Manuscript accepted on :01-07-2021
Published online on: 15-07-2021
Plagiarism Check: Yes
Reviewed by: Dr. Alamgir Ahmad Dar
Second Review by: Dr. Sujit Nair
Final Approval by: Dr. H Fai Poon
N Chandra Shakar Reddy  and K Pratap Reddy
and K Pratap Reddy
Department of Zoology, UCS, Osmania University, TS, India
Corrresponding Author E-mail: pratapkreddyou@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2244
Abstract
Fluoride is an ineluctable environmental pollutant and its chronic exposure causes nociception and inflammation. Alpha-tocopherol and Selenium (Se) are widely available compounds that are safe if taken in moderation and exert a wide range of antioxidant, analgesic and anti-inflammatory activities. This study examined the protective activity of dietary supplements, alpha-tocopherol (2 mg/kg BW) and Selenium (05 µg/kg BW), by using thermal (Hot plate test, Tail-flick test), chemical (writhing test, formalin test) and neuropathic (allodynia test) tests in fluoride (20mg/kg BW) induced pain models. In addition, anti-inflammatory activity was also assessed with paw oedema assay. The obtained data suggest that hyperalgesia in fluoride exposure group was significantly (p<0.05) exhibited in hot plate, tail flick, writhing response, formalin and allodynia tests. Moreover, inflammation in fluoride exposure group was also significantly (p<0.05) increased in paw oedema tests in comparison with the control group. The combined administration of Se and alpha-tocopherol significantly (p<0.05) increased response latency in hot plate and tail flick tests, reduced writhing responses in the writhing test, increased withdrawal duration in allodynia test, inhibited formalin induced pain response in both phases but it was more pronounced in the second phase and attenuated formalin induced paw oedema in comparison with independent treatment of Se and alpha-tocopherol against NaF suggesting their analgesic and anti-inflammatory activities. These findings conclude the synergistic effects of selenium and alpha-tocopherol against fluoride induced nociception and inflammation.
Keywords
Alpha-Tocopherol; Analgesia; Anti-Inflammation; Fluoride; Selenium
Download this article as:| Copy the following to cite this article: Reddy N. C. S, Reddy K. P. Analgesic and Anti-inflammatory Activities of Selenium and Alpha-tocopherol in Mouse Models of Pain Induced with Fluoride Exposure. Biomed Pharmacol J 2021;14(3). |
| Copy the following to cite this URL: Reddy N. C. S, Reddy K. P. Analgesic and Anti-inflammatory Activities of Selenium and Alpha-tocopherol in Mouse Models of Pain Induced with Fluoride Exposure. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/2US5OSw |
Introduction
Fluoride, exist in the soil, water, animals, plants and atmosphere, is known to be noxious for human at levels more than 1.5 mg/L in drinking water1. The excessive consumption of fluoride contributes to physiological dysfunction through many pathologies especially induction of oxidizing stress. Further, it may cross the blood-brain barrier, accumulate in the brain2, and causes neuronal changes and damage3,4. The excessive fluoride exposure enhances peripheral pain sensitivity such as mechanical, thermal, and inflammatory pain5,6. In addition, it also induces bone pain7. Pain is a serious problem globally8. It affects the quality of life as well economically burden to society. Regrettably, the pain relievers available in the market exert a wide range of adverse effects9. Hence, searching for alternatives such as dietary supplements as analgesic agents is essential. Tocopherols, dietary supplements, trap the free radical molecules such as reactive oxygen species10,11 and causes an increase in reduced glutathione, glutathione peroxidase and anti-oxidant vitamins levels and a decrement in pain intensity levels in fibromyalgia patients following alpha-tocopherol supplementation12. Selenium plays an important role in the regulation of the nervous system’s physiological functions13. It serves as a cofactor of the GPx enzyme involves in antioxidative defence mechanisms14. It acts as an analgesic in mitigating the peripheral pain15, 16, 17. Moreover, Se is more effectively prevented pain induced by fibromyalgia, oxidative stress in mitochondria and apoptosis18. Combined administration of Selenium and vitamin E exerted protective effects effectively against lipid peroxidation capacity in different brain regions of the rat such as cerebrum, cerebellum, midbrain and brainstem19. Due to the various ameliorative effects of alpha-tocopherol and selenium, mainly their role in anti-nociception and anti-inflammation has turned into global concern and in-depth investigation. Thus, in the present study, to explore the analgesic and anti-inflammatory effects of alpha-tocopherol and selenium individually and combinedly on fluoride-induced pain and inflammation, we have established mice model groups, and a series of diverse methods were applied such as Hot plate test, Tail-flick test, Writhing response test, Allodynia test, Formalin test and Paw oedema test.
