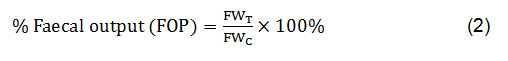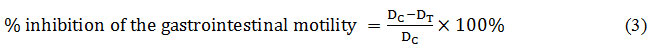Manuscript accepted on :13-08-2021
Published online on: 24-08-2021
Plagiarism Check: Yes
Reviewed by: Dr. S Parasuraman

Second Review by: Dr. Puneet Kaur Randhawa

Final Approval by: Dr I an james martin
Israt Jahan Bulbul1,2 , Md. Ekhtiar Uddin1
, Md. Ekhtiar Uddin1 , Nusratun Nahar1
, Nusratun Nahar1 , Md. Ruhul Kuddus2
, Md. Ruhul Kuddus2 , Mohammad Rashedul Haque2
, Mohammad Rashedul Haque2 , Mohammad Abdur Rashid2*
, Mohammad Abdur Rashid2*
1Department of Pharmacy, Southeast University, Banani, Dhaka-1213, Bangladesh.
2Phytochemial Research Laboratory, Department of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Dhaka, Dhaka-1000, Bangladesh.
Corresponding author E-mail: r.pchem@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2227
Abstract
The objective of the present study includes the evaluation of the antidiarrheal properties of the methanol extracts of Litsea deccanensis Gamble (MELD) bark, Litsealancifolia (Roxb.) Hook. f. MELL),Litseaglutinosa Gamble (MELG) and Litsea monopetala Roxb. (MELM) leavesin Swiss albino mice. The antidiarrheal activity was evaluated by measuring percentage inhibition of diarrheal feces, total fecal output, gastrointestinal motility and by using peristaltic indices. Castor oil was used to induce diarrhea in the experimental animal. The experiments were carried out by using three different doses (100, 200, and 400 mg/kg body weight) of these four plant extracts. The number of wet feces and total weight of the feces were significantly (p < 0.05) and dose-dependently reduced by all the plant extracts and this effect was comparable with standard drug. MELD, MELL, MELG and MELM extracts at dose of 400 mg/kg body weight demonstrated diarrheal inhibition by 43.55%, 45.16%, 32.26% and 41.94%, respectively while it was 98.39% for the standard loperamide. Percentage (%) of fecal output for MELD, MELL, MELG and MELM extracts at the dose of 400 mg/kg were 40.14%, 62.27%, 64.06%, 46.26%, respectively.The gastrointestinal motility induced by castor oil was also reduced noticeably (p < 0.05) by all the plant extracts with the increasing doses. The percentage inhibition of gastrointestinal motility at the dose of 400 mg/kg were 26.26%, 33.22%, 32.36% and 22.52% for the MELD, MELL, MELG and MELM extracts respectively, while it was 27.56% for loperamide. In most cases, all the plant extracts can reduce the peristaltic indices which were comparable to control. The obtained results from this study revealed that the methanol extracts of four different species of Litsea found in Bangladesh may have antidiarrheal potential. It also provides the basis for the traditional use of these plants to treat diarrhea.
Keywords
Antidiarrheal; Litseadeccanensis; Litsealancifolia; Litseaglutinosa; Litseamonopetala; Mice, Methanol Extracts;
Download this article as:| Copy the following to cite this article: Bulbul I. J, Uddin M. E, Nahar N, Kuddus M. R, Haque M. R, Rashid M. A. Antidiarrheal Activity of Four Different Species of Litsea Available in Bangladesh. Biomed Pharmacol J 2021;14(3). |
| Copy the following to cite this URL: Bulbul I. J, Uddin M. E, Nahar N, Kuddus M. R, Haque M. R, Rashid M. A. Antidiarrheal Activity of Four Different Species of Litsea Available in Bangladesh. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/3sMaV3h |
Introduction
Diarrhea is a foremost public health issue worldwide, particularly in developing countries the global death rate is 8% among children below age 5 in 2017. In Bangladesh during 2010–12 the rate of deaths, among children < 5 years was 4% due to acute diarrhea, 2% because of diarrhea-induced illness and 53% due to pneumonia in addition to diarrhea1.
Diarrhea is a change in normal fecal output and is characterized by frequent passing of loose and watery stool with increased fecal volume, rate of bowl movement, abdominal pain and decreased absorption of fluid2. For long years, plants with medicinal value have been used to treat various ailments counting diarrhea also3. In developing countries, most of the people rely on medicinal plants to treat diarrhea4-5.
