Manuscript accepted on :01-07-2021
Published online on: 19-07-2021
Plagiarism Check: Yes
Reviewed by: Dr. Sreedhar D
Second Review by: Dr. Salman Ahmed Pharmacognosy
Final Approval by: Dr Ayush Dogra
Uma Narayanamurthy1 , Mirunalini.R2
, Mirunalini.R2 , Subha. V1
, Subha. V1 , K. Manimekalai1
, K. Manimekalai1 , Sakthibalan K3
, Sakthibalan K3 , Arther Paul. C4
, Arther Paul. C4 Nagarajan K M4
Nagarajan K M4 and Sabarianandh JV1*
and Sabarianandh JV1*
1Department of Pharmacology, MGMCRI, SBV University, Puducherry, Tamil Nadu, India.
2Department of Pharmacology, JIPMER, Puducherry, Tamil Nadu, India.
3Investigator, Ki3, CRO Puducherry, Tamil Nadu, India.
4Manager International Business, Apex Laboratories PVT.Ltd, Chennai. Tamil Nadu, India.
Corresponding author E-Mail :crony.8681@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2248
Abstract
Aim and Objectives: This study evaluated the acute and repeated dose toxicity effects of Clevira Syrup Polyherbal formulation (CSPHF), which was prepared from ten different herbs, well known and widely used in traditional medicine for the management of viral infections and other inflammatory disease conditions. Individually these herbs (Carica papaya, Melia azedarach, Andrographis paniculata, Vetiveria zizanioides, Trichosanthes dioica, Cyperus rotundus, Zingiber officinale, Piper nigrum, Mollugo Cerviana and Tinospora cordifolia )were completely safe, but the polyherbal formulation effects were not known. Thus, this study was done for the investigation of toxicological profile of CSPHF in Wistar Albino rats. Methods: As per OECD(Organisation for Economic Co-operation and Development) guidelines 423 and 407, Acute and Repeated dose toxicity study were proceeded. In the acute toxicity study a single dose of CSPHF (2000mg/kg) was administered orally to female Wistar rats and in repeated dose toxicity study, CSPHF was administered orally in Control group and three different doses (1000, 500 and 250mg/kg body weight) to both male and female wistar albino rats for 28 days. At the end of the study, the animals were euthanized, observed the external and internal morphology (Acute Toxicity) and assessed the effect of CSPHF on histopathological and biochemical parameters (Repeated Dose toxicity study). Results: In acute toxicity study, there were no visual signs of toxicity of CSPHF (2000mg/kg) observed, whereas in Repeated dose toxicity study Ischaemia, inflammation and hematoma of the internal organs were observed at 1000mg/kg dose, but no such toxic features were seen at 500 and 250mg/kg dose of CSPHF. Conclusion: The results of the Acute and Repeated Dose toxicity study could be authenticated in future studies, which will be more useful and evidence based for the management of Viral infectionsduring pandemics.
Keywords
Acute and Repeated Dose Toxicity; Clevira syrup; Polyherbal formulation
Download this article as:| Copy the following to cite this article: Narayanamurthy u, Mirunalini R, Subha V, Manimekalai K, Sakthibalan K, Arther P. C, Nagarajan K. M, Sabarianandh J. V. Acute and Repeated Dose Toxicity Study of Clevira Syrup – A Polyherbal Formulation. Biomed Pharmacol J 2021;14(3). |
| Copy the following to cite this URL: Narayanamurthy u, Mirunalini R, Subha V, Manimekalai K, Sakthibalan K, Arther P. C, Nagarajan K. M, Sabarianandh J. V. Acute and Repeated Dose Toxicity Study of Clevira Syrup – A Polyherbal Formulation. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/3hLNQtE |
Introduction
In this modern world, the traditional medicine is more popular, curative, less toxic and with minimal side effects1. As per World Ethno Botanical reports, 70-80% of global population relies on traditional health care2. In India herbal preparations attained wide spread acceptability with lot of therapeutic value. Most of the medicinal plants in the compositionshowed pharmacological effects and it had been studied for years. Even today there are new objectives and efficacy profile determined and substantiated with scientific evidences for the benefits of these medicinalplants, whereas there are limitations for the safety and efficacy of the preparations3.
