Manuscript accepted on :
Published online on: 27-09-2021
Plagiarism Check: Yes
Reviewed by: Dr. Loai Aljerf

Second Review by: Dr. Daya Shankar Gautam

Final Approval by: Dr. Fai poon
Pratik P. Durgawale1 , Kailas D. Datkhile1*
, Kailas D. Datkhile1* , Virendra C. Patil2
, Virendra C. Patil2 , Vasant V. Devkar2
, Vasant V. Devkar2 , Sarjerao A. Dabane3
, Sarjerao A. Dabane3 , Vijaykumar S. Wader4
, Vijaykumar S. Wader4  and Satish V. Kakade5
and Satish V. Kakade5
1Department of Molecular Biology and Genetics, KIMSDU, Karad
2Department of Medicine, KIMSDU, Karad
3Physician, Sanjeevan Hospital, Islampur
4Pathologist, Modern Diagnostic Laboratory, Islampur
5Department of Community Medicine, KIMSDU, Karad
Corresponding Author E-mail: hodgeneticslab@kimskarad.in
DOI : https://dx.doi.org/10.13005/bpj/2271
Abstract
The most commonly found type of diabetes in India is type II diabetes mellitus (T2DM), which is characterized by decrease in insulin secretion and decrease in insulin sensitivity. Several environmental factors, genetic factors, socio-economic factors, life style, dietary habits have contributed to the surge of T2DM cases in India. Numerous genes involved in lipid metabolism are likely to be candidates as the markers for obesity and T2DM. In the present study, single nucleotide polymorphism (SNP) of two genes namely Apolipoprotein A5 (APOA5) and Lipoprotein lipase (LPL) involved in triglyceride metabolism were investigated using polymerase chain reaction- restriction fragment length polymorphism (PCR-RFLP). The control group comprised of non-obese, non-diabetic subjects (n=120) and T2DM cases were divided into obese (n=120), and non-obese (n=120) groups based on their body mass index (BMI). The demographic features between the control and cases were compared using Chi-square distribution. The genotype frequencies of control and cases were compared using analysis of variance (ANOVA) and binary logistic regression analysis (Odds’ ratio (OR) and adjusted Odds’ ratio). It was observed that APOA5 rs3135506 (OR = 0.46 (0.27-0.79); p = 0.007) was negatively associated, while APOA5 rs662799 (OR = 2.22 (1.28-3.84); p = 0.006) was significantly associated in non-obese diabetic patients. APOA5 rs3135506 (OR = 0.03 (0.01-0.06); p < 0.001) was negatively associated and rs662799 (OR = 4.68 (1.47-14.93); p = 0.01) was significantly associated in obese diabetic patients. Both LPL SNPs (rs285 and rs320) were found not to be associated with T2DM. The association of Apo A5 variants with T2DM may be because of post transcriptional inhibition leading to reduced Apo A5 expression or these alleles may be in linkage disequilibrium with alleles which directly affect the functioning of APOA5. The observations indicated that T2DM is a multi-factorial disease with a large number of gene-gene and gene-environment interactions.
Keywords
Apoplipoprotien A5; Lipoprotein lipase; PCR-RFLP; T2DM
Download this article as:| Copy the following to cite this article: Durgawale P. P, Datkhile K. D, Patil V. C, Devkar V. V, Dabane S. A, Wader V. S, Kakade S. V. Association of Genetic Polymorphism in Apolipoprotein A5 and Lipoprotein Lipase Genes with Type Ii Diabetes Mellitus Patients in Rural South Western Maharashtra. Biomed Pharmacol J 2021;14(3). |
| Copy the following to cite this URL: Durgawale P. P, Datkhile K. D, Patil V. C, Devkar V. V, Dabane S. A, Wader V. S, Kakade S. V. Association of Genetic Polymorphism in Apolipoprotein A5 and Lipoprotein Lipase Genes with Type Ii Diabetes Mellitus Patients in Rural South Western Maharashtra. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/3ugGNh6 |
Introduction
Diabetes is one of the most prevalent chronic metabolic disease causing adult deaths globally in developed and developing countries with 4 million deaths reported in 2017. 1 India is facing epidemic of diabetes with high prevalence in urban areas. With an estimated 65 million diabetes cases in 2016, treatment and management of diabetic cases puts a lot of burden on the nations’ economy and is a deterrent to its progress. 2 The recent advances in technology have contributed to our understanding of genetic, epigenetic and environmental factors that influence the development of this multi-factorial disease.3 Earlier reports on the heredity of T2DM indicated that individuals have 40 % or 70 % possibility of developing T2DM when one or both of their parents have T2DM, respectively. 4 Some of the newly reported variants have yet to be elucidated about their impact on the progression of diabetes such as genes involved in the triglyceride metabolism including Apolipoprotein A5 (APOA5) and lipoprotein lipase (LPL). 5 These two genes normally lower the triglyceride content in blood and help in regulating the secretion of insulin by controlling deposition of triglycerides which may lead to obesity. 6,7 Triglycerides are transported from liver through blood in the form of lipoproteins which consist of triglycerides, cholesterol, cholesterol esters, and apolipoproteins. Apolipoproteins act as structural members of lipoproteins to package the insoluble triglycerides and other lipids. Apolipoprotein B is the most abundantly found apolipoprotein. Other important apolipoproteins include Apolipoprotein CII which acts as an activator for lipoproten lipase enzyme and initiates lipolysis of triglycerides. Apolipoprotein AV has a stimulatory effect on the activty of lipoprotein lipase and helps lower plasma triglyceride levels. On the other hand, Apolipoproten CIII inhibits activity of lipoprotein lipase and causes increase in plasma triglyceride level. As such, ApoCIII and ApoAV act antagonistically to regulate blood triglyceride level.8
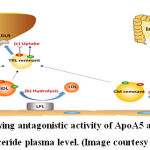 |