The Lauraceae family contains of nearly 55 genera and more than 2,000 species throughout the world6.Litsea genus belongs to the laurel family, Lauraceae and the plants are deciduous or evergreen shrubs or trees. There are more than 600 species widely distributed in tropical and subtropical regionsincluding South America, North America, Asia, Australia and New Zealand. More than 407 phytochemicals of diverse varieties including sesquiterpenes, lactones, flavonoids, lignans, alkaloids etc. have been reported from Litsea species7. Most of these secondary metabolites possess significant bioactivities as antidiarrheal, antimicrobial, insecticidal, anti-HIV, antioxidant, analgesic, anti-inflammatory etc.In Bangladesh,there are few references on this genus and 11 species of Litsea are listed by the Ministry of Environment and Forest, Bangladesh. Among them we have selected four species for our study.
Litsea deccanensis Gamble is distributed in Chattogram, Bangladesh.In Andhra Pradesh, India L. deccanensis leaves are used in chest pain and the extract of this plant was studied to possess compelling antioxidant and reducing capacities and cardioprotective effect in rat models. α-humulene, β-Caryophyllene, caryophyllene epoxide, bicyclogermacrene, germacra-3,9,11-triene; Squalene, quassin, stigmasterol, vitamin E, oleic acidand several alkaloids boldine, corytuberine, dicentrine, nordicentrine, laurolitsine, isocorydine, magnoflorine were isolated from L. deccanensis8-10.
Litsea lancifolia (Roxb.) Hook. f. named by Chakma tribes as Judijaylla belongs to the Lauraceae family is distributed in the south-eastern portion of Bangladesh. L. lancifolia root is used to treat diarrhea by Chakma tribes in Rangamati, Bangladesh11. L. lancifolia exhibited promising anti-diabetic effect and found nontoxic to 3T3L1 cells12. Bulbul et al. (2020) reported L. lancifolia to have anti-oxidant, analgesic, antimicrobial, CNS depressant and hypoglycemic activities13.Sulaimanet al. (2011) reported the alkaloids namely lancifoliaine, boldine, norboldine, actindaphnine, etc in the bark ofthis plant14. Li et al. (2008) reported alkaloids litseferine, juziphine, phanostenineand a steroid β-sitosterol. Aristotetralone, dehydrodi isoeugenol, 4′-methylenedioxyflavan-3-ol, 5,7-dimethoxy-3′, β-hydroxybenzoic acid, β-sitosterol, vanillin and dihydro dehydrodiconiferyl alcohol were also isolated from L. lancifolia15-16.
Litsea glutinosa Gamble (Lauraceae) is a medium-sized tree, growing in the forest of Chattogram and Sylhet districts in Bangladesh17. In Bangladesh, China, India, Myanmar, Sri Lanka, Malaysia L. glutinosabark, leaves, roots and fruits are used for diarrhea, abscess and traumatic injury while the essential seed oil is used traditionally for rheumatism18. Previously L. glutinosa was evaluated for its thrombolytic, analgesic, anti-inflammatory, antipyretic, antibacterial, anti-diabetic (type II), antioxidant, hepatoprotective activities19-21. Several alkaloids such as isoboldine, laureliptine, Liriodenine, actinodaphnine, n-methylactinodaphnine, laurotetanine, n-methyllaurotetanine, laurolitsine, boldine, litseferine, litsine and glutinosine A were previously reported in L. glutinosa22-24. Some flavonoids such as 2′,5,7-trihydroxy-6-methoxyflavone2′-O-beta-D-glucopyranoside25; sesquiterpenes like β-Caryophyllene, Caryophyllene oxide and monoterpenes like(E)-β-Ocimene, (Z)-β-Ocimene were isolated from L. glutinosa26.
Litsea monopetala Roxb.Pers. belongs to the Lauraceae family and spreads in the hill tract forests, Sal forests and in the village areas of Bangladesh. In Bangladesh, Nepal, Myanmar, India, China L. monopetala is traditionally used in treating fracture and dislocation, skin disease, gonorrhea, diarrhea and also to cure pains27. This plant species was also reported for its antioxidant, antimicrobial, analgesic, hypoglycemic, CNS depressant and antidiarrheal activities27-29. Chalcone and its derivatives and eugenol27, caryophyllene oxide, α-caryophyllene alcohol, humulene oxide, tricosane and pentacosanein the flower;capric acid, nonanol and decanal in fruit and myristic acid, tridecanol, tridecanal and tetradecanal in bark were also reported from L. monopetala30.