Material and methods
Chemicals
NaF and selenium from Loba Chemie, Bombay and alpha-tocopherol acetate from Merck Company were purchased. The other chemicals used in the study were of analytical grade.
Swiss albino mice weighed about 30+2 g were procured from National Infrastructural facility for laboratory animals, NIN, Hyderabad, India. The animals were maintained in plastic cages at room temperature at 25-27oC in hygienic conditions with 12 hrs light/dark cycle. The animals were provided with water and standard rat chow procured form NIN, Hyderabad, India.
They were divided into 5 groups. Each group consists of five animals. (1) Controls group (saline) (2) NaF treated group (20mg/ kg BW) (3) Selenium alone-treated group (5 µg/ kg BW) (4) Alpha-tocopherol alone treated group (2 mg/kg BW). (5) NaF+Se+alpha-tocopherol (20mg/kg bw+5 µg/ kg bw+2mg/kg BW) treated group. The doses were given daily between 8.00 – 9.00 hours for 15 days. The experiments were carried out as per the procedure of institutional ethical committee (No: 383/01/a/CPCSEA). The necessary steps were taken to lessen the number of animals used in the study.
Analgesic & Anti inflammatory tests
Most prominent methods of nociceptive assessments like a hot plate, tail-flick, allodynia, formalin, writhing (acetic acid induced) tests & anti-inflammatory test such as formalin induced paw oedema test were conducted.
Tail flick test
The tail-flick test was carried out to test the nociceptive response20. The animals were held in one hand and the distant part of the tail was kept in a beaker containing water maintained at 48+ +20C. The time that elapsed between immersion and removal of the tail from the water noted as reaction time and expressed the results in seconds. The tissue damage was avoided by keeping the cut off time at 10 seconds.
Hot plate method
A modified version of the hot plate method was used to test the nociceptive response21. The animals were kept on a hot plate maintained at 50+20C. The time taken for the first hind paw withdrawal or licking was noted and expressed in seconds. The tissue damage was avoided by keeping the cut off time at 15 seconds.
Acetic acid induced writhing method
The writhing method was used to test nociceptive response22. Acetic acid (0.8%) solution was administered to induce writhing responses. After 10 mins of injecting, the writhing responses were noted and calculated the inhibitory rate.
Inhibitory rate (%)= (1- number (treatment)/ number (model)] ×100%.
Allodynia test
The Allodynia test was used to test the nociceptive response23. The animals were confined beneath an inverted Plexiglas case, placed upon a metal floor cooled by the ice-cold water. Each animal was exposed to the floor which had 6+20C surface temperatures for 20 mins. The withdrawal duration was noted and expressed in seconds.
Formalin test
Formalin test was carried out to test nociceptive response24. The animals were injected intraperitoneally with formalin (2.5%) solution and the animals were placed in the beaker and observed over next 30 mins. Time spent on biting or licking was measured and expressed as the pain score.
Paw Oedema Test
Paw oedema test was used to assess the magnitude of oedema25. A 0.05 ml of 1.25% of formalin was injected intraperitoneally at the base of the right hind paw of the mice, while the other hind paw was injected with the equal amounts of distilled water served as relevant control. After 8 hours, the mice were sacrificed by chloroform inhalation. The paws were amputated from the ankle and weighed. The magnitude of oedema was determined by the difference in the weight of test paw from that of control of corresponding opposite paws. The mean arithmetical magnitude of oedema by weight average method was measured.
Statistical analysis
The experimental data were subjected to one way ANOVA followed by multiple comparisons between the groups by Turkey’s studentized range test (HSD). In each test p<0.05 was considered significant.
