Manuscript accepted on :08-06-2021
Published online on: 22-06-2021
Plagiarism Check: Yes
Reviewed by: Dr. Salman Ahmed Pharmacognosy
Second Review by: Dr. Mandal, Amritlal
Final Approval by: Dr. Francesca Gorini
Safinaz Ebrahim El-Toukhy1 , Omar M.E. Abdel-Salam2
, Omar M.E. Abdel-Salam2 , Amr M M Ibrahim1
, Amr M M Ibrahim1 and Eman R. Youness1*
and Eman R. Youness1*
1Department of Medical Biochemistry, National Research Centre, Cairo, Egypt.
2Department of Toxicology and Narcotics, National Research Centre, Cairo, Egypt
Corresponding Author E-mail: hoctober2000@yahoo.com
DOI : https://dx.doi.org//10.13005/bpj/2186
Abstract
We aimed to investigate the oxidative, inflammatory, angiogenic biomarkers and vitamin D status in serum of Egyptian patients with cancer breast. Seventy patients with known cancer breast (non-metastatic and metastatic) were evaluated and compared to and healthy women. We observed significant decreases in serum malondialdehyde, nitric oxide, total antioxidant capacity (TAC) and uric acid in patients with non-metastatic and metastatic cancer. Subjects with metastatic cancer exhibited significantly lower nitric oxide and TAC compared with non- metastatic cancer. Meanwhile, significant increases in serum VEGF, HGF, and MMP-9 occurred in both pre- and post-menopausal patients with either non-metastatic or metastatic cancer with significantly higher values in metastatic disease. Significant increase in serum TNF-α was observed with significantly higher values in metastatic disease. Serum 25 hydroxy vitamin D (VITD) decreased in both types of cancer with significantly lower values in pre-menopausal compared to post-menopausal patients. Pre-menopausal subjects showed significantly lower serum VITD level compared to their post-menopaual counterparts, but there were no differences between those who were –ve for PR receptor and +ve patients. These results suggest that vascular and inflammatory markers VEGF, HGF, MMP-9 and TNF-α increased in serum in advanced stages of breast cancer and could monitor disease progression and/or severity.
Keywords
Angiogensis; Breast Cancer; Inflammation; Oxidative Stress; Vitamin D
Download this article as:| Copy the following to cite this article: El-Toukhy S. E, Abdel-Salam O. M. E, Ibrahim A. M. M, Youness E. R. Oxidative, Inflammatory, Angiogenic Markers and Vitamin D Status in Pre- and Postmenopausal Breast Cancer Egyptian Patients. Biomed Pharmacol J 2020;14(2). |
| Copy the following to cite this URL: El-Toukhy S. E, Abdel-Salam O. M. E, Ibrahim A. M. M, Youness E. R. Oxidative, Inflammatory, Angiogenic Markers and Vitamin D Status in Pre- and Postmenopausal Breast Cancer Egyptian Patients. Biomed Pharmacol J 2020;14(2). Available from: https://bit.ly/3j2of0v |
Introduction
Breast cancer is the most common cause of cancer-related deaths in women particularly in industrialized nations, and it is a significant public health problem. It is the leading cause of cancer related death for women aged between 35 and 55 years worldwide 1. Distant metastases are the principal cause of death. An essential process in forming distant metastases is the degradation of the extracellular matrix allowing tumor cells to invade local tissue, intravasate and extravasate blood vessels and build new metastatic formations 2. This process is primarily influenced by the activity of proteinases secreted by the tumor. Currently, at least four classes of proteinases are known: serine proteinases, aspartatic proteinases, cystein proteinases and matrix metalloproteinases3. Collectively, these proteinases are capable of breaking down all components of the extracellular matrix. Under physiological conditions (e.g. tissue remodeling, angiogenesis, ovulation, wound healing) there is a precise regulation between proteolytic degradation and regulatory inhibition of proteolysis 4. This physiological balance seems to be disrupted in cancer. Matrix metalloproteinases (MMPs) are up regulated in almost every type of cancer and their expression is often associated with a poor prognosis for patients 5.
Methods have been developed to assess the expression or concentrations of certain angiogenic factors. Among these angiogenic factors, vascular endothelial growth factor (VEGF) has been studied most extensively and is probably the most essential factor for differentiation and development of the vascular system. VEGF is a highly specific and selective mitogen for vascular endothelial cells 6. It induces proliferation and migration of endothelial cells in vitro 7 while inhibiting apoptosis 8. In vivo, VEGF is necessary for vasculogenesis 9, promotes angiogenesis, and enhances vascular permeability 10. In several different experimental conditions, overexpression of VEGF was accompanied by marked tumor growth and neovascularization 11. On the other hand, therapeutic blockade of VEGF has been shown to inhibit primary and metastatic tumor growth in animal models 12,13.
Although significant improvements in therapy have occurred recently, most deaths from breast cancer are still caused by metastases that are resistant to conventional treatment. Therefore, novel approaches to the management of breast cancer need to be developed. VEGF have been implicated as the major angiogenic factor in human cancers. VEGF promotes angiogenesis and invasion and increases vascular permeability 14. MMP-2 and MMP-9 are related to tumor invasion and metastasis by their capacity for tissue remodeling via extracellular matrix as well as basement membrane degradation and induction of angiogenesis 15. Vitamin D has emerged as the most prolific topic in the last decade with work connecting it with risk reduction in various epithelial cancers. Vitamin D exerts a wide range of immunogenic and antiproliferative activities in the body 16.