CSPHF is a combination of ten ingredients, used in traditional medicine for management of viral infection and other disease conditions. The syrup consists of Carica papaya, Meliaazedarach, Andrographispaniculate, Vetiveriazizanioides, Trichosanthesdioica, Cyperusrotundus, Zingiberofficinale, Piper nigrum, Mollugocerviana and Tinosporacordifolia. These ingredients were found to have anti-inflammatory, anti-pyretic, anti-bacterial, anti-microbial, anti-cancer, antihelmintic, larvicidal, hepatoprotective, anti-diabetic, anti-obesity and hypolipidemic activity4-13. Apex Laboratories Private Limited, Chennai, has proved that CSPHF has antiviral effects in cell lines with fewer side effects14.
CLEVIRA Syrup Composition
Each 10ml contain (Aushadh Ghana) extracts derived from medicinal plantsof Carica papaya, Melia azedarach, Andrographis paniculata, Vetiveria zizanioides, Trichosanthes dioica, Cyperus rotundus, Zingiber officinale, Piper nigrum, Mollugo cerviana and Tinospora cordifolia in mg as described in the Table of Clevira Syrup Composition.
| S.No. | Botanical Name | Common Name (Sanskrit Name) | Plant Parts Used | Label Claim (mg) |
| 1. | Carica papaya | Erandakarkati | Leaves | 1000.00 |
| 2. | Melia azedarach | Mahanimba | Leaves | 1000.00 |
| 3. | Andrographis paniculata | Kalmegh | Herb | 250.00 |
| 4. | Vetiveria zizanioides | Usira | Root | 250.00 |
| 5. | Trichosanthes dioica | Patola | Whole plant | 250.00 |
| 6. | Cyperus rotundus | Musta | Rhizome | 250.00 |
| 7. | Zingiber officinale | Sunthi | Rhizome | 250.00 |
| 8. | Piper nigrum | Maricha | Fruit | 250.00 |
| 9. | Mollugo cerviana | Grismachatraka | Whole plant | 250.00 |
| 10. | Tinospora cordifolia | Guduchi | Stem | 250.00 |
Materials and Methods
Clevira Syrup was procured from Apex Laboratories private limited, Guindy, Chennai, India.
Ethical Clearance
Adult female wistar Albino rats (weighing 170-200g) were used for acute toxicity study and for Repeated dose toxicity animals of either sex were used after approval by the Institutional Animal Ethics Committee, MGMCRI. (for Acute toxicity study, Ref. 03/IAEC/ MGMC /06/2018 – I and Repeated Dose 28 days Toxicity study, Ref. No. 05/IAEC/11/2018 – II).
Animals
The animals were acquired from TANUVAS breeding center, Chennai. The animals were housed in a propylene cage with sterile bedding material, and fed with standard pellet feed and water, which was maintained at a room temperature of 26 ± 2°C and a relative humidity 45-55% in the Central Animal House, MGMCRI, throughout the study period.
Acute Oral Toxicity
Acute Toxicity testing was done as per the OECD (Organisation for Economic Co-operation and Development)guidelines 42315,16. Female wistar rats were randomly selected and kept in their cages for at least 5 days prior to the initiation of dosing for acclimatization to the laboratory conditions. Animals were divided into 3 groups (N=3) for testing acute oral toxicity. Following overnight fasting, the animals were weighed and the CSPHF (2000mg/kg, PO) was administered and food was withheld for a further 3-4 hours, whereas the control group only received normal saline.
All rats were allowed free access to food and water, and it was observed individually after dosing during the first 30minutes then periodically during the first 24 hrs with special attention given during the first 4 hours and daily thereafter, for a total of 14 days. In this next 14 days, the animals were observed once daily for visual observations mortality, physical appearance, behavioural changes and any injury or illness and was recorded with individual records being maintained for each animal. Additional observations were done to see if the animals showed anysigns of toxicity (Observations should include changes in skin and fur, eyes and mucous membranes, and also respiratory, circulatory, autonomic and central nervous systems, and somatomotor activity and behaviour pattern. Attention was directed towards observations of tremors, convulsions, salivation, diarrhoea, lethargy, sleep 4/14 OECD/OCDE 423 and coma).Individual weights of animals were determined before the test substance was administered and then weekly thereafter. The weight changes were recorded. At the end of the test, animals would be weighed and humanely killed.
All test animals were subjected to gross necropsy and observed for gross pathological changes for each animal. The same procedure was repeated for the second set of test 2 (n=3) using 2000 mg/kg dose(Table-1).