Figure 1: Illustration showing antagonistic activity of ApoA5 and ApoC3 in maintaining triglyceride plasma level. (Image courtesy Dai)8 |
The gene cluster at 11q23 was reported to be involved in lipid transport from liver to various organs, metabolism of triglycerides and transport of free fatty acids. Lipids in the form of triglycerides, cholesterol, cholesterol esters being insoluble; are solubilized by apolipoproteins along with phospholipid monolayer assembled in a globular structure. Apolipoprotein B is the most abundant apolipoproteins present in HDL closely associated with cholesterol. Apo AI lipoproteins are found in chylomicrons, VLDL, HDL. Apo AI proteins have hydrophobic surface that interact with lipids and a hydrophilic end that interacts with aqueous environment. ApoA IV is secreted along with chylomicrons and transferred to HDL in plasma. Apo CII initiates or triggers the activity of lipoprorotein lipase to catabolise triglycerides present in chylomicrons and VLDL. Apo CIII inihibits the lipolytic activity of lipoprotein lipase leading to increase in the plasma triglyceride content. 9,10 Apo AV is present in chylomicrons/VLDL/ HDL at low concentrations but has a stimulatory effect on the activity of lipoprotien lipase leading to decrease in plasma triglyceride content and greater production of free fatty acids from triglycerides. 7 In-vivo studies on adenovirus-mediated over-expression of ApoAV in mice, indicated that Apo AV lowers VLDL-triglyceride content in a dose-dependent manner without affecting VLDL production. The report suggested a role of Apo AV in the liquidation of ApoB which affects the further assembly of VLDL. The other mechanism by which it lowers plasma VLDL-TG was by stimulating the activity of LPL. However, the study does not report any influence on the expression of lipid metabolism pathway genes.11 Although free APOA5 is found in the plasma, a fraction of it is also localized in the membrane of epithelial cells and intracellular compartment. 12,13 Several knockout studies have linked lower presence of triglycerides with increased activity of APOA5. 14,15 Single nucleotide polymorphisms in the promoter and coding regions of APOA5 gene have been reported to be associated with abnormal triglyceride blood levels and Type 2 diabetes mellitus (T2DM) among Asian populations such as Chinese, Japanese, Indian, and Pakistani. 16–21 Although rs662799 is located in the non-coding region, a study has proposed that the SNP generates a microRNA (microRNA 3201) binding spot that leads to lower expression of ApoAV and subsequent lower activity of lipoprotein lipase. Due to the reduced lipolytic activity, free fatty acids captured from chylomicrons/VLDL would reduce. Other explanation for this association of SNP with reduced lipolysis is that, the SNP might be in linkage disequilibrium with other SNP/haplotype that directly affects the lipolytic activity of lipoprotein lipase which leads to lower the free fatty acid production from chylomicrons/VLDL.22
The activity of Lipoprotein lipase (LPL) dictates the rate of triglyceride metabolism pathway and is activated by APOA5. 23 LPL gene is located on chromosome 8p22 and expressed as a 448 amino acid long protein. 6,24 Lipoprotein lipase is expressed in the sub-endothelial space and dimerizes into its active form. Glycosylphosphatidylinositol (GPI)-anchored high-density lipoprotein– binding protein 1 (GPIHBP1) binds to LPL, stabilizes its structure and anchors it in the capillary lumen. It then interacts with chylomicrons and VLDL to hydrolyze the triglycerides into free fatty acids leading to decrease in plasma triglycerides and increase in HDL. The anchoring of LPL enables its interaction with triglycerides of chylomicrons, VLDL and with catalytic apolipoproteins. 25 The activity of LPL is initiated by Apo CII and its activity is significantly increased by Apo AV. Apo CIII inhibits the activity of LPL leading to increase in plasma triglycerides. Earlier, the importance of anchoring/ localization of LPL were studied with injecting heparin which leads to sudden release of LPL in the blood. This lead to a subsequent drop in blood plasma levels of triglycerides followed by an increase due to exhaustion of expressed LPL.
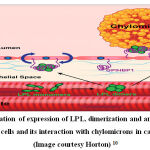 |
Figure 2: Illustration of expression of LPL, dimerization and anchoring of LPL to endothelial cells and its interaction with chylomicrons in capillary lumen. (Image courtesy Horton) 10 |
The resulting free fatty acids taken up by cells are either utilized for synthesis of triacylglycerol derivatives or stored as ‘lipid droplets’. 26 Several gain-of-function mutations in LPL have been associated with lower plasma triglycerides, confirming its role in triglyceride metabolism. 24 Interestingly, several SNPs were identified in the introns and exons of LPL gene and were reported to be associated with triglyceride levels and T2DM in Mongolian, Russian, Brazilian populations but no association was reported in Chinese population. 27–30 Considering these varying reports of association of SNPs in APOA5 and LPL genes with T2DM, it is imperative to study this association among rural population of south western Maharashtra.
Materials and Methods
Sample Collection
Clinically diagnosed T2DM patients visiting the Out-patient department and In-patient department of Department of Medicine at the Krishna Hospital and Medical Research Centre, Karad (KHMRC) were enrolled in the study. Patients were classified into obese and non-obese (number of subjects = 120 in each group) depending o their body mass index (BMI) and were enrolled in this study. 120 non-obese non-diabetic subjects were enrolled as control group from the same institute.