This study aims to explore the inflammatory and angiogenic markers as well as to evaluate the oxidative and vitamin D status in Egyptian females with breast cancer in order to assess the prognostic significance of these markers.
Patients and Methods
Seventy consecutive patients with known cancer breast (non-metastatic and metastatic), with a mean age of 50.6 ± 1.04 years (range, 33.2 – 67 years) treated in the National Oncology Institute were studied after they had given informed consent. Thirty five patients had non-metastatic cancer. Seventeen patients were pre-menopausal (mean age 42.9 ± 1.01 years; range 35.6-49 years) and 18 were post-menopausal (mean age 58.4 ± 0.92 years; range 53.0-67 years). Thirty five patients had metastatic cancer. Seventeen patients were pre-menopausal (mean age 42.6 ± 1.18 years; range 33.2-49 years) and 18 were post-menopausal (mean age 56.6 ± 0.83 years; range 51.3-64 years). The control group comprised 17 pre-menopausal (mean age 42.3 ± 1.1 years; range 33.7-50 years) and 17 post-menopausal (mean age 56.4 ± 1.03 years; range 50.3-66.1 years) healthy women.
Biochemical analyses
Determination of lipid peroxides
Malondialdehyde was determined by measuring thiobarbituric reactive species using the method of Ruiz-Larrea et al. 1994 in which the thiobarbituric acid reactive sub-stances react with thiobarbituric acid to produce a red colored complex having peak absorbance at 532 nm 17.
Determination of nitric oxide metabolites
Nitric oxide was determined in serum according to the method of Miranda 18. The level of total nitrite/nitrate in serum samples was calculated using the standard curve constructed with the prepared serial dilutions of sodium nitrite.
Determination of total antioxidant capacity
Total serum antioxidant activity was determined by the reaction of antioxidants in the sample with a defined amount of exogenously provide hydrogen peroxide (H2O2). The antioxidants eliminate a certain amount of the provided H2O2. The residual H2O2 is determined colorimetric ally by an enzymatic reaction which involves the conversion of 3, 5, dichloro-2-hydroxy benzensul-phonate to a colored product 19.
Determination of serum uric acid
Uric acid concentration was measured by the direct enzymatic method, in which uric acid was oxidized by uricase coupled with peroxidase. Uricase converts uric acid to allantoin and hydrogen peroxide. The hydrogen per-oxide formed further reacts with a phenolic compound and 4 aminoantipyrine by the catalytic action of peroxidase to form a red colored quinoneimine dye complex. Intensity of the colour formed is directly proportional to the amount of uric acid present in the sample 20.
Quantification of serum VEGF, HGF, MMP-9, TNF-a, 25 hydroxy Vitamin D.
Commercially available immunoassay kits were used according to manufacturer’s guidelines. Vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) levels were determined with human VEGF immunoassay kit from R&D system (R&D System, Minneapolis, MN, USA); tumour necrosis factor-a levels were determined by TNF-α ELISA kits (Pierce Co., Rockford, IL, USA); matrix metalloproteinase-9 (MMP-9) levels were determined using human matrix metalloproteinase-9 ELISA kit (Ray Biotech Human ELISA Kit (Ray Biotech, Norcross, Georgia,USA); 25 hydroxy Vitamin D was performed using the enzyme-linked immunosorbent assay (ELISA) (Immundiagnosti k EIA, Bensheim and Biomedica, Wien, Austria).
Statistical analysis
Results are presented as means ± SE. For statistical analysis, group comparisons were performed by one way ANOVA followed by Duncan’ multiple range test. Differences between groups and correlation coefficients were considered significant if P < 0.05. GraphPad Prism 6 for Windows (GraphPad Prism Software Inc., San Diego, CA, USA) was used.
Results
Lipid peroxidation
In premenopausal patients serum malondialdehyde (MDA) levels were significantly lower in patients with non-metastatic (mean 46.81, range 40.9 to 55.3 µmo/l; P< 0.001) and metastatic cancer (mean 43.6, range 39.9 to 46.8 µmo/l; P< 0.001) than in normal individuals (mean 70.0, range 65.2 to 76.3 µmo/l). Similarly, serum MDA levels were significantly higher in normal subjects (mean 72.67, range 68.5 to 79.2 µmo/l) compared with patients with either non-metastatic (mean 47.0, range 40.9 to 55.3 µmo/l; P< 0.001) or metastatic cancer (mean 44.0, range 40.3 to 46.8 µmo/l; P< 0.001). There were no significant differences between serum MDA levels in premenopausal and postmenopausal patients with either non-metastatic or metastatic disease (figure 1 & table 1).