Table 1: Acute Toxicity Study of CSPHF.
| Group | No. of Rats (n) | Dose of CSPHF |
| Test 1 | 3 Female Rats | 2000mg/kg Body weight |
| Test 2 | 3 Female Rats | 2000mg/kg Body weight |
Repeated Dose 28days Toxicity study
Acute Toxicity testing was done as per the OECD guidelines 40717. Following the Acute Oral Toxicity study of Clevira syrup Polyherbal formulation (2000mg/kg), Wistar Albino rats of either sex (n= 5male +5female rats) were divided into four groups (n=5+5) in each group with the allocation as follows: Group I (Control group), Group II (CSPHF 250mg/kg), Group III (CSPHF 500mg/kg) and Group IV (1000mg/kg) Table-2.
Table 2: Repeated dose Toxicity Study of CSPHF
| Study | Test Drug | Groups | Dose
mg/kg |
Species/
Strain |
No. Of Animals and Sex | Total Animals |
| Repeated Dose Toxicity Study | Clevira Syrup | Control | Sterile water 1ml/100g | Wistar Rats | 5 Male rats + 5 Female rats | 10 |
| Low Dose | 250mg/kg | Wistar Rats | 5 Male rats + 5 Female rats | 10 | ||
| Medium Dose | 500mg/kg | Wistar Rats | 5 Male rats + 5 Female rats | 10 | ||
| High Dose | 1000mg/kg | Wistar Rats | 5 Male rats + 5 Female rats | 10 | ||
| Satellite Group | ||||||
| Control Group | Sterile Water 1ml/100g | Wistar Rats | 5 Male rats + 5 Female rats | 10 | ||
| High Dose of Clevira Syrup | 500mg/kg | Wistar Rats | 5 Male rats + 5 Female rats | 10 | ||
Other two groups of rats {n=10 (5+5)} served as Control and Satellite groups based on the 14 days of follow up of the Repeated Dose toxicity study Observations of CSPHF.
The Maximum volume of CSPHF than can be administered was at the dose 1ml/100g body weight. Based on this the dosage of administration of CSPHF was calculated and given orally through oral feeding needle. Following the period of fasting, the animals were weighed and test substance was administered. After the test substance has been administered food was withheldfor 3-4 hours. The animals were administered with the test substance CSPHF at the dose of 250, 500 and 1000mg/kg body weight daily orally for a period of 28 days. Animals were observed individually after dosing at least once during the first 30 minutes, periodically during the first 24 hours, with special attention given during the first 4 hours and daily thereafter for a total of 28 chronological days. Individual animal behavioural changes were observed and recorded for each animal at the interval of 7 days as Day 0, Day 7, 14, 21 and 28 respectively. The animals were monitored for any signs of toxicity of drug from day one of the study and the observations were recorded periodically. During the process of periodical observation at the dose of 1000mg/kg body weight of CSPHF, the animals in this group started to show some of the signs of toxicity (oral ulcers and bleeding from the same, hair fall, conjunctival haemorrhage, spotting of blood at the rectal orifice, skin rashes, urethral orifice with spotting of blood during urination, high coloured urine and black coloured stools) from 15th day onwards along with conjunctival and paw edema, abdomen distension, and hemoptysis. Mortality occurred within 27 days of the study period of all the animals in Group IV (1000mg/kg of CSPHF). There were no gross pathological changes noted in Group II (250mg/kg of CSPHF) and Group III (500mg/kg of CSPHF). All the orifices, cranial, thoracic cavity, pelvic cavity and abdominal cavity on observation all the internal organs including the reproductive organs were healthy without any signs of toxicity or necrosis of tissues in Group II and Group III. But on examination of the internal organs and the orifices of the Group IV (1000mg/kg of CSPHF) animals after their mortality, the organs were observed with necrotic tissues with hematoma of the solid organs and ischemia of the colon, with necrosis and gangrenous appearance of the reproductive tissues, etc. Hence the highest safer dose was selected for dosing in the control group and satellite group17,18
In Satellite group (group V – 500mg/kg CSPHF orally), the animals (n=10) were dosed with the safer highest dose from the repeated dose toxicity (500mg/kg of CSPHF) for a period of 28 days and the control group of animals (n=10) to monitor and compare any specific observations of toxic changes in the animals during the test period.