Considering the traditional uses of above-mentioned plantsof Litsea species in the treatment of diarrhea as well as few evidence-based reports of this effect, our present study was aimed to evaluate their antidiarrheal effect in animal.
Methods
Collection of Plant Materials and Preparation of Plant Extracts
The bark of L. deccanensis (DACB–35517),the leaves ofL. lancifolia (DACB-35164), L.glutinosa(DACB-37904) and L. monopetala (DACB-38437) were collected from Chattogramhill track, Bangladesh. All the plant samples were identified by a taxonomistat Bangladesh National Herbarium, Mirpur-1, Dhaka-1216, Bangladesh.
After collection, the plant samples were washed gently with tap water and then shade dried for several days,and finally crushed to granular powder.The grounded plant materials (800 g to 1000 g) for each plant were drenched in methanol in flat bottom containers at room temperature
for seven days with occasional shaking and stirring.The extracts werethen filtered twice by using cotton mass followed by filter papers (What man No. 1). A rotary evaporator (Heidolph, Germany) was used to concentrate the filtrates at reduced pressure and temperature. All the crude extracts were evaporated to dryness and kept in a moisture free cold environment for further analysis.
Experimental animals
Swiss albino mice of either sex (Weight: 30-40g; Age: 6-8 weeks) were procured from Jahangirnagar University, Dhaka, Bangladesh for the experiment. The animals were accommodated in standard mice cases at 25°C and a proper day/night circle was maintained.Before the beginning of the experiment, the animals were adapted for seven days for acclimatization.In the complete study the animals were handled according to the guidelines by the National Research Council, Washington, DC31. Those guidelines were accepted by the committee on ethical compliance in research (SEU/Pharm/CECR/103/2021) of Southeast University.
Acute oral toxicity test
The toxicity study after oral administration of the plant extracts was conducted according to OECD 420 Standard32. Swiss albino mice (Female; weight: 30-40 g; Age: 4-6 weeks) were taken for the study. This experiment was started with one fasted mouse that was given plant extract orally at a dose of 2000 mg/kg body weight and noticed for any toxic influenceof the extract like increased motor activity, coma or death. As there was no death within 24 h then other four mice were treated with the same dose for each extract. Following the administration of four extracts, the treated animals were observed for toxic and behavioural effects every day for about 14 sequential days.
Experimental design
Extracts coding for different doses
Three different doses (100, 200 and 400 mg/kg)of the methanol extract of L. deccanensiswere coded as MELD_1, MELD_2 and MELD_3, respectively; for L. lancifoliathe codes were MELL_1, MELL_2 and MELL_3; for L. glutinosa the codes were MELG_1, MELG_2 and MELG_3 and for L. monopetala they were MELM_1, MELM_2 and MELM_3, respectively.
Animal grouping and dosing
Swiss albino mice were allocated into five groups containing five mice in each group as follows:
Group I: Control, specifiedto administer only vehicle (10 ml/kg, distilled water)
Group II: Standard control,specified to administerstandard Loperamide (3 mg/kg body weight)
Group III: Treatment group, specified to administer the plant extract (100 mg/kg body weight)
Group III: Treatment group, specified to administer the plant extract (200 mg/kg body weight)
Group III: Treatment group, specified to administer the plant extract (400 mg/kg body weight)
Antidiarrheal activity test
Castor oil-induced diarrhea
Antidiarrheal activity was evaluated by castor oil-induceddiarrheal method in mice33.The animals were divided into negative control, positive or standard group, and test groups, as discussed in the animal grouping and dosing section. After 60 min of administration of three different doses (100, 200 and 400 mg/kg body weight) of four different plant extracts (MELM, MELL, MELG and MELD) and standard Loperamide (3 mg/kg body weight), 0.5 ml castor oil was given orally to every mouse to induce diarrhea. The experimental animals were then retained separatelyin a plastic cage with the floor on which a white paper was placed to note the number of wet stools (diarrheal stool), total number and total weight of the faecal output for consequent four hours. Every hour the white paper was changed. Then, diarrheal inhibition (% inhibition of wet defecation) and the percentage of faecal output (% FOP) were calculated by the following equations:
Where, WDC= Mean wet defecation of control, WDT = Mean wet defecation of standard drug/ test samples.
Where, FWT = Mean faecal weight of each treatment group; FWC= Mean faecal weight of control group.