Nitric oxide
There was a significant decrease in serum nitric oxide in premenopausal patients with non-metastatic (mean 13.94 , range 11.2 to 16.5 µmo/l; P< 0.001) and metastatic cancer (mean 8.74 , range 6.8 to 10.8 µmo/l; P< 0.001) by 29.0% and 56.3%, respectively, compared to the control group (mean 19.5, range16.8 to 21.9 µmo/l). Similarly, serum nitric oxide levels were significantly lower in post-menopausal patients with non-metastatic (mean 14.04, range 11.2 to 16.5 µmo/l; P< 0.001) and metastatic cancer (mean 8.56, range 6.6 to 10.5 µmo/l; P< 0.001) compared to normal subjects (mean 19.78, range17.8 to 21.5 µmo/l) (figure 1 & table 1).
A significant decrease in serum nitric oxide by 37.3% and 39.0% was observed in pre- or post-menopausal patients with metastatic disease compared to non-metastatic cancer patients (figure 1 & table 1).
Uric acid
Serum uric acid showed a significant decrease in premenopausal patients with non-metastatic (mean 3.21, range 2.9 to 3.9 mg/dl; P< 0.001) and metastatic cancer (mean 2.90, range 2.1 to 3.6 mg/dl; P< 0.001) by 32.3% and 35.5%, respectively, compared to the control group (mean 4.74, range 3.7 to 6.2 mg/dl). Serum uric acid levels were also significantly lower in post-menopausal patients with non-metastatic (mean 3.13, range 2.6 to 3.9 mg/dl; P< 0.001) and metastatic cancer (mean 2.88, range 2.1 to 3.8 mg/dl; P< 0.001) compared to normal subjects (mean 4.85, range 3.9 to 6.2 mg/dl). There were no significant differences between serum uric acid levels in premenopausal and postmenopausal patients with either non-metastatic or metastatic disease (figure 1 & table 1).
Total antioxidant capacity
Serum TAC was significantly lower in premenopausal patients with non-metastatic (mean 1.63, range 1.42 to 1.85 µmo/l) and metastatic cancer (mean 1.19, range 0.96 to 1.39 µmo/l; P< 0.001) as compared to the control group (mean 1.75, range 1.62 to 1.83 µmo/l). Serum TAC values were also significantly lower in post-menopausal patients with non-metastatic (mean 1.64, range 1.42 to 1.85 µmo/l; P< 0.001) and metastatic disease (mean 1.18, range 0.95 to 1.37 µmo/l; P< 0.001) compared to their corresponding control subjects (mean 1.93, range1.82 to 2.09 µmo/l).
Patients with metastatic disease (either premenopausal or post-menopausal) exhibited significantly lower TAC values compared to those with non-metastatic cancer (figure 1 & table 1).
Table 1: Serum malondialdehyde (MDA), nitric oxide, uric acid and total antioxidant capacity (TAC) in patients with breast cancer.
| Control | Non-metastatic breast cancer | Metastatic breast cancer | |
| MDA
(mmo/l) |
|||
| Pre-menopause | 69.94 ± 0.96 | 46.81 ± 1.14* (-33.1%) | 43.64 ± 0.49* (-37.6%) |
| Post-menopause | 72.67 ± 0.9 | 47.0 ± 1.00* (-35.3%) | 44.0 ± 0.45* (-39.4%) |
| Nitric oxide
(µmol/l) |
|||
| Pre-menopause | 19.55± 0.38 | 13.94 ± 0.39* (-28.7%) | 8.74 ± 0.26*+ (-55.3%) |
| Post-menopause | 19.78 ± 0.30 | 14.04 ± 0.41* -29.0% | 8.56 ± 0.24*+ (-56.7% ) |
| Uric acid
(mg/dl) |
|||
| Pre-menopause | 4.74 ± 0.18 | 3.21 ± 0.12* (-32.3%) | 2.9 ± 0.10* (-38.8%) |
| Post-menopause | 4.85 ± 0.17 | 3.13 ± 0.12* (-35.5%) | 2.88 ± 0.12* (-40.6%) |
| TAC (µmol/l) | |||
| Pre-menopause | 1.75 ± 0.02 | 1.63 ± 0.03* (-5.7%) | 1.19 ± 0.03*+ (-32%)
|
| Post-menopause | 1.93 ± 0.02 | 1.64 ± 0.03* (-15.5%) | 1.18 ± 0.02*+ (-38.9%) |
VEGF
Pre-menopausal patients with non-metastatic or metastatic disease had significantly higher serum VEGF values (mean 227.5, range 202.8 to 239.2 pg/ml and mean 372.6, range 349.5 60 400.6 pg/ml, respectively) than did the control subjects (mean 116.39, range 102.4 to 122.8 pg/ml). In addition, post-menopausal patients with non-metastatic or metastatic breast cancer exhibited significantly higher serum VEGF (mean 224.1, range 202.8 to 235.7 pg/ml and mean 371.2, range 349.5 to 382.0 pg/ml, respectively) compared to their corresponding normal control values ( mean 108.5, range 101.6 to 119.4 pg/ml).
There was no difference between pre- or post-menopausal patients as regards their serum VEGF levels. However, patients with metastatic disease whether pre- or post-menopausal had significantly higher serum VEGF levels compared to their non-metastatic counterparts (figure 2 & table 2).
