Manuscript accepted on :24-05-21
Published online on: 02-06-2021
Plagiarism Check: Yes
Reviewed by: Dr. Ana Golez
Second Review by: Dr. Salman Ahmed
Final Approval by: Dr. Omar M. E. Abdel-Salam
Pulat B. Usmanov1., Inoyat Z. Jumayev1*., Shavkat Yu. Rustamov1., Abdisalim A. Zaripov1., Adilbay T. Esimbetov., Sherzod N. Zhurakulov2 and Valentina I. Vinogradova2.
1Institute of Biophysics and Biochemistry of National University of Uzbekistan, Tashkent, Uzbekistan.
2Institute of the Chemistry of Plant Substances, Uzbek Academy of Sciences, Tashkent, Uzbekistan.
Corresponding Author E-mail : inoyat8585@mail.ru
DOI : https://dx.doi.org/10.13005/bpj/2167
Abstract
This study investigated the positive inotropic andvasorelaxant activity ofDHQ-11, aconjugate of flavonoid dihydroquercetin with isoquinoline alkaloid 1-aryl-6,7-dimethoxy-1,2.3,4-tetrahydroisoquinoline.A study was performed using anterior papillary muscle removed from the left ventricle and thoracic aorta dissected from rats. DHQ-11 produceda concentration-dependent positive inotropic effect which was more potent than their parent compounds alone. The positive inotropic effect of conjugate DHQ-11was significantly attenuated by the β-adrenoreceptor inhibitor propranolol and L-type Ca2+ channel blocker nifedipine. Also,conjugate DHQ-11 markedly potentiated first post-rest responses indicating that it can modulate Ca2+ loading/release processes in the sarcoplasmic reticulum.These results suggest that positive inotropic effect produced by conjugate DHQ-11may be mediated through activation oftheβ-AR/AC/cAMP/PKA pathway that leads to increased Ca2+ influx and rises in Ca2+ loading/release in the SR, resulting in increased [Ca2+]i and enhanced contraction force. DHQ-11 significantly relaxed both high KCl- and phenylephrine-induced contractions of rat aortic rings whichwere significantly inhibited by lowering extracellular Ca2+ concentration and in the presence of verapamil.DHQ-11 significantly inhibited phenylephrine-induced contractions in a Ca2+-free medium, in the presence of verapamil. The vasorelaxant effect of the DHQ-11 was significantly reduced by the removal of endothelium and in the presenceof L-NAME and methylene blue as well as glibenclamide and TEA.These results suggest that the vasorelaxation produced by conjugate DHQ-11may be mediatedbyan endothelium-independent mechanism involving activation of KATP and BKCa channels and inhibition of L-type VDCCs and Ca2+ release from the sarcoplasmic reticulum and endothelium-dependent mechanism through activation of the NO/sGC/cGMP/PKG signaling pathway resulting in a decrease of intracellular Ca2+levels. These observations reveal that the conjugate DHQ-11 due to its high positive inotropic and vasorelaxant activity could be a promising compound for the design and development of new drugs for the treatment of heart failure.
Keywords
Alkaloids; Aorta; Calcium Channel; Endothelium; Nitric Oxide; Positive Inotropic Effect; Papillary Muscle; Vasorelaxation
Download this article as:| Copy the following to cite this article: Usmanov V. B, Jumayev I. Z, Rustamov S. Y, Zaripov A. A, Esimbetov A. T, Zhurakulov S. N, Vinogradova V. I.The Combined Inotropic and Vasorelaxant Effect of DHQ-11, A Conjugate of Flavonoid Dihydroquercetin with Isoquinoline Alkaloid 1-Aryl-6,7-Dimethoxy-1,2.3,4-Tetrahydroisoquinoline. Biomed Pharmacol J 2021;14(2). |
| Copy the following to cite this URL: Usmanov V. B, Jumayev I. Z, Rustamov S. Y, Zaripov A. A, Esimbetov A. T, Zhurakulov S. N, Vinogradova V. I.The Combined Inotropic and Vasorelaxant Effect of DHQ-11, A Conjugate of Flavonoid Dihydroquercetin with Isoquinoline Alkaloid 1-Aryl-6,7-Dimethoxy-1,2.3,4-Tetrahydroisoquinoline. Biomed Pharmacol J 2021;14(2). Available from: https://bit.ly/3pgIo47 |
Introduction
Heart failure (HF)is a major health problem worldwide which is the leading cause of death in many developed countries [1]. HFis a common condition in which the heart cannot pump enough blood to meet the body’s needs due to loss in cardiac contractility and ejection fraction [2]. The major pathophysiologic mechanisms leading to HF include increased hemodynamic overload, ischemia-related dysfunction, increased oxidative stress, reduced energy utilization, disrupted Ca2+ homeostasis,and reduced contractility of cardiac muscle [3]. Current pharmacological approaches for treating HF are still based on using renin-angiotensin-aldosterone system inhibitors (ACEIs), beta-blockers, positive inotropes, and vasodilators which by modulation of hemodynamics, cardiac output, and ventricular filling pressures, improve contractile performance of hearts[4]. ACEIs act by prevention the conversion of angiotensin I to angiotensin II, and inhibition of angiotensin II receptor which leads to lower blood pressure, reduced after load, and improved cardiac output [5]. The effect of beta-blockers is related to inhibition of the AMP/PKA-dependent pathway which leads to lower blood pressure, slowed heart rate, and reduced force of heart contraction [6].The most commonly used positive inotropes are β1– and β2-adrenergic receptor agonists which by activation of L-type Ca2+ channelsand Ca2+ release from the sarcoplasmic reticulum (SR) restore Ca2+ homeostasis thus improve the function of failing cardiomyocytes and cardiac contractility [7]. Other positiveinotropes that extensively used in HFare cardiac glycosides which by inhibition of Na/K-ATPase and increasing intracellular Ca2+ concentration in cardiomyocytesenhance cardiac contractility and improvecardiac output [8]. Vasodilators by dilation of arterial vessels and reducing arterial pressure decrease the left ventricular afterload and thus enhances stroke volume and increase cardiac output, while venous dilators which reduce venous pressure, decrease preload and cardiac output [9].