Results
Tail flick response
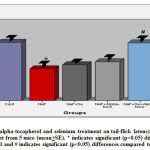
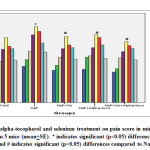
Obtained data indicates that the tail-flick latency was decreased (17.91%) significantly (p<0.05) in sodium fluoride administered group in comparison with the control animals whereas, the tail-flick latency was brought down to 14.62% in NaF+Se treated, 15.73% in NaF+alpha tocopherol treated, and 5.54% in NaF+Se+alpha tocopherol treated groups, indicates the marked recovery and it was more pronounced in NaF+Se+alpha tocopherol treated group than the selenium alone and alpha-tocopherol alone treated groups against NaF (Fig.1).
Hot plate response
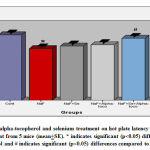
The results indicate that the hot plate latency was decreased (21%) in a significant (p<0.05) levels in NaF group in comparison with control group whereas, the hot plate latency was brought down to 15.27% in NaF+alpha tocopherol treated, 17.22% in NaF+Se treated, and 5.27% in NaF+Se+alpha tocopherol treated groups, indicates a notable reduction in fluoride intoxication. However, the recovery was more significant in NaF+Se+alpha tocopherol treated group than the selenium alone and alpha-tocopherol alone treated groups against NaF (Fig.2).
Allodynia test
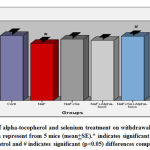
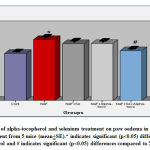
The results indicate that the hind paw cumulative withdrawal duration was decreased (12.17%) significantly (p<0.05) in sodium fluoride administered group in comparison with the control animals whereas, the hind paw cumulative withdrawal duration was brought down to 5.50% in NaF+alpha tocopherol treated, 7.30% in NaF+Se treated, and 1.10% in NaF+Se+alpha tocopherol treated groups. However, the recovery was more significant in NaF+Se+alpha tocopherol treated group than the selenium alone as well as α- tocopherol alone administered groups against NaF (Fig-3).
Writhing response
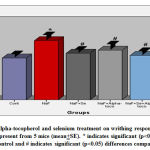
The results indicate that the writhing response of mice was significantly (p<0.05) increased (41.30%) in sodium fluoride group in comparison with control animals whereas, the number of writhers were significantly (p<0.05) brought down to 18.56% in NaF+alpha tocopherol treated, 11.30% in NaF+Se treated and to 5.98% in NaF+Se+alpha tocopherol treated groups as compared to NaF treated group suggests marked recovery in the fluoride intoxication. However, the recovery was more pronounced in NaF+Se+alpha tocopherol treated group than the selenium alone and alpha-tocopherol alone treated groups against NaF (Fig.4).
Pain score
The results indicate that the pain score of animals was significantly (p<0.05) increased (39.58%) in sodium fluoride group in comparison with control animals whereas, the pain score was brought down to 29.16% in NaF+alpha tocopherol treated, 33.33% in NaF+Se treated, and 12.5% in NaF+Se+alpha tocopherol treated groups, reveals significant inhibition of fluoride toxicity. However, the recovery was more in NaF+Se+alpha tocopherol treated group than the selenium alone and alpha-tocopherol alone treated groups against NaF (Fig.5).
Paw oedema test
The results indicate that the weight of the hind paw was significantly (p<0.05) increased (29.33%) in sodium fluoride treated group in comparison with control animals whereas, the weight of the hind paw was brought down to 21.02% in NaF+alpha tocopherol treated, 20.66% in NaF+Se treated, and 5.62% in NaF+Se+alpha tocopherol treated groups suggests a significant recovery in the weight of the hind paw. However, the recovery was more in NaF+Se+alpha tocopherol treated group than the selenium alone as well as α-tocopherol alone administered groups against NaF (Fig-6). The obtained results in all the above tests show the synergistic effect of selenium and alpha-tocopherol in the mitigation of fluoride-induced nociception and inflammation.