Gastrointestinal motility test
By using barium sulphate meal
This study was completed conferring the reported method ofChatterjee (1993), updated by Mazumdar et al. (2015) and investigated the effect of the plant extracts on the gastrointestinal motility induced by castor oil34-35. Experimental mice fasted for 18 hwere grouped as negative control, positive control and treatment group, as discussed in the previous section. One hour after treatment with standard loperamide (3 mg/kg body weight) and three different doses (100, 200 and 400 mg/kg body weight respectively) of four differentplant extracts, 0.5 ml of castor oil was administered by oral gavage to initiate diarrhea in mice. Following one hour of castor oil administration, all mice were given 1 ml of 5% barium sulphate suspension by oral gavage. White barium sulphate suspension was used in this method,because it is easily visible in normal light which can help for the measurement of the distance travelled by the barium sulphate meal of the intestine. After 30 min of barium sulphate administration, all the animals were sacrificed and the small intestine of each mouse was isolated. Then the entire length of the intestine and the intestinal length travelled by the barium sulphate meal were measured by using centimetre scale.
By using the succeeding formula, the percentage of inhibition of the gastrointestinal motility and peristaltic index were calculated.
Where, DC = Mean distance travelled by the control; DT = Mean distance travelled by the test group.
Statistical analysis
The Graph Pad Prism 8 was used to perform the statistical analysis and all values are expressed as mean ± SEM (n=5). A one-way analysis of variance (ANOVA) followed by a Dunnet test was performed to compare multiple groups. Values were measured statistically significant at P<0.05, P<0.01, P<0.001.
Results
Results acute oral toxicity test
After administration of a single oral dose of 2000 mg/kg body weight for all the plant extracts, the animals were observed for 14 days and found no significant toxic effects and death incidence. Water and food intake by the animals were normal during this observation period. So, it can be concluded that MELD, MELL, MELG and MELM have a broader safety margin and their LD50 value will be more than 2000 mg/kg.
Effect of test extracts on castor oil-induced diarrhea
In this experiment, the tested plant extractsMELD, MELL, MELG and MELM exhibited a dose-dependent reduction in the number of wet feces (diarrheal stool), total number and total weight of the feces and the results were significant (p<0.05) for all the plant extracts at a dose of 400 mg/kg, as compared with control.Castor oil-induced diarrheal feces werenoticeably reduced by all the extracts andthe maximum diarrheal inhibition was observed for MELD, MELL, MELG and MELM extracts at 400 mg/kg and that were 43.55%, 45.16%, 32.26% and 41.94%, respectively,while it was 98.39% for the standard Loperamide. Though all the extracts exhibited a similar effect, the order for the inhibition of wet defecation was MELL>MELD>MELM>MELG. Percentage (%) of fecal output for MELD, MELL, MELG and MELM extracts at 400 mg/kg were 40.14%, 62.27%, 64.06% and 46.26%, respectively and the order of the plant extracts for fecal output was MELD>MELM>MELL>MELG (Table 1).
Table 1: Effect of the methanol extracts of L. monopetala, L. lancifolia, L. glutinosaand L. deccanensison castor oil-induced diarrhea in mice.
| Treatment Groups | Dose(mg/kg, p.o) | Total number of feces | Total number of wet feces | % inhibition of wet defecation | % of fecal output |
| Control | – | 22.5±1.85 | 15.5±2.02 | ||
| Loperamide | 3 | 2±0.71* | 0.25±0.25* | 98.39 | 12.28 |
| MELD_1 | 100 | 12.25±2.06* | 9.750±1.60 | 37.10 | 46.62 |
| MELD_2 | 200 | 10.25±1.70* | 9.25±1.89 | 40.32 | 39.86 |
| MELD_3 | 400 | 9.75±1.49* | 8.75±1.65* | 43.55 | 40.14 |
| MELL_1 | 100 | 16.25±1.03 | 9.5±0.28 | 38.71 | 74.02 |
| MELL_2 | 200 | 16.75±1.32 | 9.250±1.11 | 40.32 | 70.11 |
| MELL_3 | 400 | 14.75±0.85* | 8.5±0.5* | 45.16 | 62.27 |
| MELG_1 | 100 | 19.5±1.041 | 13.25±1.32 | 14.52 | 81.14 |
| MELG_2 | 200 | 17.5±0.65 | 13±1.41 | 16.13 | 77.58 |
| MELG_3 | 400 | 15.5±3.66* | 10.5±3.23 | 32.26 | 64.06 |
| MELM_1 | 100 | 17±0.71 | 10.25±0.95 | 33.87 | 55.87 |
| MELM_2 | 200 | 14±1.0* | 9.75±0.63 | 37.10 | 52.31 |
| MELM_3 | 400 | 14±1.68* | 9±0.71* | 41.94 | 46.26 |
All values are stated as mean ± SEM (n = 5); One way ANOVA then a Tukey post hoc test was carried out for data analysis. Here, * values are statistically significant at P<0.05
Effects of test extracts on gastrointestinal motility
In this test, all the extracts (MELD, MELL, MELG and MELM) also exhibited significant (p<0.05) and a dose-dependent reduction in the gastrointestinal motility induced by castor oil. The plant extracts MELL, MELG, MELD and MELM at the dose of 400 mg/kg showed maximum influence by 33.22%, 32.36%, 26.26% and 22.52%, respectively whereas standard loperamide (3 mg/kg body weight) treated group showed a greater antimotility effect by 27.56% than all of the plant extracts. The order of the plant extracts for inhibition of intestinal motility was MELL>MELG>MELD>MELM. The peristaltic indices were reduced by the plant extracts at all doses, compared to control except MELD at dose of 100mg/kg body weight (Tables 2).