However, despite the benefits of the drugs commonly used for the treatment of HF almost all of them are far from ideal because they cannot completely correct underlying abnormalities implicated in its pathogenesis and have adverse effects.The most common side effect of these drugs is; reduced kidney function, hyperkalemia, reduced blood pressure, increased metabolic demand, slowing the heart rate and rhythm disturbances, that limit their usefulness [10,11,12 ]. Therefore, current strategies for the treatment of HF focuses on the development of a new generation of effective drugs that would avoid undesirable side effects and act through novel mechanisms involving potential therapeutic targets contributing to the progression of the disease. Recent advances in understanding the pathophysiology of HF have provided insights into novel pathways and molecular sites as promising therapeutic targets to improve the treatment efficacy of HF. According to numerous experimental evidence, the most effective therapeutic strategy in HF is to improve blood supply by dilation of the coronary artery through the restoration of altered endothelial function and bioavailability of nitric oxide (NO), and enhancing contractility of cardiac muscle bycorrection of impaired Ca2+signaling and Ca2+ homeostasis in cardiomyocytes[13,14]. In the last decade, natural compounds like plant flavonoids and alkaloids due to their high bioavailability and low cytotoxicityare recognized as the most efficient candidates for the development of novel approaches to treatment HF. There is growing evidence, that plant flavonoids and alkaloids displays antioxidant, antihypertensive, antiarrhythmic effects and protect the heart from ischemia-reperfusion injury mediated through several different mechanisms including free-radical scavenging, vasorelaxant and inotropic activities [15, 16].
Nowadays, one of the promising approaches for the rational design of novel drugs for the treatment of HF is molecular hybridization based on the combination of compounds with distinct pharmacologic activities in one moleculeto produce a new hybrid compound with improved affinity and efficacy [17].Recently, using a hybridization technique and Mannich reactions, a conjugate of flavonoid dihydroquercetin with isoquinoline alkaloid 1-aryl-6,7-dimethoxy-1,2.3,4-tetrahydroisoquinoline DHQ-11, both exhibiting potentvasodilatory and positive inotropic activity, respectively,was synthesized [18, 19].
In the present study, we aim to evaluate how the hybridization of flavonoid dihydroquercetin with isoquinoline alkaloid 1-aryl-6,7-dimethoxy-1,2.3,4-tetrahydroisoquinoline (F18) affects their vasorelaxant and inotropic activity. This study would provide evidence for the use of DHQ-11 in the treatment of HF.
Materials and Methods
All experimental protocols and conditions for preoperative care were approved by the animal use committee of our institution. Adult male Wistarrats weighing 200 – 250 g were anesthetized with sodium pentobarbital and their hearts were removed rapidlyand placed in oxygenated Kreb’s solution.The left ventricle was opened and an anterior papillary muscle was removed. The papillary muscles about 0,5-0,8 mm in diameter and 1-3 mm in length were mounted in an organ bath (STEIRT, HSE,Germany) between a fixed hook and an isometric tension transducer(Type F30, HSE). The muscle was super fused with oxygenated (gas mixture 95% oxygen, 5% carbon dioxide; pH 7.4) Krebs solution contained (in mmol/l);NaCl, 118; KCl, 4.7; MgSO4 , 1.2; KH2PO4, 1.2; glucose 5.8; NaHCO3, 24; CaCl2, 2.54)maintained at 370 C by a thermostat controlled water bath. The muscle was stretched to a length at which maximum developed force was evoked and allowed to equilibrate for at least 1 h before the commencement of the experiments. The muscle was stimulated at a rate of 0,1-5 Hz through the platinum field electrodes with rectangular pulses of 5ms duration at twice the threshold voltage. The amplitudes of elicited maximal isometric contraction were used as the control (100%) and changes in the contraction after drug action was expressed as a percentage of the maximal response. Contractions were recorded on a chart recorder (TZ 4620, Chech Rep.) and after digitalization stored on an online computer. To characterize the mechanism of inotropic action of conjugate DHQ-11its effect on contractile responses of papillary muscles at the various experimental conditions were investigated. To examine the effects of conjugate DHQ-11 on calcium homeostasis its effect on the cumulative dose-response curve of Ca2+was studied. In these experiments, the papillary muscle was washed three times in a Ca2+ free Krebs solution containing 2.5 mM EGTA. To further clarify the possible involvement of the Ca2+ channels and ß-adrenoreceptor in the inotropic action of the conjugate DHQ-11 its effect in the presence of their blockers nifedipine and propranolol, respectively, were studied. To test the effect of the conjugate DHQ-11 on loading and release functions of sarcoplasmic reticulum (SR) itseffect on post-rest potentiation was examined. The post-restpotentiation of contraction was studied at an [Ca2+]o of 0.5 mM, after a rest period of 30 s and at a stimulation frequency of 1Hz.