Exclusion criteria: Subjects currently using hypolipidemic drugs and known cases of hypothyroidism, hyperthyroidism, tuberculosis, malignancy, pregnancy, Cushing’s syndrome were excluded from the study. The patients and controls were explained about the study and upon receiving their informed consent, 3 ml whole blood was collected in EDTA-containing vacutainer.
Genomic DNA extraction
Following the provided manufacturer’s instructions, genomic DNA was extracted with blood DNA extraction kit (Make: Qiagen). The blood and DNA samples were stored at -80 °C until required.
Polymerase Chain Reaction – Restriction Fragment Length Polymorphism (PCR-RFLP) analysis
Apolipoprotein A5 (SNP rs3135506)
Genotyping of APOA5 (rs3135506) was performed by PCR-RFLP method using appropriate PCR and RFLP conditions represented in Table I. 157 bp PCR product was digested with TaqI restriction enzyme and analyzed on 3 % agarose gel. The restriction digestion yielded homozygous wild-type allele fragments were 134 bp and 23 bp, homozygous variant-type allele was undigested (157 bp); while the heterozygous allele was digested into 157 bp, 137 bp, 23 bp fragments. 31
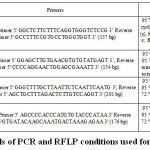 |
Table I: Details of PCR and RFLP conditions used for SNP analysis. |
Apolipoprotein A5 (SNP rs662799)
Genotyping of APOA5 (rs662799) was performed by PCR-RFLP method using appropriate PCR and RFLP conditions represented in Table I. 154 bp PCR product was digested with MseI and analyzed on 3 % agarose gel. The homozygous wild-type allele fragments were 133 bp and 21 bp, homozygous variant-type allele was undigested (154 bp); while the heterozygous allele was digested to yield 154 bp, 133 bp, 21 bp fragments. 31
Lipoprotein lipase intron 6 (SNP rs285)
Genotyping of LPL (rs285) was performed by PCR-RFLP method using appropriate PCR and RFLP conditions represented in Table I. 201 bp PCR product was digested with PvuII and analyzed on 3 % agarose gel. The homozygous wild-type allele was undigested (201 bp), homozygous variant-type allele fragments were 160bp and 41 bp; while the heterozygous allele was digested into fragments of 201 bp, 140 bp and 61 bp. 32
Lipoprotein lipase intron 8 (SNP rs320)
Genotyping of LPL (rs320) was performed by PCR-RFLP method using appropriate PCR and RFLP conditions represented in Table I. 176 bp PCR product was digested with HindIII and analyzed on 3 % agarose gel. The homozygous wild-type allele fragments were 116 bp and 60 bp, homozygous variant-type allele was undigested (176 bp); while the heterozygous allele was digested into fragments 176 bp, 116 bp and 60 bp. 32
Validation of test results
Validation was done by blinded study where the results of analysis of PCR-RFLP were analyzed by expert other than the person who performed the test. All the results were replicated thrice and hence the results of the study were validated. The obtained RFLP data was analyzed to see the genetic association by different software to confirm the significance of the results which are represented in Figure 3.
Statistical analysis
The demographic features of the study population of the three groups were tested with Chi-square test with the help of Instat software (Make Graphpad version 3.06). p < 0.05 was considered statistically significant. The allelic distribution of genes was studied by performing analysis of variance (ANOVA) and binary logistic regression analysis (odds’ ratio (OR) and adjusted odds’ ratio) using SPSS Statistics software (Make IBM version 20).
Results and Discussion
Two pathological changes that determine onset of T2DM namely increased insulin resistance and decreased insulin secretion is influenced by variety of factors including environmental factors, and genetic factors. Dysfunctional lipid metabolism leads to increase in adipose tissue storage and eventually obesity. 33–35 APOA5 and LPL represent major factors in the hydrolysis of triglycerides in the form of chylomicrons, low-density lipoprotein plasma and the way they are transported into the cells and stored. 36 Changes in the normal functioning of these metabolic proteins may lead to increase in free fatty acids in the blood which ultimately leads to insulin resistance or loss of insulin sensitivity in tissues which may contribute to progression of T2DM. 24,31 Hence, the study of association of genetic polymorphism of APOA5 and LPL genes with T2DM may provide clues to the possibility of SNPs being used as biomarkers for prediction of this disease state. 37 Apolipoproteins associated with lipoproteins of various types such as chylomicrons, VLDL, LDL, and HDL have varied functions. Apolipoproteins B are important for the structural integrity of lipoproteins. Apolipoprotein C2 activates lipoprotein lipase which is anchored to endothelial cells via GBIHBP1. Apolipoprotein C3 inhibits the triglyceride hydrolytic activity of lipoprotein lipase. On the other hand, APO A5 is present in very less concentration in the blood and has a stimulatory role towards lipoprotein lipase. Thus, APO A5 and APO C3 regulate the activity of lipoprotein lipase and help maintain plasma triglyceride level.10 APO E is also associated with the formation of VLDL and chylomicrons. This apolipoprotein gene cluster present has been associated with triglyceride metabolism through various genome wide association studies (GWAS).38 GWAS have reported rs662799 to be more closely related to triglyceridemia in Asians and rs331506 to be strongly associated in European population. The ‘Triglyceride Coronary Disease Genetics Consortium and Emerging Risk Factors Collaboration’ conducted plasma triglyceride association study with rs662799 and found that presence of one minor allele increased plasma triglyceride levels by 0.25 mmol/L. The other Apo A5 SNP rs331506 has not been found to be significantly associated in clinical studies among African, European and Asian population.28 Variants in Apo E are associated with disturbed triglyceride metabolism arising from reduced hepatic binding, uptake and catabolism of chylomicron and VLDL remnants.38 LPL SNPs rs285 and rs320 have been found to be associated with hypertiglyceridemia as part of haplotypes rather than individual SNPs among Mexican Americans. However, no association was found in Saudi Arabian population. A Canadian study reported rs320 to be associated with dyslipidemia in patients with high visceral adipose tissue. The European Atherosclerosis Research Study reported that the rs285 SNP was in strong linkage disequilibrium with another functional SNP (rs328) which leads to lower triglycerides and higher amount of free fatty acids in the blood. 39–41 Besides the study of genotype distribution in control and patient groups, it is also important to study their demographic characteristics.
