippocampus or CPF was mixed with 1.5 ml of trichloroacetic acid (10%), vortexed and incubated at room temperature for 10 min. Then it was added to the mixture 1,5 ml of thiobarbituric acid (0.67%), and heatedin boiling bath water for 15 min.After a cooling, 1.5 ml of n-butanol was mixed to the solution and strictly vortexed. The sample was centrifuged at 800 rpm for 5 min, and the supernatant was collected. The absorbance was determined spectrophotometrically at 532 nm. The results were expressed as MDA level nmol/g of tissue.
Statistical analysis
The experimental results data were expressed as means ± S.E.M (Standard Error of Mean). Statistical analysis were done using one-way analyses of variance followed by Tukey post-hoc test for multiple comparison, with value of p<0.05 considered statistically significant.
Results
Effects of Chrysophyllum perpulchrum extracton cognitive abilities in AD-like rats model induced by i.c.v Aβ1-40injection
Spatial working memory in Y-maze
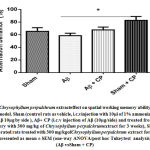
Thei.c.v injection of Aβ1-40 caused an alteration of spatial working memory through a significant reduction of the percentageof spontaneous alternation behaviour (p<0.05), when compared to the performance of sham-operated rats and treated with 300mg/kg of Chrysophyllum perpulchrum extract. However, even if, the treatment with 300 mg/kg of Chrysophyllum perpulchrum extract improved Aβ1-40-induced working spatial memory impairment that was not significant (Fig.2).
Recognition memory in NORT
As indicated on the Fig. 3A, i.c. vinjection of Aβ1-40 impaired significantly STM recognition performance in Aβ rats when compared to vehicle rats (p<0.01) and sham operated rats treated with 300 mg/kg of crude extract (p<0.05). The treatment of AD-like rats during 3 weeks with 300 mg/kg of crude extract of Chrysophyllum perpulchrumim proved the short-term recognition capacity as reflected by anincrease of the index.
Regarding to the LTM recognition, the treatment with Chrysophyllum perpulchrum extract prevented the impairments induced by Aβi.c.v injection through a significant increase of recognition index above the threshold of 50% (p<0.05) (Fig.3 B).
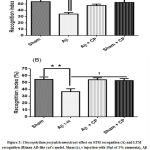 |
Figure 3: Chrysophyllum perpulchrum extract effect on STM recognition (A) and LTM recognition (B)inan AD-like rat’s model. |
Spatial learning and memory in MWM
We used MWM to assess spatial learning and memory which are hippocampal functions dependents. Our results showed that the experiment groups performed differently to locate the hidden platform in the maze (Fig.4). The i.c.v Aβ injected rats learnt with difficulty to find the platform during training day sessions, remarkably at day 1 (p<0.001), day 3 (p<0.01), day 4 (p<0.001), day 5 (p<0.001) compared to others groups. On the other hand, the treatment of i.c.v Aβ rats with 300mg/kg of plant extract over 3 weeks relieved significantly spatial learning and memory deficit. Interestingly, the latency time to find the platform was shortened by the training day’s sessions in the rats treated only with 300 mg/kg of Chrysophyllum perpulchrumcrude extract.
 |
Figure 4: Chrysophyllum perpulchrum extract effect on spatial learning and memory abilities inan AD-like rat’s model.Sham (control vehicle, i.c.v with 10µl of 1% ammonia) |
Effects of Chrysophyllum perpulchrum extract on oxidative stress status in AD-like rats model induced by i.c.v Aβ1-40 injection
Analysis of NP-SHs level
In prefrontal cortex, a significant decrease of NP-SH level was found in therats of Aβ group (p<0.001)when compared to others ones. We noted that the Aβ rats treated with 300 mg/kg of crude extract of Chrysophyllum perpulchrum did not showed significant increase of NP-SH level. However, the highest concentration of the NP-SH level was found in sham-operated rats treated with 300 mg/kg of plant extract (Fig.5). The dosage of NP-SH in hippocampal area revealed the lowest level in Aβ rats. However, the given treatment with Chrysophyllum perpulchrum extract to Aβ rats caused significant increase of the level of NP-SH (p<0.01, Aβ + CP vs Aβ group). The same observation was done in the sham-operated rats treated with extract of Chrysophyllum perpulchrum (Fig. 5).