The vasorelaxant effect of conjugate DHQ-11 was evaluated using the thoracic aorta, dissected from the rat.The isolated aorta was immediately placed in Krebs solution contained (in mmol/l); 118 mMNaCl, 5 mMKCl, 25 mM NaHCO3, 1.2 mM MgSO4, 2 mM CaCl2, 1.2 mM KH2 PO4, 11 mm glucose. The aorta was cleaned of adipose and connective tissue and cut into rings (2–3 mm long). The rings were mounted using two stainless hooks with one fixed to the bottom of the organ bath and the other connected to a force transducer. The organ bath was superfused with Krebs solution, bubbled with a 95% O2-5% CO2 gas mixture, maintained at 37°C. The aortic rings were equilibrated in Krebs solution under the tension of 1 g, for 60 min during which period the Krebs solution was replaced at least twice. Aortic ring contraction was recorded, isometrically using a force transducer (FT-03; Grass Instrument Company, USA) connected to a chart recorder Endim 621-02 ( Germany). The rings were contracted with 1 μM of phenylephrine (PE) or 50 mm KCl and allowed to plateau before the addition of tested drugs. After the contraction had reached a stable plateau, conjugate DHQ-11 was added cumulatively. The vasorelaxant effect of conjugate DHQ-11 was expressed as a percentage relaxation of the pre-contraction induced by PE orKCl(50mM). To assess the effect of conjugate DHQ-11on extracellular Ca2+ influx, concentration–response curves to CaCl2 were tested using Ca2+-free Krebs solution with 100 mM EGTA and high-K+ (50 mM), prepared by replacing an equimolar concentration of NaCl with KCl. To investigate the role of Ca2+ released from intracellular stores in the vasorelaxant action of conjugate DHQ-11, its effectson aortic rings contraction induced by 1 μM PE in Ca2+-free Krebs solution contained 50 mM EGTA, were studied. To examine the participation of the K+ channelsin the vasorelaxant action of conjugate DHQ-11,its effectsin presence of the 4-aminopyridine (4-AP), a specific blocker of voltage-dependent Kv channels, tetraethylammonium (TEA), a nonspecific blocker of thecalcium-activated large conductance BKCachannels, BaCl2 , a specific blocker of the inward rectifying KIR channels, and glibenclamide, a specific inhibitor of ATP-sensitive KATPchannels were studied. To determine the involvement of endothelium in vasorelaxant action of conjugate DHQ-11 the endothelium was removed from ring specimens by rubbing the intimal surface with a cotton ball and the absence of ACh-induced relaxation was taken as an indicator of successful denudation. To further clarify the role of the endothelium in the vasorelaxantaction of conjugate DHQ-11 its effects on PE-induced contraction of endothelium-intact aortic rings preincubated with L- NAME (nitro-L-arginine methyl ester, a NO synthase inhibitor) and methylene blue (a guanylyl cyclase inhibitor) were studied.
Drugs and reagents
All chemicals were of analytical grade commercially available. Nifedipine, verapamil, phenylephrine, propranolol, L-NAME, and methylene blue wereobtained from Sigma Ltd Co., (St. Louis, MO, USA). Conjugate DHQ-11 was synthesized at the Institute of Chemistry of Plant Compounds of Uzbek Academy of Sciences and was kindly provided by V.I. Vinogradova.
Data and statistical analysis
Throughout this article, alldata are represented as the mean±standard error of the mean (s.e.m.) of n observations. Statistical analysis was performed using an unpaired Student’s t-test. The EC50and IC50 values, the concentration of drugs causing a 50% contraction or relaxation of the maximal response (EMax), were obtained from the concentration-response curveand calculated using the sigmoidal curve fitting routine in Origin 6.0 (Microcal, Northampton, MA, U.S.A.). The differences between control and experimental values were considered significant at p < 0.05.