The demographic features of the patients and control group were analyzed using Chi-square test. Some of the demographic factors which were differently distributed among patient and control groups included economic status, education, family history of diabetes, tobacco and alcohol consumption. Additionally, sedentary lifestyle and associated disorders such as hypertension and coronary heart disease were significantly more in T2DM patients. These factors have been reported earlier to contribute to obesity and mutagenesis leading to progression of T2DM. 33–35, 39, 42, 43 The genotype frequency of APOA5 and LPL genes among control and non-obese diabetic group were as mentioned in table II.
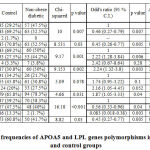 |
Table II: Genotype frequencies of APOA5 and LPL genes polymorphisms in non-obese diabetic and control groups |
Table II: Genotype frequencies of APOA5 and LPL genes polymorphisms in non-obese diabetic and control groups
When we studied APOA5 gene SNPs rs3135506 and rs662799, we observed that the distribution of rs3135506 was significantly different among control and non-obese diabetic patients and the heterozygous genotype was found to be negatively associated with T2DM in non-obese patients (OR 0.46(0.27-0.79), p = 0.007). This observation showed that the heterozygous genotype provides protective action against development of T2DM among non-obese diabetic subjects. Similarly, rs662799 of Apolipoprotein A5 was also found to be associated with occurrence of T2DM in non-obese diabetic patients (OR 2.22 (1.28-3.84), p = 0.006). This suggests that the presence of heterozygous genotype may be associated with occurrence of T2DM among non-obese individuals. Likewise, the LPL rs285 was similarly distributed among control and non-obese diabetic patients. The LPL rs320 was differently distributed among control and non-obese diabetic patients but was not associated with occurrence T2DM. The genotype frequency of APOA5 and LPL genes among control and obese diabetic group were as mentioned in table III.
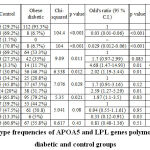 |
Table III: Genotype frequencies of APOA5 and LPL genes polymorphisms in obese diabetic and control groups |
Table III: Genotype frequencies of APOA5 and LPL genes polymorphisms in obese diabetic and control groups
The APOA5 (rs3135506) was found to be differentially distributed among control and obese diabetic groups; moreover, the heterozygous genotype was negatively associated with occurrence of T2DM (OR 0.03 (0.01-0.06) p < 0.001). This suggests that the heterozygous genotype may provide protection against development of T2DM among obese subjects. rs662799 was also found to be differently distributed among control and obese diabetic groups and the variant genotype was associated with development of T2DM (4.68 (1.47-14.93) p = 0.01). It is possible that this variant genotype was associated with development of T2DM in obese diabetic patients. The LPL rs285 was found not to be associated with occurrence of T2DM in obese diabetic group. The LPL rs320 was similarly distributed among control and obese diabetic groups.
Polymorphisms in the 5’ promoter, coding and 3’ UTR have been reported across various populations with varying frequency. The SNP -1131T>C (rs662799) is associated with increased plasma triglyceride levels and the minor allele -1131C is reported in 15 % of Caucasians and as high as 40 % in Asian population. The other important SNP (rs3135506) C56G leads to lower secretion in the plasma and higher triglyceride levels. The frequency of minor allele of rs3135506 is about 12 % in Caucasian and African population, 2% in Asians and as high as 25 % in Hispanic population. Apart from these important SNPs, A-3 > G, IVS+476 G˃A and T1259 > C have been reported but are not associated with changes in triglyceride levels. Another SNP (rs2075291) G553T has been reported in coding region. Its minor allele frequency is extremely low in Caucasian, African, Hispanic population and about 5 % in Asian population.9,10,22 Since polymorphism of Apo AV is associated with plasma triglyceride levels, it is possible that it may affect obesity/weight changes. Some studies claim that rs662799 may help in weight loss after lower consumption of fat-rich diet or after physical exercise. Howerver, contradictory statements have also been made and the heterogeneity in selected study subjects and intervention from these studies are too varied to arrive at a conclusion. In-vivo studies have proposed that the association of rs662799 with hypertriglyceridemia may be due to formation of a microRNA binding site on the transcribed mRNA which may lead to lower secretion of the protein. Another study performed on human subjects reported that SNP along with methylation pattern of Apo A5 exons together affect the triglyceride level.44 The rs662799 located in the promoter region of the APOA5 gene has been associated with elevated levels of triglycerides, cholesterol in the plasma of patients from Chinese, Japanese, Korean and Caucasian populations. 16–19 The association of rs662799 and T2DM has also been reported in Asian population including Chinese, Korean, Japanese, Indonesian, Pakistani, Indian population in a meta-analysis study. 20,21 However, no such association was observed in Croatian and other European populations. 