Discussion
The present study investigated the analgesic and anti-inflammatory properties of alpha-tocopherol and selenium in fluoride intoxicated mice model. The analgesic properties were assessed using various tests such as thermal (hot plate, tail flick), chemical (formalin test, writhing test) and neuropathic (allodynia) tests. Further, the anti-inflammation properties were also assessed with the paw oedema test. The hypothalamus neurons, nociceptive receptors and mechanoreceptors are involved in analgesia. In earlier studies, it is reported that the fluoride was reduced the small myelinated fibres26. NaF mimics the effect of PGE2 on catecholamine release from adrenal chromaffin cells27. The inhibitor of Na+ K+ ATPase also alters the catecholamine release28. The fluoride has shown influence on 5-HT metabolism in rabbits29. The turnover rate of serotonin is decreased in the hypothalamus of the rat after NaF exposure30. The depletion in central serotonin content can result in sleep disturbances, lowered pain tolerance31, and the depletion of serotonin also reduces neuronal responses to stimuli on nociceptive terminals influencing the prostaglandins which sensitize all nociceptors. Altered serotonin level influences the pain threshold. The above studies indicate that fluoride can modulate pain reception. Tail flick latency is a spinal reflex that measures the time taken to the mice to flick its tail away from the source32. Usage of tail flick test is necessary as the effective pain bring in this test is mostly related to the pain relief in humans33. The tail-flick test is a sharp spine mediated reflex to harmful thermal stimuli,33 which induces pain through spinal reflexes arbitrated by µ2, δ2, κ1opioid receptors34. In the present study, NaF treatment resulted in hyperalgesia condition and which is in an accordance with the earlier studies reported that fluoride modulates the pain reception resulting hyperalgesic conditions through altering the catecholamine levels26,27, and this hyperalgesia condition was significantly mitigated by combined administration of selenium and alpha-tocopherol than the Se as well as α-tocopherol alone administered groups (Fig-1). The tolerance capacity of the mice indicates the prevention of pain progression in the spine probably due to the ameliorative action of selenium and alpha-tocopherol.
Hot plate test is generally used to assess centrally acting analgesic agents 35, 36. In the present study, NaF decreased the latencies of hot plate test suggesting the hyperalgesic conditions and which were reversed in NaF+Se, NaF+alpha tocopherol and NaF+Se+alpha tocopherol administered groups. But, the combined administration showed more significant reversal than individual administration of alpha-tocopherol and Selenium (Fig.2). The results are corroborated with earlier reports that showing the diminished response of spinal neurons to heat therefore, inversed latency against heat was observed37. An increase in sensitivity to threshold levels of heat pain may also suggest a lesion in unmyelinated afferents. In a study, it is suggested that the reduction or degeneration of myelinated fibres, large A-type neurons and unmyelinated ‘C’ fibres result in the decrease of hot plate latencies21. In another study, it is reported that the neuro histological and signal fibre studies on neural nerve during skeletal fluorosis show reduction in small myelinated fibres due to axonal degeneration and secondary demyelination26.
The allodynia test was conducted to evaluate the neuropathic pain. The test consists of two distinct stages. Early stage occurs in few hours after dosage administration and later stage occurs after few days38. The serotonergic system mediates the pain responses at central and peripheral sites. Furthermore, adenosinergic and opioidergic systems represent essential pathways involved in analgesia39. The results showed decreased duration to allodynia test in mice treated with NaF indicating increased pain sensitivity may be due to the modulation of serotonergic, adenosinergic and opioidergic systems whereas, it was significantly reverted with the treatment of Se and alpha-tocopherol alone and combinedly along with NaF. But, the result was more significant in NaF+Se+alpha-tocopherol treated animals suggest the restored functioning of serotonergic, adenosinergic and opioidergic systems (Fig.3). The results are in line with earlier studies indicating that touch invoked allodynia is mediated via A fibres40 and also altering spinal glycinergic and GABAergin systems41. These may result in induced changes in dorsal horn along with nociceptive C fibres.