Table 2: Effect of the methanol extracts of L. monopetala, L. lancifolia, L. glutinosaand L. deccanensison gastrointestinal motility in mice.
| Treatment Groups | Dose (mg/kg, p.o) | Total Length (cm) of Intestine | Distance (cm) traveled by Barium Sulphate | % of Inhibition | Peristalsis Index (%) |
| Control | – | 53.1±7.12 | 47.943±4.06 | 90.3 | |
| Loperamide | 3 | 52.070±1.80 | 34.73±1.59 | 27.56 | 66.7 |
| MELD_1 | 100 | 47.158±3.31 | 44.45±1.80 | 7.29 | 94.3 |
| MELD_2 | 200 | 47.568±2.98 | 37.748±1.66 | 21.26 | 79.4 |
| MELD_3 | 400 | 59.860±2.87 | 35.353±5.06 | 26.26 | 59.1 |
| MELL_1 | 100 | 48.013±2.10 | 35.268±6.14 | 26.44 | 73.5 |
| MELL_2 | 200 | 53.658±2.10 | 32.385±9.53 | 32.45 | 60.4 |
| MELL_3 | 400 | 50.8±1.56 | 32.018±7.42 | 33.22 | 63.0 |
| MELG_1 | 100 | 50.165±1.10 | 35.242±2.66 | 26.49 | 70.3 |
| MELG_2 | 200 | 51.435±2.67 | 32.933±2.31 | 31.31 | 64.0 |
| MELG_3 | 400 | 54.928±3.38 | 32.428±6.66 | 32.36 | 59.0 |
| MELM_1 | 100 | 48.135±1.74 | 41.225±2.39 | 14.01 | 85.6 |
| MELM_2 | 200 | 48.955±0.62 | 39.688±3.57 | 17.22 | 81.1 |
| MELM_3 | 400 | 47.05±1.29 | 37.148±3.64 | 22.52 | 79.0 |
All values are stated as mean ± SEM (n = 5); One way ANOVA then a Tukey post hoc test was carried out for data analysis. Here, * values are statistically significant at P<0.05
Discussion
Traditionally many medicinal plantshaving antidiarrheal effect are used in our folk medicine for the management of diarrheal disease.It is therefore imperative to find out available natural drugs as alternatives to commonly used synthetic antidiarrheal drugs, which are associated with serious adverse effects. In Bangladesh, a range of medicinal plants with antidiarrheal properties has been widely used by common people36.
In our current study the antidiarrheal properties of the methanol extract of four different species of Litsea, i.e., L. deccanensis(MELD), L. lancifolia (MELL),L. glutinosa(MELG) and L. monopetala(MELM) was determined and the results of our study clearly portrays significant antidiarrheal properties in mice model. All the plant extracts showed significant (p<0.05) and a dose-dependent inhibition of castor oil-induced diarrhea and gastrointestinal motility in animal. This inhibitory effect justifies the folkloric use of L. lancifolia, L. glutinosaand L. monopetalain the treatment of diarrhea. The antidiarrheal effect of L. monopetalaobserved in this study has supported the previous reports28,whileforL. lancifolia, L. glutinosaand L. monopetala, this is the first report of their antidiarrheal activity.