21,45 Other studies have linked this SNP with coronary heart disease, acute myocardial infarction, and atherosclerosis in Czech, Spanish populations indicating its important function in triglyceride metabolism 46,47. Although APOA5 is found in extremely low levels in plasma, its stimulatory effect on lipoprotein lipase leads to faster hydrolysis of triglycerides in chylomicrons and VLDL. It has also been proposed to lower secretion of VLDL from liver. SNPs in the promoter, exon, and 3’ UTR regions have been reported to be associated with increased triglyceride levels in plasma among various populations. In the promoter region, SNP rs662799 has been reported in various studies to be associated with hypertriglyceridemia in Caucasian population with reproducible results. Additionally, this SNP was also reported to be associated with hypertriglyceridemia in European and Chinese populations. In vivo study has reported that the reason behind this association may be post-transcriptional inhibition of APO A5 mRNA by micro-RNA or it may be because this SNP is in linkage disequilibrium with some other SNP which is directly associated with plasma triglyceride level. SNP rs3135506 causes a missense mutation at amino acid 19 position and may lead to a change in the signal peptide which results in lower extracellular secretion of APOA5 from hepatic cells. 22,44 This SNP has also been reported to be strongly associated with hypertriglyceridemia among Hispanics. However, its association among Asian, Caucasian, and African population is not consistent among various reports. Further, this association may be sex-dependent which adds to the heterogeneity of studied subjects. Another SNP located in the 3’ UTR region (rs2266788) was found to be in linkage disequilibrium with rs662799 and was reported to be associated with hypertriglyceridemia among Korean and Kuwaiti populations. 48,49 Many studies have reported association of this SNP with obesity, coronary artery disease, acute myocardial infarction. 50,51 The association between rs315506 with T2DM has been reported for Polish population but no association was found in Tunisian and English studies. 31,52,53
In case of LPL gene, rs285 indicates the transition of cytosine to thymine in intron 6 and rs320 indicates the transition of guanine to thymine in intron 8. Such intronic sites often contain regulatory elements for expression of genes and mutations in these sites may have effects on the normal expression and function of genes. 54 rs285 SNP was reported to be associated with hypertriglyceridemia and coronary heart disease. 54–56 A Chinese study failed to find any association between rs285 and T2DM, while a Mongolian study reported this to be a causative factor for T2DM. 27,30 rs320 SNP was reported to be associated with hypertriglyceridemia and T2DM among Russian and Mexican population. 28,29 Similar studies in Saudi and Chinese Han patients failed to report any association with development of T2DM. 54,57 Another interesting study among Polish and Russian athletes showed that rs320 SNP has variable phenotypic effects of T2DM depending on the type and intensity of physical activity. Thus, current study sheds light on the association of variant genotypes of APOA5 and LPL genes with development of T2DM among rural population of south western Maharashtra.
Conclusion
When we correlated our results with other reports, we observed rs3135506 of APOA5 had a negative association with T2DM and rs662799 had a causative effect with T2DM with both obese and non-obese individuals. Though these SNPs show positive and negative associations towards development of T2DM, their phenotypic expressions are influenced by physical activity, interaction with other genes and environmental factors. Since T2DM is a polygenic disorder, we need to investigate a larger study population or cohort to assess our findings.
Acknowledgement
The authors are grateful for the financial assistance provided by Krishna Institute of Medical Sciences Deemed to be University.
Conflict of Interest
The authors declare no conflict of interest
References
- Ajjemami, M.; Ouatou, S.; Charoute, H.; Fakiri, M.; Rhaissi, H.; Benrahma, H.; Rouba, H.; Barakat, A. Haplotype Analysis of the Apolipoprotein A5 Gene in Moroccan Patients with the Metabolic Syndrome. Diabetes Metab. Disord. 2015; 14 (1):29.
CrossRef - Aouizerat, B. E.; Kulkarni, M.; Heilbron, D.; Drown, D.; Raskin, S.; Pullinger, C. R.; Malloy, M. J.; Kane, J. P. Genetic Analysis of a Polymorphism in the Human ApoA-V Gene Effect on Plasma Lipids. Lipid Res. 2003; 44 (6):1167–1173.
CrossRef - Aljerf, L.; Alhaffar, I. Salivary Distinctiveness and Modifications in Males with Diabetes and Behçet’s Disease. Res. Int. 2017;2017.
CrossRef - Banting, L. K.; Pushkarev, V. P.; Cieszczyk, P.; Zarebska, A.; Maciejewska-Karlowska, A.; Sawczuk, M. -arek; Leońska-Duniec, A.; Dyatlov, D. A.; Orekhov, E. F.; Degtyarev, A. V. Elite Athletes’ Genetic Predisposition for Altered Risk of Complex Metabolic Traits. BMC Genomics 2015;16 (1):25.
CrossRef - Johansen, C. T.; Kathiresan, S.; Hegele, R. A. Genetic Determinants of Plasma Triglycerides. Lipid Res. 2011; 52 (2):189–206.
CrossRef - Baum, L.; Tomlinson, B.; Thomas, G. N. APOA5-1131T> C Polymorphism Is Associated with Triglyceride Levels in Chinese Men. Genet. 2003; 63 (5):377–379.
CrossRef - Bhaskar, S.; Ganesan, M.; Chandak, G. R.; Mani, R.; Idris, M. M.; Khaja, N.; Gulla, S.; Kumar, U.; Movva, S.; Vattam, K. K. Association of PON 1 and APOA 5 Gene Polymorphisms in a Cohort of Indian Patients Having Coronary Artery Disease with and without Type 2 Diabetes. Test. Mol. Biomark. 2011;15 (7–8):507–512.