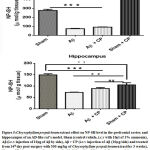 |
Figure 5: Chrysophyllum perpulchrum extract effect on NP-SH level in the prefrontal cortex and hippocampus of an AD-like rat’s model. |
Analysis of MDA level
The i.c.v Aβinjection showed an increased level of lipid peroxidation which is reflected by high amount of MAD in both the prefrontal cortex and hippocampus(Fig.6). In the prefrontal cortex, there was very high significant increase of the MDA level in the Aβ rats group (p<0.001). The treatment with Chrysophyllum perpulchrum extract help to counteract the lipid peroxidation observed in Aβ rats. At the same manner, in hippocampus, there was high significant raising of MDA concentration in Aβ rats group (p<0.001), while the treatment with bark crude extract of Chrysophyllum perpulchrum avoided high level of lipid peroxidation. The sham-operated rats with plant extract treatment were not significantly affected compared to Aβ rats with plant treatment (p<0.05).
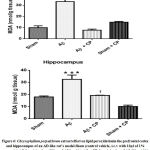 |
Figure 6: Chrysophyllum perpulchrum extract effect on lipid peroxidationin the prefrontal cortex and hippocampus of an AD-like rat’s model. |
Discussion
The increase of risk factors of AD sporadic form could rise the prevalence in SSA population during upcoming years. Thus, as none drug is yet efficient against AD, it becomes now worthwhile to conduct further experiments with goal to find drugs based on natural products from African’s endemic medicinal plants. To our knowledge, there is no yet researches having studied pharmacologically and clinically the beneficial effects of any Ivoirian’s medicinal plants in the neurodegenerative diseases context. The present study is a first one launched to evaluate whether methanolic extract of Chrysophyllum perpulchrum could relieve memory and cognitive deficits, and alleviate oxidative stress status in an AD-like rats induced by i.c.v Aβ1-40 injection. We found as main findings significant deficits of short-term and long-term object recognition, and spatiallearning and memoryabilities in Aβ rats. However, the treatment of AD-like rats with crude extract of Chrysophyllum perpulchrum rescued cognitive impairments.Interestingly, AD-like rats treated with plant extract performed some times behavioral tests at the same manner as the rats of control or sham operated-treated with plant extract groups. Also, the treatment by Chrysophyllum perpulchrum extract prevents oxidative stress and lipid peroxidation in hippocampus or prefrontal cortex observed in AD- like rats. Here, we discuss our results with others possible actions supposed to be due to bioactive compounds from Chrysophyllum perpulchrum against AD.
It has been reported that object recognition task is a suitable behavioral test to evaluate AD-related cognitive impairments as well as hippocampus-dependent spatial learning and memory assessed with MWM.14 Poor performances of i.c.v Aβ rats compared to others in behavioral testing arise the question to know the chronology events occurred in the physiopathology mechanisms according to our experimental procedure.Firstly, we suggest that the target of one bio active component of Chrysophyllum perpulchrum namely catech in is AChE inhibitor. In fact, AChE is largely recognised to promote acceleration of Aβ sheet polymerization, fibrillization and its aggregation in amyloid plaque.19,20
A previous study mentioned that polyphenolic compounds are well known to inhibit AChE which is a key enzyme in AD.21More specifically, Olasehende et al22 when testing the anti-amyloidogenic effects of Catech in and derived compounds isolated from seaweeds, have in vitro highlighted a significant reduction of aggregated Aβ1-42 level. Another experiment using in vivo mice’s model of AD confirmed that catechin from Rhizophora mucronata enhanced cognitive functions by inhibiting AChE and others cholinesterases.23 The neuroprotective effects of Chrysophyllum perpulchrum extract on memory performances suggest partly to be related to its effect on Aβ clearance. It is clearly established that Aβ mediates oxidative stress by different processes leading to production of ROS like super oxide anion radical, hydrogen peroxide or hydroxyl radical among others.24 Oxidative stress occurred when the level of free radicals generated by Aβ exceeds the cells natural antioxidants including glutathione and antioxidant enzymes as superoxide dismutase, catalase and glutathione peroxidase. Oxidative stress in brain is a major factor that underlies neurotoxicity in the AD, with consequence of cells damage or death by apoptosis.25 An approach of supplementation with exogenous antioxidants from natural substance may be effective to prevent Aβ-induced toxicity or halt AD progression.26 In our study, there was an increased of NP-SH level (90% represented by gluthation) in hippocampus of Aβ rats after treatment with Chrysophyllum perpulchrum extract, when compared to Aβ rats without treatment. However, we found that this difference was less observed in the prefrontal cortex. Our results are in agreement with previous study that showed significant increase in glutathione in a rat’s model of AD treated with natural antioxidant plant Cantella asiatica.27 In fact, it has been demonstrated that Aβ is able to cause high-depleted level of glutathione in brain and reduce synaptic density.28