Various chemical agents are generally used as nociceptive stimuli to evaluate antinociceptive drugs42. In the current investigation, acetic acid is used in the writhing test. The nociception induced by acetic acid possibly due to the release of tumour necrosis factor-α, interleukin-1β and interleukin-8 by the macrophages and mast cells43. Pain is classified into visceral and somatic types. The visceral type of pain is linked with various substances i.e. histamine, ach, kinin and prostaglandins44, 45. In earlier studies, it is reported that NaF exposure is characterized by painful symptoms of gastrointestinal, muscle-skeletal, respiratory and other visceral systems46. The NaF alters the PGE2 and catecholamine secretions which are essential mediators of sympathetic system functions that implying the NaF influence on visceral pain through the sympathetic system27. The majority of the visceral symptoms may be due to the toxicity of fluoride on various target organs47, 48. The writhing responses of mice after NaF treatment in this study confirm the aforesaid inferences. In the present study, mice with writhing responses serve as a model for visceral pain. The NaF exposure resulted in maximum writhing response suggesting the presence of visceral pain, whereas the writhing responses were decreased significantly in NaF+alpha tocopherol, NaF+Se and NaF+Se+alpha tocopherol administered groups whereas, a marked decrease was noted in NaF+Se+α-tocopherol group (Fig.4). The antinociceptive effects of tocopherol and selenium probably due to the prevention of the synthesis of the inflammatory mediators (tumour necrosis factor-α, interleukin-1β and interleukin-8).
Formalin test is used for quantitative assessment of pain and analgesia which has the advantage of allowing quantification of toxic pain component32. The test involves an evaluation of two different stages of nociceptive sensitivity. 1st stage occurs within five minutes and the 2nd stage occurs in between 15 to 20 minutes after the injection49. Analgesic drugs like narcotics and morphine, which act centrally, inhibit two stages. But, corticosteroids and NSAIDs, which act, peripherally inhibit only 2nd stage50. In the current investigation, the treatment of selenium and tocopherol individually and combinedly against NaF decreased pain response in 2nd stage (fig.5), which suggest that the analgesic activity of both compounds attributable to their peripheral action. The combined administration showed marked recovery than the individual indicates a synergistic effect. Here, it may be concluded that the protective effects of Se and alpha- tocopherol are connected with the anti-inflammatory effects (a reduction of tumour necrosis factor-α levels and nitric oxide) involve in the pain sensitization process 51,52.
Oedema is an inflammatory response that is dependent on the integrity of peripheral nociceptors. It is induced by irritant substances and modulated by poly Model nociceptor ‘C’ fibres and A-delta fibres. The touch and mechanoreceptors are mediated by ‘A’-beta and A-delta and C mechanoreceptors. Many of unmyelinated fibres converge on to the spinal neurons which obtain information from A-delta afferents involved in pain process53. In the present study, the rate of paw oedema was significantly increased in fluoride exposure group and which may be due to the formation of inflammatory mediators i.e. serotonin, histamines, prostaglandins and bradykinins44,45,49,54intensifying the polymorphonuclear chemotaxis and the inflammatory process whereas, paw oedema was significantly decreased may be due to the inhibition in the formation of inflammatory mediators in Se+NaF, alpha-tocopherol+NaF and Se+alpha-tocopherol+NaF treated groups (Fig.6). Collectively, the results specify that combined administration of selenium and alpha-tocopherol showed much recovery than individual administration indicating a synergistic effect.
Conclusion
The results of the present study showed increased nociceptive sensitivity and inflammation in mice after NaF exposure whereas which were significantly reverted after selenium and alpha-tocopherol administration individually and together along with NaF. However, the combined administration of Se and alpha-tocopherol was shown a more significant reversal of the nociceptive sensitivity and inflammation indicating a synergistic effect. The increased nociceptive sensitivity during NaF exposure might be due to significant accumulation of fluoride in the important centres of pain pathways of the nervous system which might result in sensitization of neurons, inhibition of Na+K+ATPase, altered catecholamine levels, depleted serotonin levels and other endogenous pain modulating substances and increased inflammation due to the formation of inflammatory mediators. The reversal trend observed might be due to lowered fluoride levels and reversal of concomitant neuronal changes. Further studies are required to have a specific mechanism which accounts for the observed nociceptive sensitivity and inflammation in fluorosis.
Acknowledgment
The author thanks the Head Department of Zoology, UCS, Osmania University TS, India, for providing laboratory facilities.
Conflict of interest
No conflict of interest.
Funding Source
The authors received financial assistance from UGC-SAP-DSA (F.5-26/2015/DSA-(SAP-II), a programme of Department of Zoology, Osmania University, TS, India.