CrossRef - Dai, W.; Zhang, Z.; Yao, C.; Zhao, S. Emerging Evidences for the Opposite Role of Apolipoprotein C3 and Apolipoprotein A5 in Lipid Metabolism and Coronary Artery Disease. Lipids Health Dis. 2019;18 (1):1–7.
CrossRef - Pennacchio, L. A.; Rubin, E. M. Apolipoprotein A5, a Newly Identified Gene That Affects Plasma Triglyceride Levels in Humans and Mice. Thromb. Vasc. Biol. 2003; 23(4):529–534.
CrossRef - Horton, J. D. Intravascular Triglyceride Lipolysis Becomes Crystal Clear. Natl. Acad. Sci. 2019; 116 (5):1480–1482.
CrossRef - Schaap, F. G.; Rensen, P. C.; Voshol, P. J.; Vrins, C.; van der Vliet, H. N.; Chamuleau, R. A.; Havekes, L. M.; Groen, A. K.; van Dijk, K. W. ApoAV Reduces Plasma Triglycerides by Inhibiting Very Low Density Lipoprotein-Triglyceride (VLDL-TG) Production and Stimulating Lipoprotein Lipase-Mediated VLDL-TG Hydrolysis. Biol. Chem. 2004; 279 (27):27941–27947.
CrossRef - Bielicki, P.; Plywaczewski, R.; Brzoska, K.; Kumor, M.; Barnas, M.; Jonczak, L.; Stepkowski, T. M.; Piechuta, A.; Chazan, R.; Sliwinski, P. Impact of Polymorphism of Selected Genes on the Diagnosis of Type 2 Diabetes in Patients with Obstructive Sleep Apnea. Pol Arch Intern Med. 2018.
CrossRef - Bogari, N. M.; Aljohani, A.; Dannoun, A.; Elkhateeb, O.; Porqueddu, M.; Amin, A. A.; Bogari, D. N.; Taher, M. M.; Buba, F.; Allam, R. M. Association between HindIII (Rs320) Variant in the Lipoprotein Lipase Gene and the Presence of Coronary Artery Disease and Stroke among the Saudi Population. Saudi J. Biol. Sci. 2020; 27(8): 2018–2024.
CrossRef - Burdon, K. P.; Langefeld, C. D.; Beck, S. R.; Wagenknecht, L. E.; Carr, J. J.; Freedman, B. I.; Herrington, D.; Bowden, D. W. Association of Genes of Lipid Metabolism with Measures of Subclinical Cardiovascular Disease in the Diabetes Heart Study. Med. Genet. 2005; 42(9):720–724.
CrossRef - Cao, L.; Li, Q.; Chen, X. The HindIII and PvuII Polymorphisms of Lipoprotein Lipase (LPL) Gene Reduce the Risk of Ischemic Stroke (IS): A Meta-Analysis. Medicine (Baltimore) 2018;97(18).
CrossRef - Celap, I.; Simundic, A.-M.; Nikolac, N.; Kackov, S.; Katalinic, D. Association of APOA5- 1131T> C Polymorphism and Serum Lipid Levels in Patients with Type 2 Diabetes. DNA Cell Biol. 2013; 32 (10): 589–593.
CrossRef - Chaaba, R.; Attia, N.; Hammami, S.; Smaoui, M.; Mahjoub, S.; Hammami, M.; Masmoudi, A. S. Association of SNP3 Polymorphism in the Apolipoprotein AV Gene with Plasma Triglyceride Level in Tunisian Type 2 Diabetes. Lipids Health Dis. 2005; 4 (1):1–6.
CrossRef - Chamberlain, J. C.; Thorn, J. A.; Oka, K.; Galton, D. J.; Stocks, J. DNA Polymorphisms at the Lipoprotein Lipase Gene: Associations in Normal and Hypertriglyceridaemic Subjects. Atherosclerosis 1989; 79 (1): 85–91.
CrossRef - Chen, H.; Ding, S.; Zhou, M.; Wu, X.; Liu, X.; Wu, Y.; Liu, D. Association of Rs662799 in APOA5 with CAD in Chinese Han Population. BMC Cardiovasc. Disord. 2018; 18 (1): 2.
CrossRef - Chuluun-Erdene, A.; Sengeragchaa, O.; Altangerel, T.-A.; Sanjmyatav, P.; Dagdan, B.; Battulga, S.; Enkhbat, L.; Byambasuren, N.; Malchinkhuu, M.; Janlav, M. Association of Candidate Gene Polymorphism with Metabolic Syndrome among Mongolian Subjects: A Case-Control Study. Sci. 2020; 8 (3):38.
CrossRef - De Andrade, F. M.; Maluf, S. W.; Schuch, J. B.; Voigt, F.; Barros, A. C.; Lucatelli, J. F.; Hutz, M. H. The Influence of the S19W SNP of the APOA5 Gene on Triglyceride Levels in Southern Brazil: Interactions with the APOE Gene, Sex and Menopause Status. Metab. Cardiovasc. Dis. 2011; 21 (8): 584–590.
CrossRef - Dallinga-Thie, G. M.; van Tol, A.; Hattori, H.; Van Vark-van der Zee, L. C.; Jansen, H.; Sijbrands, E. J. G. Plasma Apolipoprotein A5 and Triglycerides in Type 2 Diabetes. Diabetologia 2006; 49 (7):1505–1511.
CrossRef - Garelnabi, M.; Lor, K.; Jin, J.; Chai, F.; Santanam, N. The Paradox of ApoA5 Modulation of Triglycerides: Evidence from Clinical and Basic Research. Biochem. 2013; 46 (1–2):12–19.