Results and Discussion
The positive inotropic effect of the conjugate DHQ-11.
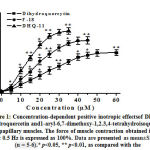
In the isometric tension recordings in isolated rat papillary muscle, it was found that the conjugate DHQ-11 exerted a pronounced positive inotropic effect (PIE) in a concentration-dependent manner. In these studies, the application of 35 µM of conjugate DHQ-11 caused a maximal increase in the contractile force of rat papillary muscle to 77.4±4.2% from the baseline value set as 100% (Fig.1).Under the same experimental conditions, the flavonoid dihydroquercetin and alkaloid 1-aryl-6,7-dimethoxy-1,2.3,4-tetrahydroisoquinoline maximally increased the force of contraction by 51.4±3.9% and 65.6±4.4% from the baseline value. EC50 values(the concentration of compounds causing 50% of the maximum effect) obtained from these results were 21.2±4.1 μM, 14.6±3.5 μM, and 9.7±4.3 μM for dihydroquercetin, alkaloid 1-aryl-6,7-dimethoxy-1,2.3,4-tetrahydroisoquinolineand conjugate DHQ-11, respectively. These data indicate that the conjugate DHQ-11 has a more strong PIE compared to flavonoid dihydroquercetin and alkaloid 1-aryl-6,7-dimethoxy-1,2.3,4-tetrahydroisoquinoline. The most commonly used positive inotropes enhanced cardiac contractility through the activation of different mechanism resulted mainly in increased intracellular Са2+ level ([Са2+]i) in cardiomyocytes [19].The increase in[Са2+]imainly may be mediated by Са2+ influx through voltage-dependent L-type Са2+channels (VDCCs) and Са2+ release from the sarcoplasmic reticulum (SR) via ryanodine receptors (RyR2) which are activated by stimulation of β-adrenergic receptors[20].
The stimulation of β-adrenergic receptors leads to activation of adenylyl cyclase (AC) and increased cAMP which by activation of protein kinase A (PKA), leads to the phosphorylation ofthe L-type VDCCsand RyR2 resulting in the elevation in [Са2+]I thereby allowing forceful contraction[21].
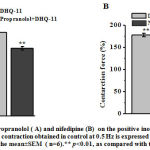
To determine the possible involvement of theβ-AR/AC/cAMP/PKA signaling pathway in the PIE of conjugate DHQ-11 its effect in the presence of propranolol, a β-adrenoreceptor blocker was examined. In these experiments, it was found that after pretreatment of papillary muscle with 10 μM propranolol the PIE of conjugate DHQ-11(35 μM) reduced from 77.4±4.2% to 43.6±3.8% (Fig. 2, A). These results indicate that the PIE of conjugate DHQ-11 possible is due to the activation of the β-AR/AC/cAMP/PKA signaling pathway, which may be accompanied by an increase in the influx of Ca2+ ions into cardiomyocytes through VDCCs. To confirm this further the experiments with nifedipine, a specific blocker of Са2+L-channels were performed. As can be seen from Fig. 2, B application of conjugate DHQ-11 (35 μM) on the background of 0.01 μM nifedipine, the concentration corresponding to its IC50 value, increased the force of contraction by 26.8±3.4% compared to a 77.4±4.2% observed in the absence of nifedipine. These data suggest that PIE of conjugate DHQ-11 possible is mediated through activation of theβ-AR/AC/cAMP/PKA signaling pathway and subsequent enhancing Ca2+ influx into cardiomyocytes through VDCCs. However, our observation that PIE of conjugate DHQ-11 was partially preserved in the presence of nifedipine suggested that its PIE may be mediated not only by enhancing Ca2+ influx via L-type VDCCsbut other mechanisms may also be involved.
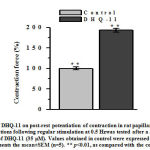
The key determinant of cardiac muscle contraction force is the Ca2+released from SRvia RyR2 mediated by calcium-induced release of calcium mechanism activated by Ca2+influx through L-type VDCCsin the sarcolemma [22].Therefore, to determine the possiblerole of Ca2+ released from SR in PIE produced by conjugateDHQ-11its effect on post-rest potentiation of the force of contraction which reflects the amount of Ca2+ accumulated within and released from the SR was studied [23]. The results obtained in these experiments showed that in the presence of conjugate DHQ-11 (35 μm), the first contraction after rest intervals( 30 s) increased from the control level taken as 100% by 93.1±3.8% (Fig. 3).
Post-rest potentiationof contractions following regular stimulation at 0.5 Hzwas tested after a 30 s rest period before and after the addition of DHQ-11 (35 μM). Values obtained in control were expressed as 100%.Each column represents the mean±SEM (n=5). ** p<0.01, as compared with the control.