References
- Singh G, Kumari B, Sinam G, Kumar N, Mallick S. Fluoride distribution and contamination in the water, soil and plants continuum and its remedial technologies, an Indian perspectivee a review. Environ. Pollut, 2018; 239: 95-108.
CrossRef - Wei N, Dong YT, Deng J, Wang Y, Qi XL, Yu WF, Xiao Y, Zhou JJ, Guan ZZ. Changed expressions of N-methyl-d-aspartate receptors in the brains of rats and primary neurons exposed to high level of fluoride. J Trace Elem Med Biol, 2018; 45: 31-40.
CrossRef - Mesram N, Nagapuri K, Banala RR, Nalagoni CR, Karnati PR. Quercetin treatment against NaF induced oxidative stress related neuronal and learning changes in developing rats. J King Saud Univ Sci, 2017; 29:221-229.
CrossRef - Nalagoni CSR, Karnati PR. Protective effect of resveratrol against neuronal damage through oxidative stress in cerebral hemisphere of aluminum and fluoride treated rats. Interdisci Toxicol, 2016; 9(2):78-82.
CrossRef - Jing Maa, Fei Liua, Peng Liub, Ying-Ying Donge, Zheng Chub, Tie-Zhou Houa,Yong-Hui Dang. Impact of early developmental fluoride exposure on the peripheralpain sensitivity in mice. Int. J. Devl Neuroscience, 2015; 47: 165–171.
CrossRef - Sudhakar K, Nageshwar M, Reddy KP. Abelmoschus moschatus extract reverses altered pain and neurohistology of a rat with developmental exposure of fluoride. J App Pharm Sci, 2018; 8(06): 094-104.
CrossRef - Barajas MR, McCullough KB, Merten JA, Dierkhising RA, Bartoo GT, Hashmi SK, Hogan WJ, Litzow MR, Patnaik MM, Wilson JW, Wolf RC, Wermers RA. Correlation of Pain and Fluoride Concentration in Allogeneic Hematopoietic Stem Cell Transplant Recipients on Voriconazole. Biol Blood Marrow Transplant, 2016; 22(3):579-83
CrossRef - Goldberg DS and McGee SJ. Pain as a global public health priority. BMC Public Health, 2011; 11: 770.
CrossRef - Crofford LJ. Adverse effects of chronic opioid therapy for chronic musculoskeletal pain. Nat Rev Rheumatol, 2010; 6:191–197.
CrossRef - Ju J, Picinich SC, Yang Z. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis, 2010; 31: 533–542.
CrossRef - Jiang Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med, 2014; 72:76–90.
CrossRef - Batista ED, Andretta A, de Miranda RC, Nehring J, Dos Santos Paiva E, Schieferdecker ME. Food intake assessment and quality of life in women with fibromyalgia. Rev Bras Reumatol, 2016; 56:105–110.
CrossRef - Schweizer U, Bräuer AU, Köhrle J, Nitsch R, Savaskan NE. Selenium and brain function: a poorly recognized liaison. Brain Res Brain Res Rev, 2004; 45: 164–178.
CrossRef - Lawrence RA and Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun, 1976; 71: 952–958.
CrossRef - Kahya MC, Nazıroğlu M, Övey İS. Modulation of diabetes-induced oxidative stress, apoptosis, and Ca(2+) entry through TRPM2 and TRPV1 channels in dorsal root ganglion and hippocampus of diabetic rats by melatonin and selenium. Mol Neurobiol, 2017; 54: 2345–2360.
CrossRef - Nazıroğlu M, Özgül C, Küçükayaz M, Çiğ B, Hebeisen S, Bal R. Selenium modulates oxidative stress-induced TRPM2 cation channel currents in transfected Chinese hamster ovary cells. Basic Clin Pharmacol Toxicol, 2013; 112: 96–102.
CrossRef - Balaban H, Nazıroğlu M, Demirci K, Övey İS. The Protective Role of selenium on scopolamine-induced memory impairment, oxidative stress, and apoptosis in aged rats: The involvement of TRPM2 and TRPV1 channels. Mol Neurobiol, 2017; 54: 2852–2868.