It plays neuroprotective role from oxidative stress by reacting with prooxidant hydroxyl peroxide to give rise to the oxidized form of glutathione. We can suggest that active compounds of Chrysophyllum perpulchrum extract help to combate Aβ1-40-induced oxidative stress by enhancing NP-SH level.In another way, high concentration of NP-SH in hippocampus is thought to be linking to its great vulnerability to oxidative stress,29it is the primary brain site affected in AD condition before extending toward the cerebral cortex for advanced cognitive disorders.Healthy brain contains much macromolecules which are susceptible to oxidative stress. One of subsequent effects of Aβ-facilitated extensive oxidative stress is peroxidation of membrane lipids, leading to neuronal damage and death by apoptosis.25 It has been clearly found a positive correlation between Aβ plaques and lipid peroxidation in AD brain.30 Our experiment highlighted high level of MDA in hippocampus and prefrontal cortex in Aβ rats, certainly due to high lipid peroxidation of neuronal membranes.However, the treatment with Chrysophyllum perpulchrum extract attenuated Aβ1-40-induced membrane lipid peroxidation. A significant lessening of MDA level was observed in brain ofAβ rats treated with plant crude extract. Similar results were reported by Schimidt et al31which sustain that catechin from Camellia sinensis avoids lipid peroxidation in AD rat model, and relieves oxidative stress and memory deficits. Another key toxicity action of Aβ isbased on its high affinity toward redox-active metalcopper or iron which releases hydrogen peroxide and hydroxyl and responsible for membrane lipid peroxidation.32
The supplementation of catechin contained in Chrysophyllum perpulchrum extract could be considered as potent redox metal-chelator for the prevention membrane peroxidation and cognitive disorders related to AD. Otherwise, oxidative stress condition promotes progressively the neuronal loss mainly by apoptotic pathway.33 In this sense, a previous study investigating the beneficial effects of Catechin-rich from methanolic extract of Rhuzophora mucronata demonstrated aninhibition of hippocampal neurons apoptosis by down–regulating caspase 3 in a mice model of i.c.v administration of aggregated Aβ.23
We remind that Chrysophyllum perpulchrum extract contains two dimeric procyanidin compounds.[7] An important factor suggested to prevent Aβ-induced cognitive deficits is probably the neuroprotective effects of procyanid in. For this purpose, procyanid in B1 from a medicinal plant (Uncaria hook) suppressed actively Aβ oligomeric-induced neurotoxicity, their neuroprotective action consists so to attenuate activation of Caspase 3 by inhibiting the pro-caspase 8 or 9.34 All results regarding Chrysophyllum perpulchrum or others plant species mentioned rich in catechin and procyanidin suggest that this should be proposed asphytotherapeutic drug for AD because of their antioxidante, anti-apoptosis, and likewise their anti-AChE properties.
Although we found some neuro protection effects of crude extract of Chrysophyllum perpulchrum spon memory and cognitive performances in i.c.v injected Aβ rats, we cannot exclude the possibles mechanisms of Aβ clearance from lateral ventricle in order to validateour experimental model. The processes of Aβ influx or efflux beetwen brain parachyma and blood-cerebrospinal fluid via brain barriers remains poorly understood as well as the threshold amount of Aβ level to provoke AD. It has been experimented in mice’s model of AD that the efflux of Aβ from central nervous to plasma increasing the blood baseline level 200 pg/mL to 5-10 ng/mL ((0.5-1).10-2 µg/µl) within 24 h.35 That suggest that the burden of i.c.v Aβ injected in our experiment (10µg of Aβ/side) could be sufficient to extend half-life avoiding so a faster Aβ clearance. However, some authors conclude their experiment in vitro by suggesting that choroid plexus of CSF reduces potentially Aβ from normal brain or AD brain because that area contains potent mechanism of Aβ efflux via lipoprote in receptor-related protein,36 as also early demonstrated in vivo.37 Another limit is the effectiveness of therapeutic concentration of bioactive compounds catech in and procyanidins contained in the dose of Chrysophyllum perpulchrum (300mg/kg for 21 days)used in our experiment. Even if we found positive cognitive and antioxidant outcomes with our medicinal plant, some pharmachokinetic properties including bio availibilty and excretion must be take account. In fact, none study regarding catech in or procynad in from Chrysophyllum perpulchrum has been addressed in this sense, but a past report revealed for instance that by oral route catech in from green tea reached a blood peak 1-2 hours followed by an elimination by 5-6 hours.38 Those authors suggested that an administration every 4 h should allow to maintain high blood level of catech in.It could be interesting to investigate using larger or lower concentration, and duration of treatment in order to further appreciate the dose and time-dependant effects.