These results indicate that treatment of papillary muscle with conjugate DHQ-11 caused an additional accumulation of Ca2+ in the SR that lead to an increased Ca2+ release from the SR resulted in enhanced contraction force. These findings suggest that conjugate DHQ-11 can activate not only Ca2+ influx via VDCCs but also modulate Ca2+ loading/release processes in SR. This suggestion was supported by fact that nifedipine inhibits PIE produced by conjugate DHQ-11(Fig.2), presumably through suppression of Ca2+influx via L-type VDCCs which play important role in refilling Ca2+ store [20]. The data obtained in these study suggest that PIA produced by conjugateDHQ-11may be mediated through activation ofβ-AR/AC/cAMP/PKA signaling pathway that leads to increased Ca2+ influx and rises in Ca2+loading/release in the SR, which results in increased [Ca2+]i and enhanced contraction force.
The vasorelaxant effect of the conjugate DHQ-11.
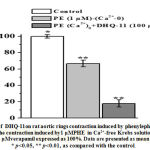
In rat aortic rings precontracted with high KCl (50 mM) and phenylephrine (PE), the conjugate DHQ-11produced a significant vasorelaxant effect in a concentration-dependent manner. In these experiments, the conjugate DHQ-11maximally reduced the KCl-induced contraction by94.7±3.2%, of control,at a concentration of 100 μM (Fig 4, A). At the same concentration (100 μM) the conjugate DHQ-11causedthe maximal relaxant effect up to 90.3±3.4%in rat aortic rings precontracted with PHE (1 µM) ( Fig 4, B). Under similar experimental conditions, the flavonoid dihydroquercetin and alkaloid1-aryl-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline maximally reduced the KCL-induced contraction by 81.1±3.8% and 89.5±3.1%, as well as, PE-induced contraction by 83.6±3.7% and 75.2±3.2% respectively (Fig.4, A, B).
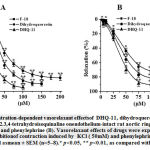 |
Figure 4: Concentration-dependent vasorelaxant effectsof DHQ-11, dihydroquercetin and 1-aryl-6,7-dimethoxy-1,2.3,4-tetrahydroisoquinaline onendothelium-intact rat aortic rings precontracted with KCl (A) and phenylephrine (B). |
The IC50 values(the concentration to produce a 50% maximal relaxant effect) for DHQ-11,dihydroquercetin, and alkaloid 1-aryl-6,7-dimethoxy-1,2.3,4-tetrahydroisoquinoline were 23.7 μM, 30.9 μM and, 41.6 μM, respectively. These data indicated that tested compounds produced a significant vasorelaxant effect and that the vasorelaxant potency of the conjugate DHQ-11 was markedly greater than that of flavonoid dihydroquercetin and alkaloid1-aryl-6,7-dimethoxy-1,2.3,4-tetrahydroisoquinoline.
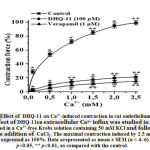
The contractility of smooth muscle cells (SMC)isdependent on [Ca2+]iwhich is mainly regulated by Ca2+influx from the extracellular space through L-type VDCCs and by Ca2+ release from the SR [24].KCl-induced contraction of SMC is mainly related to the extracellular Ca2+ influx through L-type VDCCs activated by the SMC membrane depolarization[25]. Therefore, to evaluate the role of the L-type VDCCs in the vasorelaxant action of conjugate DHQ-11 its effects on aortic rings contractions induced by the cumulative addition of Ca2+ in Ca2+-freeKrebs solution containing 50 mM KCl were studied. Fig.5 shows that preincubation of the aortic ring with conjugateDHQ-11 (100 μM), reduced the contractile response to 2.5 mM CaCl2 by 78.5±32% of the control obtained in a normal Krebs solution containing Ca2+.
These results suggest that the vasorelaxant effect of conjugate DHQ-11 possible is related to inhibition of the Ca2+ influx via the L-type VDCCs. This is confirmed in experiments withL-type VDCCs blocker verapamil that have shown that despite the great difference between the used concentrations,the percentage of inhibition ofCa2+ -induced contractionsproduced by verapamil was almost similar to that produced by conjugate DHQ-11 (data not shown). These results support the notion that the vasorelaxant effect of conjugate DHQ-11 is associated with the blockage of Ca2+ influx through L-type VDCCs.
In contrast to KCl, phenylephrine (PE), an α-adrenergic agonist, induced SMC contraction mainly by releasing intracellular Ca2+ from the SR via activation of inositol-1,4,5-trisphosphate (IP3) receptors,To investigate the involvement of Ca2+ released from SR in the vasorelaxant action of conjugate DHQ-11its effect on the PE-induced contractions of aortic rigs in Ca2+-free Krebs solutions containing 100 mM EGTA (100 mM) and verapamil (1 μM)was examined. As shown in Fig.6 in the presence of conjugate DHQ-11 (100 μM) the PE-induced (1 μM) contraction of aortic rings in Ca2+-free Krebs solution reduced from control level 66.7±4.2% to 17.9±4.3%. These results indicate that this effect of conjugate DHQ-11 is due to inhibition ofCa2+ release from SR through IP3R, suggesting that this mechanism could be involved in its vasorelaxant action.