CrossRef - Yüksel E, Nazıroğlu M, Şahin M, Çiğ B. Involvement of TRPM2 and TRPV1 channels on hyperalgesia, apoptosis and oxidative stress in rat fibromyalgia model: Protective role of selenium. Sci Rep, 2017; 7:17543
CrossRef - Mohsen M, John B, Macauley, Jeffrey B. Blumberg Effect of Dietary Vitamin E and Selenium on Susceptibility of Brain Regions to Lipid Peroxidation. LIPIDS, 1988; 23(5): 405-409.
CrossRef - Sewell and Spencer PS. Modifications of nociceptive activity of narcotic agonists and antagonists by intra ventricular injection of biogenic amines in mice. Br. J. Pharmacol, 1974; 51:140-147.
- Hiura A, Villalobs EL, Ishizoka H. Age dependent attenuation of the decrease of C-fibres by capsaicin and its effects on responses to nociceptive stimuli. somatosens. Res, 1992; 9: 37
CrossRef - Chen Y, Tao S, Zeng F, Xie L, Shen Z. Antinociceptive and anti-inflammatory activities of Schefflera octophylla extracts. Journal of ethnopharmacology. 2015; 171: 42-50.
CrossRef - Bennet J and Xie YK. A peripheral neuropathy in rat produces disorder of pain sensation like those seen in man. Pain, 1988; 33:107
CrossRef - Rapacz A, Kamiński K, Obniska J, Koczurkiewicz P, Pękala E, Filipek B. Analgesic, antiallodynic, and anticonvulsant activity of novel hybrid molecules derived from N-benzyl-2-(2,5-dioxopyrrolidin-1-yl)propanamide and 2-(2,5-dioxopyrrolidin- 1-yl)butanamide in animal models of pain and epilepsy. Naunyn Schmiedebergs Arch. Pharmacol, 2017; 390: 567–579.
CrossRef - Zanoni I, Ostuni R, Barresi S. CD14 and NFAT mediate lipopolysaccharide-induced skin edema formation in mice. J Clin Invest, 2012; 122:1747–1757
CrossRef - Sesikiran B, Harinarayana Rao S, Krishnamurthy D, Raja Reddy D. Studies on sural nerve biopsies in endemic skeletal fluorosis. Fluoride, 1994; 22:33-9.
- Ito S, Negishi M, Oda ., Yokohama H, Hayaish O. Sodium fluoride mimics the effects of prostaglandin E2 on catecholamine releases from bovine adrenaline chromaffin cells. Journal of neurochemistry, 1991; 56:44-251.
CrossRef - Tanaka T, Yokohom H, Negsi H, Hayaishi H, Ito S, Hayaishi O. Synergestic effect of prostaglandin E2 and ovabian on catecholamine release from cultured bovine and resilan chromatin cells. J. Neuochemistry, 1990; 54:86-95.
CrossRef - Geerearts F. Effects of administration of NaF on urinary excretion of tryptophan metabolism. Fluoride, 1981; 14: 155-160
- Yuan shude, Qiweurin Xie, Furing Lu. Changes of serotonin content and turn over rate in hypothalamus of female rat during fluorosis. Fluoride, 1993; 26(1): 57-60.
- Measing RB and Lytle LD. Serotonin containing neurons. Their possible role in pain and anaesthesia. Pain , 1977; 4:1-21.
CrossRef - Dubbuison and Dennis SG. The formalin test a quantitative study of analgesic effects of morphine meperidine and brain stimulation in rats and cats. Pain, 1977; 4:161-174
CrossRef - Sharma BR, Park CM, Choi JW, Rhyu DY. Anti-nociceptive and anti inflammatory effects of the methanolic extract of Opuntia humifusa stem. Avicenna. J. Phytomed, 2017; 7(4): 366–375
- Adnan M, Nazim Uddin Chy M, Kamal ATMM, Barlow JW, Faruque MO, Yang X, Uddin SB. Evaluation of anti-nociceptive and anti-inflammatory activities of the methanol extract of Holigarna caustica (Dennst.) Oken leaves. J. Ethnopharmacol, 2019; 236: 401–411.