Conclusions
The present study is a first to promote neuroprotective actions of ivorian’s traditional plant Chrysophyllum perpulchrumsp using AD-like rat model induced by i.c.v Aβ injection.We can predict that the bioactive compounds catechin and dimer procyanidins (catechin + Hexose) act to attenuate oxidative stress status and relieve memory deficits in the AD condition. However, the challenge remains to tackle its full pharmacological targets.
Acknowledgement
We thank IBRO ARC committee which supporting travel and stay grant at the Cajal Institute (Madrid) for stereotaxic method training.
Conflict of Interest
No conflict of interest was reported by authors
References
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Rev. Mol.Cell Biol 2007;8: 101–112.
CrossRef - Kalaria RN, Maestre GE, Arizaga R, et al. Alzheimer’s disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol 2008; 7:812-826.
CrossRef - Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin. Neurosci2009; 11: 111–128.
CrossRef - Butterfield DA, Boyd-Kimball D.The critical role of methionine 35 in Alzheimer’s amyloid beta-peptide (1–42)-induced oxidative stress and neurotoxicity. Biochim Biophys Acta 2005; 1703 (2): 149–156.
CrossRef - Citron M. Alzheimer’s disease: strategies for disease modification. Rev. Drug Discov2010;9: 387–398.
CrossRef - Adewusi E A, Steenkamp V. Medicinal plants and their derivatives with amyloid beta inhibitory activity as potential targets for drug discovery.Asian Pac J Trop Dis2015;5(6): 430-440.
CrossRef - Bidie AP, Ndjoko K, Attioua K B, Zirihi GN, N’guessan J D, Djaman, A Joseph, et al. Bio-guided Isolation of Antioxidant Compounds from Chrysophyllum perpulchrum, a Plant Used in the Ivory Coast Pharmacopeia.Molecules2010; 15: 6386-6398.
- Bidié AP, Djyh B N, SoroYR, Yapi HF, Zirihi GN, N’guessan JD, et al. Acute, subacute toxicity and cytotoxicity of Chrysophyllum perpulchrum. Scientific Research and Essays2011; 6(28): 5855-5864.
CrossRef - El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature 1996; 382: 716–719.
CrossRef - Maurice T, Lockhart BP, Privat A. Amnesia induced in mice by centrally administered _-amyloid peptides involves cholinergic dysfunction. Brain Res 1996;706:181–193.
CrossRef - AlkamT, Nitta A, Mizoguchi H, Saito K, Seshima M, Itoh A, et al. Restraining tumor necrosis factor-α by thalidomide prevents the amyloidβ-induced impairment of recognition memory in mice. Behav Brain Res2008;189:100–106.
CrossRef - Wahl D, Coogan SC, Solon-Biet SM, De Cabo R, Haran JB, Raubenheimer D, et al. Cognitive and behavioral evaluation of nutritional interventions in rodent models of brain aging and dementia. Clin. Interv. Aging 2017; 12: 14-19.
CrossRef - Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci. Biobehav. Rev 2004; 28: 497–
- Bryan KJ, Lee H, Perry G, Smith M A, Casadesus G. ed J J Buccafusco. In Methods of Behavior Analysis in Neuroscience. Frontiers in Neuroscience.; 2009.
- Ennaceur A, Delacour J A. New one-trial test for neurobiological studies of memory in rats. Behav Brain Res1988; 31: 47–59.
CrossRef - Morris MC, Evans DA, Bienias JL, et al. Vitamin E and cognitive decline in older persons. Arch Neurol2002; 59:1125–1132.
CrossRef - Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959; 82:70–77
CrossRef - Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta1978; 90:37–43.
CrossRef - Bartolini M, Bertucci C, Cavrini V, AndrisanoV.β-Amyloid aggregation induced by human acetylcholinesterase: inhibition studies. Biochem. Pharmacol2003; 65: 407-416.
CrossRef - Anand P, Singh B, Singh N A. review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg Med Chem 2012; 20(3):1175–
CrossRef - Dias KST, De Paula CT, Riquinel MM, Lago ST, CostaKCM, Vaz SM, et al. Aplicacoesrecentes da abordagem de f_armacosmultialvo para o tratamento da Doenc¸a de Alzheimer. Rev Virtual Quim2015 ;7(2):609–648.