Weight changes were calculated and recorded weekly. Measurement of food consumption was made at least once weekly. At the end of the study the animals were fasted overnight prior to necropsy and blood samples for Hematological and Biochemical parameters estimation were collected from retro-orbital puncture after anaesthetising the animals with Intraperitoneal Inj. Thiopentone sodium, following which the samples for histopathological examination were collected after euthanasia of the animals.
Blood Analysis
For haematological analysis different parameters such as Hemoglobin (Hb), Red Blood Cell (RBC) count, White Blood Cell (WBC) count, Platelet Count (PLT), Differential Count (DC), Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH) and Mean Corpuscular Hemoglobin Concentration (MCHC) were estimated. Biochemical analysis of serum samples were performed to analyse various parameters such as Serum Creatinine, Aspartate Aminotransferase, Alanine Aminotransferase, Serum Albumin, Globulin, Albumin and Globulin ratio (A/G ratio), total bilirubin, Blood Urea, Blood Glucose and Alkaline Phosphatase.
Histopathological Examination
After blood collection, the animals were sacrificed by Inj. Thiopentone Sodium i/p and different organs such as Ovary, Testes, Liver, Kidney, Heart, Adrenals and Pancreas were collected from each rat in all groups to observe for any changes is histopathology. The organs were then fixed in 10% neutral buffered formalin for 18hrs at 4 degree Celsius and processed by conventional techniques. Paraffin sections were stained with hematoxylin and eosin, following the standard laboratory procedures. The stained sections were examined under Oil Immersion Microscope under magnification for any change in morphology and for any cellular damage.
Results and Discussion
Acute Toxicity Study of Clevira Syrup Polyherbal Formulation (Table:3 and 4)
Table 3: Weight changes in wistarrats baseline, day 7 and day 14 for Acute toxicity study.
| Groups
N=3 |
Dose of CSPHF | Baseline weight on Day 0 (in gm) | Weight on Day 7 (in gm) | Weight on Day 14 (in gm) |
| Test 1 | 2000mg/kg | 165+173+169
Avg: 169 |
170+188+165
Avg: 174.3 |
167+190+160
Avg: 172.3 |
| Test 2 | 2000mg/kg | 180+179+173
Avg: 177.3 |
189+190+184
Avg: 187.6 |
185+189+170
Avg: 181.3 |
Table 4: Observation on signs of Acute toxicity.
| GROUPS | Day 0 | Day 7 | Day 14 |
| Test 1 | Nil | Nil | Nil |
| Test 2 | Nil | Nil | Nil |
On Observation
The animals in each test group (Test 1 and Test 2) after overnight fasting were observed for their activity and looked for signs of toxicity. The Orifices and internal organs were carefully examined and observations recorded on Individual basis. During the study none of the animals showed signs of toxicity or had a moribund status. After Inj. Thiopentone Sodium IP the animals were sacrificed and necropsy was done. There was no gross pathological changes noted in any of the groups. Hence from the data obtained after the acute toxicity study the Repeated dose toxicity study of CSPHF was done in the Group of Male and Female Wistar Rats for a period of 28 days.
Repeated Dose 28 days toxicity study of Clevira Syrup Polyherbal formulation in Wistar Albino rats(Table-5 and 6).
Table 5: Weight changes in wistarrats baseline, day 7, day 14, day 21 and day 28
| Group designation | Baseline weight in kg | Weight on Day 7 | Weight on Day 14 | Weight on Day 21 | Weight on Day 28 |
| Test 1(n=5)
Dose of Clevira syrup 250mg/kg |
183.4 ± 1.89 | 180.5 ± 1.17 | 174.9 ± 2.84 | 179.3 ± 1.903 | 183.7 ± 1.202 |
| Test 2 (n=5)
Dose of Clevira syrup 500mg/kg |
178.5 ± 1.14 | 181.4 ± 1.91 | 176.8 ± 1.404 | 174.8 ± 0.785 | 171.4 ± 0.66 |
Table 6: Observation on signs of Repeated dose toxicity.
| DAYS | Day 0 | Day 7 | Day 14 | Day 21 | Day 28 |
| Test 1 (250mg/kg) | Nil | Nil | Nil | Nil | Nil |
| Test 2
(500mg/kg) |
Nil | Nil | Nil | Nil | Nil |
The animals from each group were monitored from day one of the study and the observations were done on the external features for signs of toxicity. All systems were carefully examined and recorded on individual basis on every seventh day (Day 0, 7, 14, 21 and 28 days). During the study period none of the animals showed signs of toxicity or had a moribund status in the dose of 250mg/kg and 500mg/kg, but to mention the dose of 1000mg/kg started to show the signs of toxicity from Day 15 onwards with conjunctival and paw edema, abdomen distension and hemoptysis, and mortality occurred within day 27 of all the animals in the group.