A critical role in the regulation of smooth muscle contractility plays a variety of the K -channels, which as the dominant ionic conductance in the regulation of the membrane potential contribute to the modulation of L-type VDCCs activity and regulation of [Ca2+]i, as well as the Ca2+ release/loading in SR [ 26]. To evaluate the participation of K+ channels in the vasorelaxant action of conjugate DHQ-11, its effect in the presence of the K+ channels blockers 4-AP, TEA, BaCl2, and glibenclamide were studied. In these experiments, the endothelium-intact aortic rings were precontracted with KCl 20 mM to depolarize the SMC membrane and enhance K-channel activity.The results present in Table 1 show that the vasorelaxant effect of conjugate DHQ-11 was significantly attenuated by glibenclamide and TEA and partially by 4-AP. The greater potency of glibenclamide and TEA to inhibit the effect of conjugate DHQ-11 suggest that KATP and BKCachannels involved in its vasorelaxant action indicating that this effect ofconjugate DHQ-11 may be related to the activation of these channels.
Table 1: The participation of K-channels invasorelaxation induced by DHQ-11.
|
K–channelblockers |
Vasorelaxation% | |
| Emax | IC50 (µМ) | |
| Control | 97.7±1.3%** | 11.7 |
| Glibenclamide(50 µM) | 38.7±3.5%* | 19.1 |
| TEA (1 mМ) | 59.3±3.5%* | 17.2 |
| 4-АP (1 mМ) | 77.6±3.9%** | 14.4 |
| BaCl2 (0,1 mМ) | 90.9±3.4%** | 12.3 |
The results are expressed as the percentage vasorelaxation of rat aortic rings contraction
induced by 20 mM KCl.Data arepresented as mean ± SEM (*p<0,05, **p<0,01; n=5-6).
A key role in the control of vascular tone plays endothelial cells through the production of a variety of relaxing and constricting factors thatmodulate the smooth muscle cells reactivity [27].The nitric oxide (NO) produced from its precursor L-arginine by endothelial NO synthase (eNOS)i, is one of the major relaxing factors responsible forthe endothelium-dependent dilatation in various vasculature [28].
To assess the role of the endothelium in the vasorelaxation produced by conjugate DHQ-11, its effects on aortic preparations with the removed endothelial layer were studied.In this study, was found that the removal of the endothelium significantly blunted the vasorelaxant effect of conjugate DHQ-11. As illustrated in Table 2 the vasorelaxant effect of conjugate DHQ-11 (100 μM) in aortic rings precontracted with PE (1 μΜ) significantly decreased from 90.3±3.4% to 60.5±3.1% after removal of the endothelium. The IC50 values for conjugateDHQ-11 obtained in aortic rings with and without endothelium were 23.7 μM and 38.2 μM, respectively. These results showed that there was a significant difference in the vasorelaxant potency of conjugate DHQ-11 in the aortic rings with and without endothelium indicating that the vasorelaxant effect of conjugate DHQ-11 is endothelium-dependent and may involve the NO/sGC/cGMP/PKG pathway.
To further examine the role of NO/sGC/cGMP/PKG pathway in the vasorelaxant action of conjugate DHQ-11 its effect on PE-induced contraction of endothelium-intact aortic rings preincubated with NOS inhibitor L-NAME and guanylate cyclase inhibitor methylene blue, as well as with indomethacin, a cyclooxygenase inhibitor were studied. The results presented in Table 2 showed that pretreatment of the intact aortic ring with L-NAME (100 μM), significantly reduced the vasorelaxant effect of conjugateDHQ-11 from 90.3±3.4% to 60.5±3.9%.Similarly, the pretreatment of the intact aortic ring with methylene blue (10 μM) reduced the vasorelaxant effect of conjugateDHQ-11 to 69.8±3.3% of control (Table 2).
Table 2: The involvement of endothelium invasorelaxation induced by DHQ-11.
| Treatment | Vasorelaxation % | |
| Emax | IC50 (µМ) | |
| Endotheliun(+) | 90.3±3.4** | 23.7 |
| Endothelium(-) | 60.5±3.9** | 28.7 |
| L-NAME (100 µM) | 65.8±3.2* | 26.9 |
| Мethylene blue (10 µM) | 69.8±3.3** | 26.5 |
| Indomethacin (10 µM) | 86.4±3.3* | 24.9 |
The results are expressed as the percentage vasorelaxation of rat aortic rings contraction induced by 1 μΜ phenylephrine. Data are presented as mean ± SEM (*p<0,05, **p<0,01; n=5-6).