CrossRef - Carvalho de, Ropero PR, Pinheiro DR, Fernandes MM, Boylan PD. Quinoline Alkaloids Isolated from Choisya Aztec-Pearl and Their Contribution to the Overall Antinociceptive Activity of This Plant. Plos one, 2016; 11 (10) – e0164998.
CrossRef - Patel MK, Mandavia DR, Patel TK, Barvaliya MJ, Tripathi CB. Evaluation of Anti-Inflammatory, Analgesic, and Antipyretic Effects of Ethanolic Extract of Pedalium Murex Linn. Fruits. African Journal of Traditional Complementary and Alternative Medicines, 2013; 10:94-100.
CrossRef - Fitzgerald M and Wolf CJ. The time course and specificities of the changes in the behavioural and dorsal horn cell responses to noxious stimuli following peripheral nerve capsarian treatment in rat. Neuroscience J, 1982; 7 (9): 2051-56.
CrossRef - Flatters SJL, Dougherty PM, Colvin LA. Clinical and preclinical perspectives on chemotherapy-induced peripheral neuropathy (CIPN): a narrative review. Br J Anaesth, 2017; 119(4):737–749.
CrossRef - Luchese C, Prigol M, Acker CI, Nogueira CW. Antinociceptive effect of butyl (2-phenylethynyl) selenide on formalin test in mice: evidences for the involvement of serotonergic and adenosinergic systems. Eur J Pharmacol, 2010; 644:49–54
CrossRef - Shir Y and Seltzen. A fibres mediate touch evoked allodynia and hyperesthesis and C-fibres mediate thermal hyperalgesia in a rat model of sympathetically maintained neuropathic pain. Neurosci, 1990; 115: 62-67.
CrossRef - Yaksh TL. Behavioural and autonomic correlates of tactyl evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonist. Pain, 1989; 37: 111-123.
CrossRef - Barrot M. Tests and models of nociception and pain in rodents. Neuroscience, 2012; 211: 39–50.
CrossRef - Ribeiro RA, Vale ML, Thomazzi SM, Paschoalato AB, Poole S, Ferreira SH, Cunha FQ. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur J Pharmacol. 2000; 387:111–118.
CrossRef - Golshani S, Karamkhani F, Monsef-Esfehani HR, Abdollahi M. Antinociceptive effects of the essential oil of Dracocephalum kotschyi in the mouse writhing test. Pharm. Pharm. Sci. 2014; 7:76-79.
- Nwidu LL, Airhihen B, Ahmadu A. Anti-inflammatory and anti-nociceptive activities of stem-bark extracts and fractions of Carpolobia lutea (Polygalaceae). Journal of Basic Clinical and Pharmacy, 2016; 8(1): 25-32.
CrossRef - Reddy DR, venken PJ, Grynn GW. 1979. Skeletal fluorosis in: Hand books of clinical neurology vol 36 eds. North Holland Publishing Company NY Oxford: 465-504.
- Waldbott GL, Burgstahler AW, McKinney HL. 1978. Fluoridation: the great dilemma. Lawrence, Kansas: Coronado Press Inc
- Monsour PA and Kruger BJ Effect of fluoride on soft tissues in vertebrates (a review). Fluoride, 1985; 18(1): 3-61.
- Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain, 1992; 51: 5–17.
CrossRef - Chi-Ren Liao, Yuan-Shiun Chang, Wen-Huang Peng, Shang-Chih Lai, Yu-Ling Ho. Analgesic and Anti-Inflammatory Activities of the Methanol Extract of Elaeagnus oldhamii Maxim. in Mice. The American Journal of Chinese Medicine, 2012; 40(3): 581–597.
CrossRef - Luo ZD and Cizkova D. The role of nitric oxide in nociception. Curr Rev Pain, 2000; 4:459–466.
CrossRef - Zarpelon AC, Cunha TM, Alves-Filho JC. IL-33/ST2 signalling contributes to carrageenin-induced innate inflammation and inflammatory pain: role of cytokines, endothelin-1 and prostaglandin E2. Br J Pharmacol, 2013; 169:90–101.
CrossRef - Hamnard DL and Yaksh TL. Peripheral and central pathways in pain. Pharmacol Ther, 1981;14: 459-475.
CrossRef - Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol. Rev, 2001; 53:597‑