At the end of the study animals were anasthetizedby Inj. Thiopentone sodium I.P Blood samples were collected for Biochemical and Hematological parameters, animals were sacrificed and necropsy was done Table (7 and 8). There were no gross pathological changes of the internal organs noted in group 1 (250mg/kg) and group 2 (500mg/kg). All the orifices, cranial, thoracic, pelvic and abdominal cavity and the internal organs including the reproductive organs were healthy, no signs of toxicity and necrosis of tissues were identified in them on gross examination.
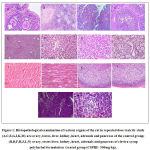
On Histopathological examination, the liver, kidney, stomach, heart, adrenals, ovary, uterus, testes and pancreas were found to be normal without any signs of inflammation at the dose of 250mg/kg and 500mg/kg of Clevira Syrup. [Figure -1]
Table 7: Clevira syrup-Repeated dose toxicity blood analysis parameters Hematological and Biochemical analysis.
|
Blood analysis |
Group 1 Control |
Group 2 (250mg/kg) |
Group 3 (500mg/kg) |
| Platelets (103/ µL) | 707.8 ± 1.61 | 768.0 ± 1.49 | 811.0 ± 4.08 |
| MCHC (g/dL) | 33.32 ± 0.18 | 34.91 ± 0.15 | 35.09 ± 0.19 |
| MCH (pg) | 81.21 ± 0.87 | 18.54 ± 0.21 | 19.08 ± 0.15 |
| Hemoglobin (g/dL) | 14.43 ± 0.20 | 14.25 ± 0.10 | 14.50 ± 1.95 |
| Red blood cells (106/ µL) | 8.13 ± 0.14 | 8.11 ± 0.07 | 8.70 ± 0.149 |
| White Blood cells (103/ µL) | 4.49 ± 0.14 | 4.67 ± 0.24 | 7.22 ± 0.10 |
| MCV [fL(µm3)] | 45.45 ± 0.19 | 48.6 ± 0.15 | 58.61 ±0.29 |
| % Neutrophils | 16.20 ± 1.31 | 17.80 ± 1.61 | 17.20 ± 1.619 |
| % Lymphocytes | 80.90 ± 1.52 | 80.0 ±1.49 | 81.70 ±1.76 |
| % Monocytes | 2.60 ± 1.74 | 2.60 ±1.174 | 2.20 ± 1.22 |
| % Eosinophils | 2.00 ± 0.94 | 0.10 ± 0.326 | 0.10 ±0.316 |
| % Basophils | 0.10 ± 0.316 | 0.10 ±0.316 | 0.10 ± 0.316 |
| Total Bilirubin | 0.70 ± 0.14 | 0.09 ± 0.16 | 0.72 ± 0.10 |
| Alkaline Phosphatase | 78.0 ± 1.49 | 80.20 ± 1.31 | 81.80 ± 1.61 |
| Aspartate Aminotransferase | 89.0 ± 1.49 | 86.0 ± 1.49 | 89.0 ±1.49 |
| Alanine Aminotransferase | 38.0 ± 1.49 | 40.0 ± 1.49 | 40.0 ±1.37 |
| Blood Urea | 14.66 ± 0.30 | 15.26 ± 0.11 | 15.97 ± 0.25 |
| Serum Creatinine | 0.34 ± 0.14 | 0.33 ± 0.14 | 0.38 ± 0.015 |
| Albumin | 3.73 ± 0.12 | 3.55 ±0.15 | 4.14 ± 0.15 |
| Globulin | 1.88 ± 0.014 | 1.77 ± 0.10 | 1.86 ±0.15 |
| A/G Ratio | 2.10 ± 0.01 | 2.13 ± 0.12 | 2.14 ± 0.015 |
Effect of Repeated dose of CSPHF on Hematological and biochemical Values are expressed as mean ±SEM, P<0.05 when compared to control group CSPHF.