From these data is evident that treatment of the aortic rings with L-NAME and methylene blue attenuated the vasorelaxant activity of conjugate DHQ-11 to a comparable extent as did by the mechanical removal of the endothelium. In contrast, pretreatment of the aortic rings with indomethacin did not affect the vasorelaxant activity of conjugate DHQ-11. These results indicate that the activation of NO/sGC/cGMP/PKG pathway but not the prostaglandin signaling pathwayis involved invasorelaxation induced by conjugate DHQ-11. These results revealed that conjugate DHQ-11 may exert a marked vasorelaxant effect mediated by endothelium-dependent and -independent mechanisms. Taken togetherobtained results suggested that the endothelium-dependent mechanism is likely involved NO/sGC/cGMP/PKG pathway, while blockage of the L-type VDCCs and inhibiting intra cellular Ca2+ release as well as activating KATP and BKCa channels, might contribute to the endothelium-independent mechanism.
Conclusion
We have been demonstrated that DHQ-11, a conjugate of flavonoid dihydroquercetin with is oquinoline alkaloid 1-aryl-6,7-dimethoxy-1,2.3,4-tetrahydroisoquinoline, exertpronounced positive inotropic and vasorelaxant effects mediated by multiple mechanisms. In the rat papillary muscle, the conjugate DHQ-11 increased the force of contraction in a concentration-dependent manner. The blockage ofβ-adrenoreceptor and L-type VDCCs with propranolol and nifedipine, respectively, significantly attenuated the conjugate DHQ-11-induced positive inotropic effect suggesting that it may activate the β-AR/AC/cAMP/PKA pathway and thus enhance the Ca2+ influx in cardiomyocytes through L-type VDCCs. Also, the conjugate DHQ-11 significantly increased the first contraction after rest intervals indicating that it enhanced the post-rest potentiation of force contraction.These findings suggest that the conjugate DHQ-11maycause an additional accumulation of Ca2+ in the SR that leads to an increased Ca2+ release from the SR resulted in enhanced contraction force. This suggestion was supported by fact that nifedipine inhibits the positive inotropic effect produced by conjugate DHQ-11, presumably through suppression of Ca2+influx via L-type VDCCs which play important role in refilling Ca2+ store. These results demonstrate that positive inotropic effect produced by conjugate DHQ-11 mediated through activation ofβ-AR/AC/cAMP/PKA signaling pathway that leads to increased Ca2+ influx and rises in Ca2+ loading/release in the SR, resulting in increased [Ca2+]I and enhanced contraction force.
The conjugate DHQ-11 significantly relaxed both high KCl- and phenylephrine-induced contractions of rat aortic rings in a concentration-dependent manner. The vasorelaxant effect of the conjugate DHQ-11was significantly reduced by lowering extracellular Ca2+ concentration in both high KCl- and phenylephrine and the presence of verapamil. Also, the conjugate DHQ-11 significantly inhibited phenylephrine-induced contractions in a Ca2+-free medium, indicating inhibition of Ca2+ release from the sarcoplasmic reticulum (SR). At the same time, the vasorelaxant effect of conjugate DHQ-11 was more potent in aortic rings precontracted with 20 mM KCl- than 50 mM KCl and significantly attenuated by glibenclamide and TEA. Furthermore, the vasorelaxant effect of the conjugate DHQ-11 was significantly reduced by the removal of endothelium and in the presence of L-NAME and methylene blue, an NO synthase and guanylate cyclase inhibitors.. indicating that it is endothelium-dependent and related to the stimulation of the NO/sGC/cGMP/PKG signaling pathway. These results suggest that the vasorelaxation produced by conjugate DHQ-11 may be mediatedbyan endothelium-independent mechanism involving activation of KATP and BKCachannels and inhibition of L-type VDCCs and Ca2+ release from the sarcoplasmic reticulum and endothelium-dependentmechanism through activation of the NO/sGC/cGMP/PKG signaling pathway resulting in a decrease of intracellular Ca2+ levels.
These observations reveal that the conjugate DHQ-11 due to its high positive inotropic and vasorelaxant activity could be a promising compound for the design and development of new drugs for the treatment of heart failure.
Conflict of Interest
The authors have declared that no conflict of interest exists.
Acknowledgement.
This work was supported by a grant FA-F6-004 from the Coordinating Committee for Development of Science and Technology under the Cabinet of Ministers of the Republic of Uzbekistan.
References
- Roger V.L.Epidemiology of heart failure. Circ Res. 2013, vol.113, pp.646-59
CrossRef - Braunwald E, The war against heart failure: the Lancet lecture. The Lancet. 2015, 38, No 15. pp. 1838–1845
- Dassanayaka S., Jones, S.P. Recent developments in heart failure. Res.2015, vol. 117, e58–e63.