Castor oil-induced diarrheal model is a common technique which is used to evaluate the antidiarrheal activity of plant extracts in animal. Castor oil produces diarrheain mice by changing the permeability of electrolytes through the intestinal mucous membrane37-38. Ricinoleic acid, an active metabolite of castor oil, inducesmucosal irritation and inflammation via augmented secretion of prostaglandins which ultimately increases GI motility and secretion.
In this experiment,the methanol extracts of L. deccanensis,L. lancifolia, L. glutinosaand L. monopetalaeffectively exhibited antidiarrheal action by the inhibition of castor oil-induced prostaglandin synthesis.The antidiarrheal activity might also be due to inhibition of ricinoleic acid secretion, resulting in the stimulation of Na+, K+ ATPase activity which enhances absorption of electrolytes in the intestinal mucosa39. It can be presumed that this antidiarrheal effect of the tested plant extracts is due to their antisecretory and antimotility properties, a similar mechanism produced by loperamide, a commonly used antidiarrheal drug. So, the reduced intestinal motility and fluid accumulation within the gastrointestinal tract of the treated animal by the plant extracts may be mediated through the similar mechanism by the standard loperamide.
This antidiarrheal effect could probably be associated with the occurrence of phytochemicals such as terpenoids, tannins and flavonoids present in the plant extract, MELD, MELL, MELG and MELM which are shown to inhibit the prostaglandin release and intestinal absorption of electrolyte. Many studies have confirmed several phytochemicals in medicinal plants to produce antidiarrhealactivity by increasing antispasmodic effects, suppressing gut motility, delaying intestinal transit, stimulating water adsorption orreducing electrolyte secretion40. Phytochemicals such as flavonoids and tannins are reported to exhibitantidiarrheal activity by stimulatingelectrolyte and water reabsorption from small intestine41-42. Four of our studied plants have been reported previously to have many of these phytochemicals predominantly alkaloids14-15, 22-25.
The obtained results from this study revealed that the methanol extracts of four different species of Litsea found in Bangladesh have remarkable antidiarrheal potential. All these four plant extracts may produce the antidiarrheal activity by stimulating the reabsorption of electrolyte andwater, and by checking intestinal motility as all of these plants are rich sources of alkaloids, flavonoids and tannins. This result also provides the basis for the traditional uses of these plant species as antidiarrheal agent.However, more detailed phytochemical analysis will be necessary to identify the active constituents responsible for the pharmacological activities of Litsea species.
Acknowledgement
We would like to thank Jahangirnagar University, Dhaka, Bangladesh for providing us experimental animals. The authors would also like to acknowledge the support of Bangladesh National Herbarium for the identification of the studied plants.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Funding Source
This is a self-funded research project.
Reference
- Makhdum Ahmed JA, Alam KF, Al Mamun A, Paul RC, Rahman M, Iuliano AD, Sturm-Ramirez K, Parashar U, Luby SP, Gurley ES. Incidence of acute diarrhea-associated death among children< 5 years of age in Bangladesh, 2010-12. The Am J Trop Med Hyg.2018; 98(1):281.
CrossRef - Abdela, J. Evaluation of in vivo antidiarrheal activities of hydroalcoholic leaf extract of Dodonaeaviscosa (Sapindaceae) in Swiss albino mice. J. Evid. Based Integr. Med., 2019; 24:1-10.
CrossRef - Teferi, M.Y., Abdulwuhab, M. and Yesuf, J.S. Evaluation of in vivo antidiarrheal activity of 80% methanolic leaf extract of OsyrisquadripartitaDecne (Santalaceae) in Swiss albino mice. Evid. Based Integr. Med., 2019; 24:1-9.
CrossRef - Mahmud, K.A.A. and Rahmatullah, M. Rural home remedies: Medicinal plants used in a village of Tangail district, Bangladesh. Med. Plants Stud., 2020; 8(1):11-14.
- Rahman, M.K., Chowdhury, M.A.U., Islam, M.T., Chowdhury, M.A., Uddin, M.E. and Sumi, C.D. Evaluation of antidiarrheal activity of methanolic extract of Maranta arundinacea Leaves. Adv. Pharmacol. Pharm. Sci., 2015; 2015: Article ID 257057, 6 pages.
CrossRef - Lim, T.K. Couroupitaguianensis. In Edible Medicinal and Non-Medicinal Plants 2012 (pp. 133-137). Springer, Dordrecht.