Table 8: Clevira syrup-Repeated dose toxicity blood analysis parameters Hematological and Biochemical analysis: Satellite group
| Blood Analysis | Satellite Control | Satellite Group (500mg/kg) |
| Platelets (103/ µL) | 707.8 ± 1.61 | 822.20 ± 1.619 |
| MCHC (g/dL) | 33.32 ± 0.18 | 34.38 ± 0.175 |
| MCH (pg) | 81.21 ± 0.87 | 18.88 ± 0.15 |
| Hemoglobin (g/dL) | 14.43 ± 0.20 | 16.40 ± 0.14 |
| Red blood cells (106/ µL) | 8.13 ± 0.14 | 8.99 ± 0.23 |
| White Blood cells (103/ µL) | 4.49 ± 0.14 | 7.40 ± 0.149 |
| MCV [fL(µm3)] | 45.45 ± 0.19 | 51.15 ± 16.18 |
| % Neutrophils | 16.20 ± 1.31 | 20.00 ± 1.49 |
| % Lymphocytes | 80.90 ± 1.52 | 79.00 ± 2.00 |
| % Monocytes | 2.60 ± 1.74 | 2.20 ± 1.033 |
| % Eosinophils | 2.00 ± 0.94 | 2.00 ± 0.94 |
| % Basophils | 0.10 ± 0.316 | 0.20 ± 0.422 |
| Total Bilirubin | 0.70 ± 0.14 | 0.18 ± 0.09 |
| Alkaline Phosphatase | 78.0 ± 1.49 | 80.30 ± 1.160 |
| Aspartate Aminotransferase | 89.0 ± 1.49 | 87.20 ±1.37 |
| Alanine Aminotransferase | 38.0 ± 1.49 | 39.10 ± 0.504 |
| Blood Urea | 14.66 ± 0.30 | 15.50 ± 0.14 |
| Serum Creatinine | 0.34 ± 0.14 | 0.38 ± 0.015 |
| Albumin | 3.73 ± 0.12 | 4.30 ± 0.156 |
| Globulin | 1.88 ± 0.014 | 1.87 ± 0.014 |
| A/G Ratio | 2.10 ± 0.01 | 2.42 ± 0.307 |
Effect of Repeated dose -satellite group of CSPHF on hematological and biochemical Values are expressed as mean ±SEM, P<0.05 when compared to control group CSPHF.
In case of utilization of polyherbal formulations we should be clear with some merits and demerits of the combined use of multiple components to demarcate the salient features of each component in the formulation. From the present compound CSPHF during the Acute Toxicity study period the animals in each group were observed for their activity and looked for signs of toxicity from Day 0 to Day 14 once in every 7 days. Every system was carefully examined and recorded on individual basis for each animal. In this group of animals there were none of the animals which showed signs of toxicity or had a moribund status during the acute toxicity study period.
At the end of the study after gross pathological examination there was no changes noted in any of the groups. Hence from the data obtained the CSPHF was evident to be non-toxic up to single high oral dose of 2000mg/kg body weight without any mortality. Acute toxicity studies showing toxicity signs and mortality for a polyherbal formulation in Wistar Albino rats were meager in the past. 19,20.Based on this data we further proceeded for Repeated dose toxicity study of CSPHF.
During the repeated dose toxicity study of CSPHF the animals from each group we monitored from day one of the study and the animals were observed for any external features of signs of toxicity. All systems were carefully examined and recorded on individual basis on every seventh day (Day 0, 7, 14, 21 and 28) for 28 days. During the study period at the dose of 250mg/kg and 500mg/kg, none of the animals showed signs of toxicity or ended up with moribund status. But to mention in specific at the dose of 1000mg/kg PO of CSPHF the animals started showing the signs of toxicity (Conjunctival and paw edema, distension of abdomen and hemoptysis, blood stained oral and rectal orifices, increased respiratory rate) from day 15 onwards and then mortality occurred within 27 days, for all the animals in this group with high dose of CSPHF (1000mg/kg).
At the end of the study, animals on examination for gross pathological changes after necropsy the changes noted in Group 1 (250mg/kg, PO, CSPHF) and Group 2 (500mg/kg, PO, CSPHF) were healthy without any signs of inflammation or any other signs of toxicity of the internal organs and normal orifices. But in animals from Group 3 (1000mg/kg, PO, CSPHF), the morbidity and mortality of animals were high even before completion of the study period with inflamed organs on gross pathological examination immediately after the animal mortality.