CrossRef - El Yaagoubi, F. L.; Charoute, H.; Bakhchane, A.; Ajjemami, M.; Benrahma, H.; Errouagui, A.; Kandil, M.; Rouba, H.; Barakat, A. Association Analysis of APOA5 Rs662799 and Rs3135506 Polymorphisms with Obesity in Moroccan Patients. Biol. 2015; 63 (6):243–247.
CrossRef - Ajong, A. B.; Kenfack, B.; Ali, I. M.; Yakum, M. N.; Aljerf, L.; Telefo, P. B. Hypocalcaemia and Calcium Intake in Pregnancy: A Research Protocol for Critical Analysis of Risk Factors, Maternofoetal Outcomes and Evaluation of Diagnostic Methods in a Third-Category Health Facility, Cameroon. PloS One 2020;15 (11): e0241812.
CrossRef - Girona, J.; Guardiola, M.; Cabré, A.; Manzanares, J. M.; Heras, M.; Ribalta, J.; Masana, L. The Apolipoprotein A5 Gene–1131T→ C Polymorphism Affects Vitamin E Plasma Concentrations in Type 2 Diabetic Patients. Chem. Lab. Med. CCLM 2008; 46 (4): 453–457.
CrossRef - Guilherme, A.; Virbasius, J. V.; Puri, V.; Czech, M. P. Adipocyte Dysfunctions Linking Obesity to Insulin Resistance and Type 2 Diabetes. Rev. Mol. Cell Biol. 2008; 9 (5): 367–377.
CrossRef - Hubacek, J. A. Apolipoprotein A5 and Triglyceridemia. Focus on the Effects of the Common Variants. Chem. Lab. Med. CCLM . 2005; 43 (9): 897–902.
CrossRef - Kahn, S. E.; Hull, R. L.; Utzschneider, K. M. Mechanisms Linking Obesity to Insulin Resistance and Type 2 Diabetes. Nature 2006; 444 (7121): 840–846.
CrossRef - Kaveeshwar, S. A.; Cornwall, J. The Current State of Diabetes Mellitus in India. AMJ 2014, 7, 1, 45-48. Http. Dx Doi Org104066AMJ 2013.
CrossRef - Kefi, R.; Hechmi, M.; Dallali, H.; Elouej, S.; Jmel, H.; Halima, Y. B.; Nagara, M.; Chargui, M.; Fadhel, S. B.; Romdhane, S. Association of Apolipoprotein A5 Gene Variants with Metabolic Syndrome in Tunisian Population. In Annales d’endocrinologie; Elsevier. 2017; 78:146–155.
CrossRef - Kochetova, O. V.; Avzaletdinova, D. S.; Sharipova, L. F.; Korytina, G. F.; Akhmadishina, L. Z.; Morugova, T. V.; Mustafina, O. E. An Analysis of the Associations of Polymorphic Variants of the LEPR (Rs1137100), LRP5 (Rs3736228), and LPL (Rs320) Genes with the Risk of Developing Type 2 Diabetes Mellitus. J. Genet. 2019; 55 (4):495–503.
CrossRef - Li, L.-L.; Kang, X.-L.; Ran, X.-J.; Wang, Y.; Wang, C.-H.; Huang, L.; Ren, J.; Luo, X.; Mao, X.-M. Associations between 45T/G Polymorphism of the Adiponectin Gene and Plasma Adiponectin Levels with Type 2 Diabetes. Exp. Pharmacol. Physiol. 2007; 34 (12):1287–1290.
CrossRef - Mahrooz, A.; Zargari, M.; Ansari, V.; Makhlough, A.; Hashemi-Sooteh, M.-B. Association of APOA5 Gene Promoter Region-1131T> C Polymorphism (Rs662799) to Plasma Triglyceride Level in Patients with Type 2 Diabetic Nephropathy. Clin. Diagn. Res. JCDR 2016; 10 (5):09.
CrossRef - Muñoz-Barrios, S.; Guzmán-Guzmán, I. P.; Francisco, J.; Salgado-Bernabé, A. B.; Salgado-Goytia, L.; Parra-Rojas, I. Association of the HindIII and S447X Polymorphisms in LPL Gene with Hypertension and Type 2 Diabetes in Mexican Families. Markers 2012; 33 (6):313–320.
CrossRef - Larsson, M. Endogenous and Exogenous Factors Affecting Lipoprotein Lipase Activity. PhD Thesis, Ume\aa University, 2014.
- Prieur, X.; Huby, T.; Joan, R.; Couvert, P.; Chapman, J. Apolipoprotein AV: Gene Expression, Physiological Role in Lipid Metabolism and Clinical Relevance. Future Lipidol. 2008; 3 (4):371–384.
CrossRef - Eichner, J. E.; Dunn, S. T.; Perveen, G.; Thompson, D. M.; Stewart, K. E.; Stroehla, B. C. Apolipoprotein E Polymorphism and Cardiovascular Disease: A HuGE Review. J. Epidemiol. 2002; 155 (6):487–495.
CrossRef - Murea, M.; Ma, L.; Freedman, B. I. Genetic and Environmental Factors Associated with Type 2 Diabetes and Diabetic Vascular Complications. Diabet. Stud. RDS 2012; 9 (1): 6.
CrossRef - Humphries, S. E.; Nicaud, V.; Margalef, J.; Tiret, L.; Talmud, P. J.; EARS, for the. Lipoprotein Lipase Gene Variation Is Associated with a Paternal History of Premature Coronary Artery Disease and Fasting and Postprandial Plasma Triglycerides: The European Atherosclerosis Research Study (EARS). Thromb. Vasc. Biol. 1998; 18 (4):526–534.