Table 2: Serum VEGF, HGF, MMP-9, VITD and TNF-α in patients with breast cancer.
| Control | Non-metastatic breast cancer | Metastatic breast cancer | |
| VEGF (pg/ml) | |||
| Pre-menopause | 116.39 ± 1.33 | 227.5 ± 2.56*(95.5%) | 372.64 ± 3.56*+ (220.1%) |
| Post-menopause | 108.55 ± 1.54 | 224.14 ± 2.00* (106.5%) | 371.19 ± 2.11*+ (241.9%) |
| HGF (ng/ml) | |||
| Pre-menopause | 307.97 ± 2.6 | 429.12 ± 3.15* (39.3%) | 542.99 ± 2.39*+ (76.3%) |
| Post-menopause
|
311.25 ± 3.29 | 433.32 ± 2.82* (39.2%) | 546.97 ± 2.66*+ (75.7%) |
| MMP-9 (ng/ml) | |||
| Pre-menopause | 244.16 ± 2.3 | 437.28 ± 3.37* (79.1%) | 523.3 ± 3.00*+ (114.3%) |
| Post-menopause | 259.86 ± 2.36
|
434.28 ± 3.10*(67.1%) | 524.18 ± 2.78*+ (101.7%) |
| VITD (ng/ml) | |||
| Pre-menopause
|
21.73 ± 0.72
|
8.69 ± 0.15* (-60.0%) | 8.62 ± 0.14* (60.3%)
|
| Post-menopause
|
22.02 ± 0.84
|
14.47 ± 0.19* (-34.3%) | 13.86 ± 0.29* (-37.1%) |
| TNF-α (pg/ml) | |||
| Pre-menopause | 3.52 ± 0.14
|
12.83 ± 0.39* (264.5%)
|
21.6 ± 0.49*+ (513.6%)
|
| Post-menopause | 3.49 ± 0.13 | 12.78 ± 0.41* (266.2%)
|
21.13 ± 0.52*+ (505.4%)
|
Data are expressed as mean ± SEM. *:p<0.05 vs. the corresponding control group. +:p<0.05 vs. the corresponding non-metastatic group. The percentage change from the corresponding control group is shown in parenthesis.
TNF-α
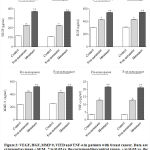
Serum TNF-α showed marked and significant increases by 264.5% and 513.6% in pre-menopausal women with both non-metastatic (mean 12.83, range 9.3 to 15.8 pg/ml) or metastatic disease (mean 21.6, range 18.4 to 24.9 pg/ml) compared with the healthy controls (mean 3.51, range 2.6 to 4.4 pg/ml). Similarly, in post-menopausal patients, significant elevations in serum TNF-α by 266.2% and 505.4% were observed in patients with both non-metastatic (mean 12.78, range 9.3 to 15.8 pg/ml) or metastatic disease (mean 21.13, range 18.4 to 24.9 pg/ml) compared with the control group (mean 3.49, range 2.7 to 4.6 pg/ml). Moreover, patients with metastatic cancer whether pre- or post-menopausal exhibited significantly higher levels of TNF-α in serum by 68.3% and 65.3% compared to their corresponding control values (figure 2 & table 2). Figure 3 shows the correlation analysis between serum VEGF and TNF-a in patients with breast cancer. There is significant +ve correlation between serum VEGF and TNF-a in pre-menopausal patients with either metastatic or non-metastatic disease and in post-menopausal patients with metastatic cancer.
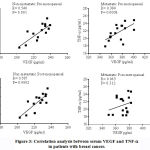 |
Figure 3: Correlation analysis between serum VEGF and TNF-a in patients with breast cancer. |
MMP-9
Premenopausal women with non-metastatic or metastatic disease had significantly higher MMP-9 serum values (mean 437.3, range 410.2 to 458.3 ng/ml and mean 523.3, range 495.3 to 539.5 ng/ml, respectively) than in healthy subjects (mean 244.2, range 110.7 to 336.9 ng/ml). Serum MMP-9 were also significantly elevated in post-menopausal patients with either non-metastatic (mean 434.3, range 410.3 to 453.6 ng/ml) or metastatic cancer (mean 524.2, range 537.6 to 498.4 ng/ml) than in normal individuals (mean 259.9, range 129.5 to 331.2 ng/ml).
There was significantly increased serum MMP-9 in pre- and post-menopausal patients with metastatic disease compared to corresponding non-metastatic patients (figure 2 & table 2). Figure 4 shows the correlation analysis between serum VEGF and MMP-9 in patients with breast cancer. There is significant +ve correlation between serum VEGF and MMP-9 in pre-menopausal patients with non-metastatic disease and in post-menopausal patients with either metastatic or non-metastatic disease.
 |
Figure 4: Correlation analysis between serum VEGF and MMP-9 in patients with breast cancer. |
HGF
Serum HGF levels were significantly higher in pre-menopausal patients with non-metastatic or metastatic disease (mean 429.1, range 410.3 to 455.7 ng/ml and mean 543.0, range 528.4 to 559.3 ng/ml, respectively) than in healthy subjects (mean 307.9, range 290.6 to 323.4 ng/ml).