CrossRef - Wang, Y.S., Wen, Z.Q., Li, B.T., Zhang, H.B. and Yang, J.H. Ethnobotany, phytochemistry, and pharmacology of the genus Litsea: An update. Ethnopharmacol., 2016; 181: 66-107.
CrossRef - Kumar, P.B., Kannana, M.M., Lavanyaa, B., Suthakaranb, R. and Quinec, D.S. GC-MS analysis of methanolic extract of Litseadecanensis gamble and its free radical scavenging activity. Pharma. Res., 2011;4(1): 100-103.
- Kumar, P.B., Kannan, M.M. and Quine, S.D. Litseadeccanensis ameliorates myocardial infarction in Wistar rats: Evidence from biochemical histological studies. Young Pharm., 2011;3(4): 287-296.
CrossRef - Irulandi, K., Kumar, J.S., Arun, K.D., Rameshprabu, N. and Swamy, P.S. Leaf essential oil composition of two endemic Litsea species from South India. Nat. Comp., 2016; 52(1): 159-161.
CrossRef - Yusuf, M., Begum, J., Hoque, M.N. and Choudhury, J.U. Medicinal plants of Bangladesh-Revised and enlarged. Bangladesh Council and Scientific of Industrial Research Lab. Chittagong, Bangladesh. 2009; 794.
- Alsawalha, M., Al-Subaie, A.M., Al-Jindan, R.Y., Bolla, S.R., Balakrishna, J.P., Ravi, P.K., Gollapalli, S.S., Veeraraghavan, V.P., Pillai, A.A., Joseph, J.P. and Mohan, S.K. Effect of Litsealancifolia leaf extract on glucose transporter 4 translocation and glucose uptake in 3T3L1 cell line. Pharm. Bioall. Sci., 2019; 11(3): 240-247.
CrossRef - Bulbul, I.J., Haque, M.R. and Rashid, M.A. Pharmacological investigations of Litsealancifolia (Roxb.) Hook. F. Bangladesh J. Bot., 2020; 49(1): 179-183.
CrossRef - Sulaiman, S.N., Mukhtar, M.R., Hadi, A.H., Awang, K., Hazni, H., Zahari, A., Litaudon, M., Zaima, K. and Morita, H. Lancifoliaine, a new bisbenzylisoquinoline from the bark of Litsealancifolia. , 2011; 16(4): 3119-3127.
CrossRef - Li, L., Yang, S. and Yang, X. Chemical constituents of Litsealancifolia. Yunnan Univ. Nat. Sci., 2008; 30(2): 187.
- Yang, S., Li, L.W., Yang, X.D., Zhao, J.F. and Li, L. Studies on the chemical constituents of Litsealancifolia. Chin. Med. Mat., 2008; 31(7): 985-987.
- Ghani, A. Medicinal plants of Bangladesh: chemical constituents and uses. Asiatic society of Bangladesh. 1998.
- Mandal, S.C., Kumar, C.A., Majumder, A., Majumder, R. and Maity, B.C. Antibacterial activity of Litseaglutinosa Fitoterapia, 2000; 71(4): 439-441.
CrossRef - Bhowmick, R., Sarwar, M.S., Dewan, S.M.R., Das, A., Das, B., Uddin, M.M.N., Islam, M.S. and Islam, M.S. In vivo analgesic, antipyretic, and anti-inflammatory potential in Swiss albino mice and in vitro thrombolytic activity of hydroalcoholic extract from Litseaglutinosa Biol. Res., 2014; 47(1): 1-8.
CrossRef - Palanuvej, C., Hokputsa, S., Tunsaringkarn, T. and Ruangrungsi, N. In vitro glucose entrapment and alpha-glucosidase inhibition of mucilaginous substances from selected Thai medicinal plants. Pharm., 2009; 77(4): 837-850.
CrossRef - Ghosh, N., Chaki, R., Pal, M. and Mandal, S.C. Hepatoprotective activity of methanol extract of Litseaglutinosa against hepatotoxin induced toxicity. Pharm. Exp. Med., 2016; 16(2): 139-146.
CrossRef - Yang, J.H., Li, L., Wang, Y.S., Zhao, J.F., Zhang, H.B. and Luo, S.D. Two new aporphine alkaloids from Litseaglutinosa. Chimi. Acta., 2005; 88(9): 2523-2526.
CrossRef - Jin, Y., Wu, Y., Li, Y., Zhang, C. and Sun, W. Litsine A: a new aporphine alkaloid from the root barks of Litseaglutinosa. Nat. Prod., 2018; 13(2): 167-171.