CrossRef - Goodarzi, M. O.; Guo, X.; Taylor, K. D.; Quinones, M. J.; Saad, M. F.; Yang, H.; Hsueh, W. A.; Rotter, J. I. Lipoprotein Lipase Is a Gene for Insulin Resistance in Mexican Americans. Diabetes 2004; 53 (1):214–220.
CrossRef - Nabika, T.; Nasreen, S.; Kobayashi, S.; Masuda, J. The Genetic Effect of the Apoprotein AV Gene on the Serum Triglyceride Level in Japanese. Atherosclerosis 2002; 165 (2): 201–204.
CrossRef - Ong, K. L.; Jiang, C. Q.; Liu, B.; Jin, Y. L.; Tso, A. W.; Tam, S.; Wong, K. S.; Tomlinson, B.; Cheung, B. M.; Lin, J. M. Association of a Genetic Variant in the Apolipoprotein A5 Gene with the Metabolic Syndrome in Chinese. Endocrinol. (Oxf.) 2011; 74 (2):206–213.
CrossRef - Oliva, I.; Guardiola, M.; Vallvé, J.-C.; Ibarretxe, D.; Plana, N.; Masana, L.; Monk, D.; Ribalta, J. APOA5 Genetic and Epigenetic Variability Jointly Regulate Circulating Triacylglycerol Levels. Sci. 2016; 130 (22):2053–2059.
CrossRef - Prasad, R. B.; Groop, L. Genetics of Type 2 Diabetes—Pitfalls and Possibilities. Genes 2015;6 (1):87–123.
CrossRef - Radha, V.; Vimaleswaran, K. S.; Ayyappa, K. A.; Mohan, V. Association of Lipoprotein Lipase Gene Polymorphisms with Obesity and Type 2 Diabetes in an Asian Indian Population. J. Obes. 2007;31 (6):913–918.
CrossRef - Rao, C. R.; Kamath, V. G.; Shetty, A.; Kamath, A. A Cross-Sectional Analysis of Obesity among a Rural Population in Coastal Southern Karnataka, India. Med. J. 2011; 4 (1):53.
CrossRef - Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A. A.; Ogurtsova, K.; Shaw, J. E.; Bright, D.; Williams, R. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019;157.
CrossRef - Speer, G.; Cseh, K.; Winkler, G.; Vargha, P.; Braun, E.; Takacs, I.; Lakatos, P. Vitamin D and Estrogen Receptor Gene Polymorphisms in Type 2 Diabetes Mellitus and in Android Type Obesity. J. Endocrinol. 2001;144 (4):385–389.
CrossRef - Srivastava, R. K.; Singh, P.; Verma, P.; Sethi, R.; Verma, A.; Ali, W.; Tiwari, S. Influence of APOA5 (Rs662799 and Rs3135506) Gene Polymorphism in Acute Myocardial Infarction Patients and Its Association with Basic Coronary Artery Disease Risk Factors. Appl. Pharm. Sci. 2015;5 (06):008–014.
CrossRef - Talmud, P. J.; Cooper, J. A.; Hattori, H.; Miller, I. P.; Miller, G. J.; Humphries, S. E. The Apolipoprotein AV Genotype and Plasma Apolipoprotein AV and Triglyceride Levels: Prospective Risk of Type 2 Diabetes. Results from the Northwick Park Heart Study II. Diabetologia 2006;49 (10):2337–2340.
CrossRef - Talmud, P. J.; Hawe, E.; Martin, S.; Olivier, M.; Miller, G. J.; Rubin, E. M.; Pennacchio, L. A.; Humphries, S. E. Relative Contribution of Variation within the APOC3/A4/A5 Gene Cluster in Determining Plasma Triglycerides. Mol. Genet. 2002;11 (24):3039–3046.
CrossRef - Tandon, N.; Anjana, R. M.; Mohan, V.; Kaur, T.; Afshin, A.; Ong, K.; Mukhopadhyay, S.; Thomas, N.; Bhatia, E.; Krishnan, A. The Increasing Burden of Diabetes and Variations among the States of India: The Global Burden of Disease Study 1990–2016. Lancet Glob. Health 2018;6 (12):1352–e1362.
CrossRef - Ukkola, O.; Savolainen, M. J.; Salmelaa, P. I.; Von Dickhoff, K.; Kesäniemi, Y. A. DNA Polymorphisms at the Lipoprotein Lipase Gene Are Associated with Macroangiopathy in Type 2 (Non-Insulin-Dependent) Diabetes Mellitus. Atherosclerosis 1995;115 (1):99–105.
CrossRef - Wang, Z.; Huang, Q.; Qu, K. Association and Meta-Analysis of LPL PvuII and HindIII Polymorphisms with Type 2 Diabetes in Chinese. Mod. Biomed. 2010;10 (9):1604–1612.
- Yin, Y.-W.; Sun, Q.-Q.; Wang, P.-J.; Qiao, L.; Hu, A.-M.; Liu, H.-L.; Wang, Q.; Hou, Z.-Z. Genetic Polymorphism of Apolipoprotein A5 Gene and Susceptibility to Type 2 Diabetes Mellitus: A Meta-Analysis of 15,137 Subjects. PloS One 2014;9 (2):89167.
CrossRef - Zhao, T.; Zhao, J. Association of the Apolipoprotein A5 Gene-1131 T> C Polymorphism with Fasting Blood Lipids: A Meta-Analysis in 37859 Subjects. BMC Med. Genet. 2010; 11 (1):120.
CrossRef