CrossRef - Massie B.M. Novel targets for the treatment of heart failure: perspectives from a heart failure clinician and trialist. J Mol Cell Cardiol. 2011,vol 51,pp. 438-440
CrossRef - Flather M.D., Yusuf S., Kober L., et al. ACE-Inhibitor myocardial infarction collaborative group. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: A Systematic overview of data from individual patients. Lancet. 2000, vol 355, pp.1575–1581
CrossRef - Bristow M.R. Treatment of chronic heart failure with beta-adrenergic receptor antagonists: a convergence of receptor pharmacology and clinical cardiology. Circ. Res.2011,vol 109, pp.1176–94
CrossRef - Petersen JW, Felker GM. Inotropes in the management of acute heart failure. Crit Care Med. 2008, vol 36, No 1, pp.106–111
CrossRef - Ambrosy P., Butler J., Ahmed A., Vaduganathan M., van Veldhuisen D.J., Colucci W. S., Gheorghiade M. The use of digoxin in patients with worsening chronic heart failure. Journal of the American College of Cardiology. 2014, vol. 63(18), pp. 1823–1832
CrossRef - Cole R.T., Kalogeropoulos A.P., Georgiopoulou V.V., et al. Hydralazine and isosorbide dinitrate in heart failure: historical perspective, mechanisms, and future directions. Circulation. 2011,vol. 123(21), pp. 2414–2422
CrossRef - Andersen M. J., and Borlaug B. A. Heart failure with preserved ejection fraction: current understanding and challenges. Cardiol. Rep. 2014,vol. 16, pp.501-507.
CrossRef - Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G., et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J. Heart Fail. 2016, vol 18(8), pp. 891-975.
- Yamamoto K. Pharmacological treatment of heart failure with preserved ejection fraction. Yonago Acta Med.2017, vol. 60, pp.71–76.
CrossRef - Gevaert A.B.,Lemmens K., Vrints K.J., Van Craenenbroeck E.M. Targeting endothelial function to treat heart failure with preserved ejection fraction: The promise of exercise training. Oxidative medicine and cellular longevity. 2017, vol. 2017, Article ID 4865756
CrossRef - Luo M., and Anderson M.E.Mechanisms of altered Ca2+handling in heart failure. Res. 2013, vol.113, pp.690–708
CrossRef - Engler M.M., and Engler M.B. The cardioprotective effects of flavonoids. Int. J. Cardiovasc. disease and diagnosis. 2020, vol. 5(2), pp. 042-046.
- Qian J.Q. Cardiovascular pharmacological effects of bisbenzylisoquinoline alkaloid derivatives. Acta Pharmacol. Sin. 2002, vol. 23 (12), pp. 1086-109
- Berube G. An overview of molecular hybrids in drug discovery.Expert opinion on drug discovery. 2016,vol 11, No3, pp. 281-305
CrossRef - Zhurakulov Sh.N., Babkin V.A., Chernyak E.I., Morosov S.V., Grigoev L.A., Levkovich M.G., V.Vinogradova V.I. Aminomethylation of 1-aryl-6,7-dimethoxy- 1,2,3,4-tetrahydroisoquinolines by dihydroquercetin. Chemistry of Natural Compounds. 2015. Vol.51, No.1, January
CrossRef - Inoyat Jumayev, Pulat Usmanov, Shavkat Rustamov, Sherzod Zhurakulov. Comparative inotropic effects of the some isoquinoline alkaloids. Biomedical & Pharmacology Journal,March 2020. India. Vol. 13(1), p. 325-333
CrossRef - Scholz H. Introduction: Mechanisms of positive inotropic effects. Basic Research In Cardiology. 1989, Vol. 84,No1, pp 3–7.
CrossRef - Bers D.Cardiac excitation-contraction coupling.Nature, 2002, Vol 10; No 415, PP.198-205.
CrossRef - Bers DM (2008) Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70:23–49
CrossRef - Bassani R. A., Altamirano J., Puglisi J. L., Bers D. M. Action potential duration determines sarcoplasmic reticulum Ca2+ reloading in mammalian ventricular myocytes. J. Physiol. (London). 2004, Vol 559. PP.591–607
CrossRef - Bers, D.M. 1985. Calcium influx and sarcoplasmic reticulum calcium release in cardiac muscle activation during post-rest recovery. Am. J. Physiol. 248: H366–H381.
CrossRef - Karaki H., Ozaki H., Hori M., Mitsui-Saito M., Amano K., Harada K, et al. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev. 1997, vol 49, PP.157–230.
- Cribbs LL. Vascular smooth muscle calcium channels. Circulation Research. 2001, Vol 89, PP. 560–562
CrossRef - Jackson WF. Potassium Channels in Regulation of Vascular Smooth Muscle Contraction and Growth.Adv Pharmacol. 2017, vol, 78, PP.89-144.
CrossRef - Furchgott R.F. Role of the endothelium in responses of vascular smooth muscle. Circ Res.1983, vol. 53, pp. 557–572.
CrossRef - Vanhoutte, P.M.; Zhao, Y.; Xu, A.; Leung, S.W.S. Thirty years of saying NO: Sources, fate, actions, and misfortunes of the endothelium-derived vasodilator mediator. Circ. Res. 2016, 119, 375–396.
CrossRef