CrossRef - Ji, Y., Wang, C., Zhang, Y., Zhang, C., Cui, D. and Zhang, X. Glutinosine A: a new morphinandienone alkaloid from Litseaglutinosa. Nat. Prod., 2019; 13(4): 363-366.
CrossRef - Wang, Y.S., Huang, R., Lu, H., Li, F.Y. and Yang, J.H. A new 2′-oxygenated flavone glycoside from Litseaglutinosa (Lour.) CB Rob. Biotechnol. Biochem., 2010; 74(3): 652-654.
CrossRef - Choudhury, S.N., Singh, R.S., Ghosh, A.C. and Leclercq, P.A. Litseaglutinosa (Lour.) CB Rob., a new source of essential oil from northeast India. Essent. Oil Res., 1996; 8(5): 853-856.
CrossRef - Ghosh, M. and Sinha, B.N. GC-MS studies on the bark extracts of Litsea polyantha Middle-East J. Sci. Res. 2010; 5: 441-444.
- Ferdous, M.R., Ashrafudolla, M., Hossain, M.S. and Bellah, S.F. Evaluation of antioxidant, analgesic and antidiarrheal activities of methanolic extract of Litseamonopetala (Roxb.) leaves. Pharmacol.Biopharm., 2018; 7(3):185.
CrossRef - Bulbul, I.J., Rashid, M.A. and Haque, M.R. Pharmacological studies of different fractions of Litseamonopetala Bangladesh Pharm. J., 2020; 23(1): 61-64.
CrossRef - Choudhury SN, Ghosh AC, Choudhury M, Leclercq PA. Essential oils of Litseamonopetala (Roxb.) Pers. A new report from India. Essent. Oil Res., 1997; 9(6): 635-639.
CrossRef - National Research Council. Guide for the care and use of laboratory animals. 8th Washington, DC: The National Academies Press; 2011.
- OECD, 2002. OECD Test No. 420. Acute oral toxicity-fixed dose procedure [adopted 17 December 2001]. OECD Guidelines for the Testing of Chemicals, Section 4: Health Effects, OECD Publishing, Paris (2002).
- Shoba, F.G. and Thomas, M. Study of antidiarrhoeal activity of four medicinal plants in castor-oil induced diarrhoea. Ethnopharmacol. 2001; 76(1): 73-76.
CrossRef - Chatterjee, T.K. Handbook of Laboratory Mice and Rats, Department of Pharmaceutical Technology. 1st India: Jadavpur University; 1993;157.
- Mazumdar, S., Akter, R. and Talukder, D. Antidiabetic and antidiarrhoeal effects on ethanolic extract of Psidium guajava (L.) Bat. leaves in Wister rats. Asian Pacific J. Trop. Biomed., 2015; 5(1):10-14.
CrossRef - Jahan, N., Ferdousi, J., Alam, M. J., Rahman, T., Rahman, M. and Shahriar, M. Antidiarrheal activity of ethanolic extract of Melochiacorchorifolia and Glochidionthomsonii in experimental animal models. Bangladesh Pharm. J., 2019; 22(2): 192-199.
CrossRef - Akanda, M.K.M. and Hasan, A.H.M.N. Characterization of pharmacological properties of methanolic seed and stem bark extracts of Ziziphus mauritiana (BAU Kul) using in-vitro and in-vivo animal (Swiss albino male mice) model. Phytosci., 2021; 7: 8.
CrossRef - Naher, S., Aziz, M.A., Akter, M.I. et al. Anti-diarrheal activity and brine shrimp lethality bioassay of methanolic extract of Cordyline fruticosa (L.) A. Chev. leaves. Phytosci., 2019; 5: 15.
CrossRef - Tadesse, E., Engidawork, E., Nedi, T. et al. Evaluation of the anti-diarrheal activity of the aqueous stem extract of Lantana camara Linn (Verbenaceae) in mice. BMC Complement. Altern. Med., 2017; 17: 190.
CrossRef - Tiwari, B.P., Kumar, M.K. and Kaur, H.K.G. Phytochemical screening and extraction-A review. Pharm. Sci., 2011;1: 98-106.
- Kumar, B., Divakar, K., Tiwari, P., Salhan, M. and D. Goli. Evaluation of anti-diarrhoeal effect of aqueous and ethanolic extracts of fruit pulp of Terminalia belerica in rats. J. Drug Develop. Res., 2010; 2: 769-779.