Significant elevations in serum HGF levels were also observed in post-menopausal patients with non-metastatic or metastatic breast cancer (mean 433.3, range 413.2 to 455.7 ng/ml and mean 546.9, range 428.4 to 562.9 ng/ml, respectively) compared to normal individuals ( mean 311.2, range 219.3 to 335.5 ng/ml).
No significant differences were observed between pre- or post-menopausal subjects as regards their serum HGF levels. In contrast, there were significantly increased HGF levels in pre- and post-menopausal patients with metastatic disease than their non-metastatic counterparts (figure 2 & table 2). Figure 5 shows the correlation analysis between serum VEGF and HGF in patients with breast cancer. There was significant inverse correlation in post-menopausal patients with metastatic cancer.
 |
Figure 5: Correlation analysis between serum VEGF and HGF in patients with breast cancer. |
VITD
Serum VITD was significantly decreased by 60.0% and 60.3% in pre-menopausal women with both non-metastatic (mean 8.68, range 7.5 to 9.7 ng/ml) and metastatic breast cancer (mean 8.62, range 7.8 to 9.6 ng/ml) compared with the control group (mean 31.7, range 15.4 to 25.2 ng/ml). Serum VITD was also significantly lower by 34.3% and 37.1% in post-menopausal women with both non- metastatic (mean 14.47, range 9.3 to 15.8 ng/ml) and metastatic breast cancer (mean 13.86, range 11.5 to 15.3 ng/ml) compared with the control group (mean 22.02, range 15.4 to 26.3 ng/ml). There were no significant differences between serum VITD in metastatic or non-metastatic patients whether pre- or post-menopausal (figure 6).
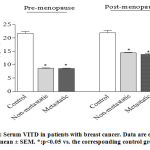 |
Figure 6: Serum VITD in patients with breast cancer. Data are expressed as mean ± SEM. *:p<0.05 vs. the corresponding control group. |
Changes in oxidative and inflammatory biomarkers according to hormonal receptor status
Out of 17 pre-menopausal patients with non-metastatic cancer 16 were +ve for the estrogen receptor and 16 were +ve for the progesterone receptor. In Post-menopausal patients with non-metastatic cancer, 18/18 were +ve for the estrogen receptor and 16/18 were positive for the progesterone receptor.
Out of 17 pre-menopausal patients with metastatic cancer, 10 were –ve and 7 were positive for the progesterone receptor. Out of 18 post-menopausal patients with metastatic cancer, 10 were –ve and 8 were positive for the progesterone receptor. Only one pre-menopausal patient and one post-menopausal patient with metastatic cancer were +ve for the estrogen receptor. Statistical comparisons were therefore made between patients with metastatic disease (pre- and post-menopausal) according to their progesterone receptor (PR) status.
No significant differences were observed between PR –ve and PR +ve patients (whether pre- or post-menopausal) as regards MDA, nitric oxide, or uric acid in serum. PR +ve patients showed lower TAC compared with PR –ve subjects. This difference reached statistical significance only in pre-menopausal patients (Table 3).
Table 3: Serum malondialdehyde (MDA), nitric oxide, uric acid and total antioxidant capacity in patients with metastatic breast cancer according to the progesterone receptor status.
| Progesterone receptor -ve | Progesterone receptor +ve | |
|
MDA (mmo/l) |
||
| Pre-menopause | 44.14 ± 0.73 | 42.91 ± 0.60 |
| Post-menopause | 43.25 ± 0.60 | 44.9 ± 0.52 |
| Nitric oxide
(µmol/l) |
||
| Pre-menopause | 8.55 ± 0.30 | 9.0 ± 0.34 |
| Post-menopause | 8.33 ± 0.36 | 9.8 ± 0.28 |
| Uric acid
(mg/dl) |
||
| Pre-menopause | 9.46 ± 0.33 | 10.73 ± 0.42 |
| Post-menopause | 9.27 ± 0.31 | 10.00 ± 0.34 |
| TAC (µmol/l) | ||
| Pre-menopause | 1.11 ± 0.04 | 1.32 ± 0.02* (p = 0.012) |
|
Post-menopause |
1.14 ± 0.05 |
1.22 ± 0.03 (p = 0.063) |
Data are expressed as mean ± SEM.
There were also non-significant differences in serum VEGF, HGF, MM9, or TNF-α between PR –ve and PR +ve patients (Table 4). Pre-menopausal patients, however, showed significantly lower serum VITD level compared to their post-menopausal counterparts, but no differences were observed between those who were –ve for PR receptor and +ve patients (Table 4).
Table 4: Serum VEGF, HGF, MMP-9, VITD and TNF in patients with metastatic breast cancer according to the progesterone receptor status.
| Progesterone receptor -ve | Progesterone receptor +ve | |
| VEGF (pg/ml) | ||
| Pre-menopause | 374.18 ± 4.72 | 370.44 ± 5.27 |
| Post-menopause | 369.28 ± 3.21 | 373.57 ± 2.24 |
| HGF (ng/ml) | ||
| Pre-menopause | 542.84 ± 3.22 | 543.21 ± 3.55 |
| Post-menopause | 545.73 ± 3.58 | 548.51 ± 3.90 |
| MMP-9 (ng/ml) | ||
| Pre-menopause | 520.01 ± 4.42 | 528.0 ± 2.80 |
| Post-menopause | 519.44 ± 4.11 | 530.11 ± 2.20 |
| VITD (ng/ml) | ||
| Pre-menopause | 8.76 ± 0.21 | 8.41 ± 0.12 |
| Post-menopause | 13.75 ± 0.33 | 14.0 ± 0.49 |
| TNF-α (pg/ml) | ||
| Pre-menopause | 21.89 ± 0.78 | 21.18 ± 0.38 |
| Post-menopause | 20.69 ± 0.62 | 21.69 ± 0.86 |
Data are expressed as mean ± SEM.