Conclusion
Hence CSPHF(Clevira Syrup Polyherbal formulation) was safe as a single dose at the maximum level (2000mg/kg, PO) in acute toxicity testing, whereas at repeated dosing of CSPHF the dosage of 500mg/kg and below was found to be safe without any signs of toxicity, morbidity and mortality. We hence plan to continue with chronic toxicity testing of CSPHF to provide more evidence for management with CSPHF without any further signs of toxicity or morbidity on further investigation of the formulation, so it can be useful in chronic viral infections like Hepatitis B, HPV etc, in near future.
Acknowlegement
We would like to thank Apex Laboratories Private Limited., Guindy, Chennai and for their support during this study.
Conflict of interest
There is no conflict of interest.
Funding Source
There is no Funding Source
References
- Santhosh B N, Pravin C D. Acute and sub-acute oral toxicity assessment of the polyherbal formulation in albino wistar rats: International Journal of Pharmacy and pharmaceutical sciences.2010;8(7):311-316.
- Mardi M, Algandaby. Assessment of acute and subacute toxic effects of the Saudi folk herb Retamaraetamin rats. J Chin Med Assoc 2015;78:691-701.
CrossRef - Kuruvila A. Herbal formulations as pharmacotherapeutic agents. Indian J. Exp. Biol.,40.7-11.
- Urooj R and Shahid S. Pharmacological activities of Carica papaya Journal of Basic & applied sciences, 2018;14:210-216.
CrossRef - Paul S D Y. Preliminary and pharmacological profile of Melia azedarachL :- An overview: Journal of applied pharmaceutical science,2013;3(12):133-138.
- Bharati B D, Sharma P K, Kumar N, Dudhe R and Bansal V. Pharmacological activity of Andrographis paniculata:-A Brief review; Pharmacology online, 2011;2:1-10.
- Bhushan B, Kumar S S, Tanuja S, Lalit S, Hema A. Vetiveriazizanioides(Linn). NASH: A Pharmacological overview; Int. Res. J. Pharm ,2013;4(7):18-20.
CrossRef - Shah B N, Seth A K. Pharmacological potential of Trichosanthesdioica– An edible Plant. Hygeia. J D. Med. 2010;2(2):1-7
- Sivapalan S R. Medicinal uses & pharmacological activities of Cyperusrotundus– A review. International Journal of scientific and Research Publications, 2013;3(5):1-7.
- Mishra R K, Kumar A& Kumar A. Pharmacological activity of Zingiber officinale. International Journal of pharmaceutical & chemical sciences, 2012;1(3):1073-78.
- Srivastava A K, Singh v K. Biological action of Piper nigrum the king of spices. European Journal of biological research. 2017; 7(3):223-33.
- Aglin A A. Medicinal effects of Mollugocerviana– A Review. International Journal of Scientific Research in multidisciplinary studies,2018;4(9): 34-37.
- Tiwari P, Nayak P, Prusty S K. Phytochemistry and Pharmacology of Tinosporacordifolia: A Review.Sys Rev Pharm,2018;9(1):70-78.
CrossRef - In vitro cytotoxic & antiviral study of clevira; JSS academy of higher education & research.
- OECD (2000) Guidance Document on Acute Oral Toxicity. Environmental Health and Safety Monograph Series on Testing and Assessment No 24.
- Schlede E., Mischke U., Diener W. and Kayser D. (1994). The International Validation Study of the Acute-Toxic-Class Method (Oral). Arch. Toxicol. 69, 659-670.
CrossRef - (2006). Report of the Validation of the Updated Test Guideline 407: Repeat Dose 28-day Oral Toxicity Study in Laboratory Rats. Series on Testing and Assessment No 59, ENV/JM/MONO(2006)26.
CrossRef - OECD(2008). Guidance for the testing of chemicals.Repeated Dose 28-Day Oral Toxicity Study in Rodents. 407.
CrossRef - Jain P, Pandey R, Shukla S S. Acute and Sub-Acute toxicity studies of polyherbal formulation of TalisadyaChurna in Experimental animal model. Mintage Journals of pharmaceutical and Medical Sciences,2015;1(1):7-10.
- Anandh SJV, Kumarappan M, Shanmuganathan P, Vinayagam S, Iyyankannu, Narayamurthy U. Antidiabetic activity of Manomani Chooranam aqueous extract on female wistar albino rats.Int J Basic Clin Pharmacol, 2019;8:2153-7.
CrossRef