CrossRef - Olasehinde AT, Olaniran AO, Okoh AI. Phenolic composition, antioxidant activity, anticholinesterase potential and modulatory effects of aqueous extracts of some seaweeds on β-amyloid aggregation and disaggregation, Pharmaceutical Biology 2019; 57 (1) 460-469.
CrossRef - SuganthyN, MalarDS, Devi KP. Rhizophora mucronata attenuates beta-amyloid induced cognitive dysfunction, oxidative stress and cholinergic deficit in Alzheimer’s disease animal model. Metab Brain 2016.
crossRef - Crouch PF, Susan-MarieEH, Anthony R W, James C, Ashley I B, Colin L M. Mechanisms of Aβ-mediated neurodegeneration in Alzheimer’s disease. The International Journal of Biochemistry & Cell Biology2008; 40: 181–198.
CrossRef - DA. Amyloid beta-peptide1-42-induced oxidative stress and neurotoxicity: implication for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic Res2002 ;36(12): 1307-13.
- Knez D, Coquelle N, Pi slarA, Zakelj S, JukicM, Sova M, et al. Multi-target-directed ligands for treating Alzheimer’s disease: Butyrylcholinesterase inhibitors displaying antioxidant and neuroprotective activities. Eur J Med Chem2018; 156:598–617.
CrossRef - Veerendra KumarMH ,Gupta YK. effect of Centella asiatica on cognition andoxidative stress in an intracerebroventriculars treptozotocin model of alzheimer’s diseasein rats. Clinical and Experimental Pharmacology and Physiology 2003; 30:336–342.
CrossRef - Prediger RDS, Franco JL, Pandolfo P, Medeiros R, Duarte FS, Di Giunta G, et al. Differential susceptibility following b-amyloid peptide-(1–40) administration in C57BL/6 and Swiss albino mice: evidence for a dissociation between cognitive deficits and the glutathione system response. Behav Brain Res2007; 177:205–213.
CrossRef - Zuo L, Hemmelgarn BT, Chuang CC, Best T M. The role of oxidative stress-induced epigenetic alterations in amyloid-beta production in Alzheimer’s disease. Oxidative Medicine and Cellular Longevity 2015. 604658.
CrossRef - Lovell M A, Robertson JD, Teesdale W J, Campbell JL, Markesbery W R. Copper, iron and zinc in Alzheimer’s disease senile plaques. Journal of the Neurological Sciences 1998;158: 47–52.
CrossRef - Schimidt HL, Garcia H, MartinsA, Pamela B, Mello-Carpesb, Felipe P C. Green tea supplementation produces better neuroprotective effects than red and black tea in Alzheimer-like rat model.Food Research International2017;100: 442–448.
CrossRef - Simunkova M, Alwasel SH, Alhazza I M, Jomova K, Kollar V, Rusko M, et al. Management of oxidative stress and other pathologies in Alzheimer’sdisease. Archives of Toxicology2019.
CrossRef - Liu Z, Li T, Li P, Wei N, Zhao Z, Liang H, Wei J. The ambiguous relationship of oxidative stress, tau hyperphosphorylation, and autophagy dysfunction in Alzheimer’s disease. Oxidative Medicine and Cellular Longevity 2015, 352723.
CrossRef - Kanno H, Kawakami Z, Tabuchi M, Mizoguchi K, karashi Y, KaseYProtective effects of glycycoumarin and procyanidin B1,active components of traditional Japanese medicine yokukansan, on amyloid β oligomer-induced neuronal death. Journal of Ethnopharmacology 2015; 159: 122–128.
CrossRef - DeMattos R, Bales K, Cummins D, Paul S, Holtzman D. Brain to plasma amyloid-b efflux: a measure of brain amyloid burden in a mouse model of Alzheimer’s disease. Science 295 2002a; 2264–2267.
- Crossgrove JS, Li GJ, ZhengThe Choroid Plexus Removes β-Amyloid from Brain Cerebrospinal Fluid. Exp Biol Med2005;230(10): 771–776.
CrossRef - Ghersi-Egea JF, Gorevic PD, Ghiso J, Frangione B, Patlak CS, Fenstermacher JD. Fate of cerebrospinal fluid-borne amyloid beta-peptide: rapid clearance into blood and appreciable accumulation by cerebral arteries. J Neurochem 1996; 67:880–883.
CrossRef - Liao S, Kao Y-H, Hiipakka RA. Green tea: biochemical and biological basis for health benefits vitamins and hormones. VitamHorm 2001; 62:1–94.
CrossRef