Discussion
Free radicals have been implicated as a possible cause for cancer. In this study, we assessed the oxidant/antioxidant status of patients with cancer breast by measuring MDA, nitric oxide, total antioxidant capacity and uric acid in the serum. We observed significant and marked decrease in serum malondialdehyde, an end product of lipid peroxidation which indicates a decrease in free radicals in these patients. This finding confirms our previous observation suggesting reduced oxidative stress in breast cancer patients undergoing chemotherapy 21. In their study, Saintot et al. 1996 found that malondialdehyde plasma concentration were lower in breast cancer patients before therapy than in controls 22. This decrease in lipid peroxidation was related to tumour size and progression i.e., tumour aggressiveness. Another study showed that the presence of nodes and/or metastases was directly associated with low plasma concentrations of cholesterol and malondialdehyde 22. Moreover, Gonenc et al. 2006 found decreased serum and tissue malondialdehyde levels in breast cancer patients compared to benign breast disease 23. The observed decrease in serum malondialdehyde in breast cancer patients could be also the result of chemotherapeutic agents. Our findings are in contrast with studies that reported increased plasma malondialdehyde in cancer breast 24,25. Other studies found increased lipid peroxidation in breast cancer compared with non-malignant tissue and also increased superoxide dismutase and glutathione peroxidase enzyme activities 26.
The measurement of antioxidant capacity (TAC) defined as the moles of radicals neutralized per 1 L of tested sample, is widely used test in biological studies that is thought to reflect the sum of endogenous antioxidants 27. Other researchers suggested that the determination of individual antioxidants might be more informative of the oxidant/antioxidant status of the tissue than a single test 28. In this study, TAC significantly decreased in subjects with metastatic cancer compared to the control group, which might indicate consumption of endogenous antioxidants and/or reduced intake of exogenous antioxidants. Other studies also indicated decreased TAC in breast cancer patients compared to the healthy control 29. Feng et al. 2012 found decreased TAC in serum and breast tissue of patients with benign breast lesions and breast cancer 30.
Significantly decreased TAC has also been reported during chemotherapy for malignancies in children which has been attributed to reduced dietary intake of antioxidants and an increase in free radicals by the effect of anticancer agents 31. In the present study, TAC decreased in patients with either non-metastatic or metastatic cancer with the lowest values being observed in subjects with metastatic disease. Whether this is due to consumption of endogenous antioxidants in the disease process, reduced dietary intake in advanced disease or the result of chemotherapy is not clear. Since the test is affected by decreased dietary intake of antioxidants it might therefore not be suitable for monitoring disease or indicate disease severity.
In this study, we found that nitric oxide also decreased in serum of breast cancer patients, with those having metastatic disease exhibiting the lowest levels, which confirms our previous studies. Similar results were provided by Güler et al. 2006 who demonstrated decreased plasma nitric oxide after chemotherapy 32. Other studies reported increased serum nitric oxide in breast cancer 29. The gaseous molecule nitric oxide is derived from L-arginine via the enzyme nitric oxide synthase (NOS) that exists in constitutive (endothelial and neuronal) and an inducible (NOS2) isoform. The latter is responsible for the sustained generation of large amounts of nitric oxide during inflammatory conditions by neutrophils and phagocytes 33. Increased expression of both endothelial (eNOS) and NOS2 was detected in invasive and in situ breast cancer 34. Nitric oxide has an important role in different stages of cancer eg. angiogenesis, cell invasion, intravasation and metastasis 35,36 . And the increased expression of NOS2 has been shown to be associated with its increased expression is associated with disease aggressiveness, and predict poor outcome in ER (-) breast cancer 37,38. High levels of nitric oxide are associated with the production of a range of reactive nitrogen species such as peroxynitrite and nitrogen dioxides and trioxides, which subsequently interact with more diverse targets, and result in chemical stress 33. Rabender et al. 2015 found that NOS of tumor cells, in contrast to normal tissues, generates peroxynitrite and superoxide anion than nitric oxide, with important consequent on tumor growth 39. Nitric oxide might also inhibit tumourigenesis by inhibiting MMP-9 activity 40. Indeed, both tumorigenic and anti-oncogenic activities have been detected for nitric oxide with low-intermediate concentrations stimulating whilst high concentrations inhibiting oncogenic signaling 41.
Uric acid, the end product of purine metabolism that results from the oxidation of xanthine and hypoxanthine by xanthine oxidase is considered an important factor in the development and mortality from cancer. Uric acid because of its pro-inflammatory properties is thought to represent a link between inflammatory states like obesity and metabolic syndrome and the occurrence of cancer. Uric acid, however, is a double edged sword because the molecule is a potent antioxidant which scavenges peroxyl, hydroxyl and superoxide radicals and inhibits oxidative damage to cell biomolecules lipids. Proteins, and nucleic acids 42,42. Uric acid might thus serve a protective role to reduce cancer 44. In the present study, serum uric acid levels were significantly lower in breast cancer patients compared to healthy controls. Uric acid levels were unaffected by the menopausal status. This reduction in serum uric acid might be due to drugs that inhibit uric acid synthesis eg. allopurinol, often given in the course of chemotherapy to prevent sudden increments in serum and tissue uric acid from tissue damage. In healthy subjects, administering uric acid was found to decrease oxidative stress and increase serum TAC during acute physical exercise 45. The decrease in serum uric acid might also contribute to the decrease in TAC observed in our study.
Our study also shows depressed serum VITD levels in metastatic and non- metastatic breast cancer patients. Significantly lower values were however detected in pre-menopausal compared to post-menopausal patients. In cells, VITD binds to its nuclear vitamin D receptor and mediates signaling pathways that involve cellar proliferation, apoptosis, angiogenesis, and metastasis and hence its significance in cancer development and progression 46,47. In recent years, serum VITD has been a focus of much interest in relation to breast cancer. Low levels of VITD in serum of patients with breast cancer were observed at diagnosis compared to controls and showed significant decrease after neoadjuvant chemotherapy 48,49. In a meta-analysis study by Li et al.
2014 higher VITD levels were significantly associated with improved disease free survival for patients with cancer breast 50. In their study, Almeido-Filho et al. 2017 found an association between the extent of VITD deficiency in post-menopausal women with breast cancer and tumours with worse prognosis i.e., high grade, locally advanced, metastatic, ER (-) and PR (+ve) tumours 51. The authors suggested that low serum VITD is a risk factor for ER (-) tumours with axillary nodes and high cellular proliferation. In their study, Cheney et al. 2018 in a follow-up study of 7 years, however, observed no significant relationship between serum VITD levels and cancer risk 52. Moreover, Charehbili et al. 2016 found significantly decreased serum VITD levels during post-neoadjuvant chemotherapy 53. However, basal and end of treatment VITD levels were not related to the pathological response following neoadjuvant chemotherapy. Similar data were provided by 48.
The development and progression of breast cancer is dependent not only on the intrinsic properties of cancer cells but also on micro enviromental factors 54. Studies indicated that the inflammatory cytokine TNF-a have an important role in tumour growth and metastasis, via inducing proagniogenic factors and matrix metalloproteinasis 54,55,56. Our results are in agreement with other studies indicating increased serum TNF-a in breast cancer patients 57,58. Saglam et al. 2009 found that plasma TNF-α levels were higher in ERBB2+ breast cancer patients than in controls 57. Ma et al. 2017 reported significantly higher serum TNF-α levels in stage III breast cancer patients than in controls. Serum TNF-a was associated with lymph node metastasis 58.
The matrix-degradative enzymes, matrix metalloproteinases (MMPs) have been implicated in cancer progression and metastasis 59,60. MMP-9 (also -1, -12 and -15) is significantly elevated in high-grade as compared with low-grade tumors and high expressions of MMP-9 (also -1, -12, -14 and -15) associates with poor overall survival 61. In the present study, we found significant increase in serum MMP-9 in both pre- and post-menopausal patients with either non-metastatic or metastatic cancer with significantly higher values in those with metastatic disease. Similar data were provided by Rashad et al. 2013 29.
Angiogenesis is the formation of new blood vessels and is a prerequisite for tumour growth and dissemination. Vascular endothelial growth factor (VEGF) is an important regulator of the growth and proliferation of blood vessels 62,63. Adams et al. 2000 found that patients with metastatic disease had higher plasma and serum VEGF levels compared to normal controls 64. Samy et al. found that serum VEGF were increased in patients with breast cancer compared to controls 65. Serum VEGF increased in advanced stages and in ER (+ve) patients. Byrne et al. found that plasma VEGF levels were increased in pre-menopausal patients with early breast cancer compared to controls 66. The authors, found no correlation between plasma and intra-tumoural VEGF. Studies, however, suggested that clincopathological prognostic parameters and tumour microvessel density were not affected by circulating VEGF levels 64,65. Moreover, Bachelot et al. 2003 reported that serum and plasma levels of VEGF are not useful indicators for prognosis in patients with metastatic breast cancer 67. Our study found significant increase in serum angiogenic factor VEGF in both non-metastatic and metastatic cancer patients with significantly higher values in those with metastatic disease. This suggests that the level of VEGF in serum increases in advanced disease. We found no difference, however, in serum levels of VEGF in relation to menopausal status of patients. We also observed significant positive correlation between serum VEGF and the inflammatory cytokine TNF-a in pre-menopausal patients with non-metastatic or metastatic disease and in post-menopausal patients with non-metastatic cancer, suggesting a link between the inflammatory response and the level of VEGF in serum. On the other hand, there was also significant association between serum VEGF and MMP-9 in non-metastatic cancer 67.
Hepatocyte growth factor (HGF) is a potent simulator of hepatocyte growth and also a tumour-disseminating factor 68. Yang et al. 2015 reported high expression of HGF in breast cancer cells which was related to lymph node metastasis, prognosis and also to the sensitivity to chemotherapy 69. Significant increase in plasma HGF levels was found both in patients with benign breast lumps and in those with breast cancer patients when compared to controls 70. We also found significant increase in HGF in serum of patients with non-metastatic or metastatic cancer. Higher levels of HGF were observed in patients with metastatic disease. There was, however, no difference in serum HGF levels in relation to menopausal status. Moreover, a negative correlation was present between serum VEGF and HGF in patients with metastatic disease post-menopausal.
In the present study, patients with metastatic breast cancer disease (pre- and post-menopausal) were further evaluated according to their progesterone receptor (PR) status. It is estimated that about 50% of breast cancer patients express PR and which parallels ER expression 71,72. We found no significant differences between PR –ve and PR +ve patients for the alterations in the oxidative, inflammatory, angiogenic biomarkers in serum of breast cancer patients.
In summary, the present study in patients with breast cancer suggests that vascular and inflammatory markers VEGF, HGF, MMP-9 and TNF-α were increased in serum in advanced stages and could monitor disease progression and/or disease severity.
Acknowledgement
Many thanks to National Research Centre for their great help during the study.
Conflict of interest
None
Funding Source
None
References
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res.; 69 (3): 562-73 (2006).
CrossRef - Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol.; 8: 211-233 (2007).
CrossRef - Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol. 10: 415-433 (2000).
CrossRef - Curran S, Dundas SR, Buxton J, Leeman MF, Ramsay R, Murray GI. Matrix metalloproteinase/tissue inhibitors of matrix metalloproteinase phenotype identifies poor prognosis colorectal cancers. Clin Cancer Res.; 10: 8229-8234 (2004).
CrossRef - Iwasaki M, Nishikawa A, Fujimoto T, Akutagawa N, Manase K, Endo T, Yoshida K, Maekawa R, Yoshioka T, Kudo R: Anti-invasive Effect of MMI-166 New Selective Matrix Metalloproteinase Inhibitor, in Cervical Carcinoma Cell Lines. Gynecol Oncol.; 85: 103-107(2002).
CrossRef - Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun.; 161:851-858 (1989).
CrossRef - Nicosia RF. What is the role of vascular endothelial growth factor-related molecules in tumor angiogenesis? [comment]. Am J Pathol.; 153:11-16 (1998).
CrossRef - Gerber HP, Hillan KJ, Ryan AM et al. VEGF is required for growth and survival in neonatal mice. ; 126:1149-1159 (1999).
CrossRef - Shalaby F, Ho J, Stanford WL et al. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell.; 89:981-990 (1997).
CrossRef - Grunstein J, Roberts WG, Mathieu-Costello O et al. Tumor-derived expression of vascular endothelial growth factor is a critical factor in tumor expansion and vascular function. Cancer Res.; 59:1592-1598(1999).
- Fong TAT, Shawver LK, Sun L et al. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res.; 59:99-106 (1999).
- Asano M, Yukita A, Suzuki H. Wide spectrum of antitumor activity of a neutralizing monoclonal antibody to human vascular endothelial growth factor. Jpn J Cancer Res.; 90:93-100 (1999).
CrossRef - Vajkoczy P, Menger MD, Vollmar B et al. Inhibition of tumor growth, angiogenesis, and microcirculation by the novel Flk-1 inhibitor SU5416 as assessed by intravital multi-fluorescence videomicroscopy. ; 1:31- 41(1999).
CrossRef - Liang Y, Brekken R and Hyder S. Vascular endothelial growth factor induces proliferation of breast cancer cells and inhibits the anti-proliferative activity of anti-hormones. Endocrine-Related Cancer.; 13: 905–919 (2006).
CrossRef - Pellikainen J, Ropponen K, Kataja V, Kellokoski J, Eskelinen M, and Kosma V. Expression of Matrix Metalloproteinase (MMP)-2 and MMP-9 in Breast Cancer with a Special Reference to Activator Protein-2, HER2, and Prognosis. Clinical Cancer Research.; 10: 7621–7628 (2004).
CrossRef - Imtiaz S, Siddiqui N, Raza S, Loya A, and Muhammad A. Vitamin D deficiency in newly diagnosed breast cancer patients. Indian J Endocrinol Metab.; 16(3): 409–413 (2012).
CrossRef - Ruiz-Larrea, M.B, Leal, A.M, Liza, M., Lacort, M. and De Groot, H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. ; 59, 383-388 (1994).
CrossRef - Miranda, K.M., Espey, M.G. and Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide.; 5, 62-71 (2001).
CrossRef - Koracevic, D., Koracevic, G., Djordjevic, V., Andrejevic, S. and Cosic, V. Method for the measurement of antioxidant activity in human fluids. Journal of Clinical Pathology.; 54, 356-361 (2001).
CrossRef - Fossati, P., Prencipe, L. and Berti, G. Use of 3,5- dichloro-2-hydroxybenzenesulfonic acid/4-aminophena-zone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clinical Chemistry.; 26, 227-231 (1980).
CrossRef - Abdel-Salam OME, Youness ER, Hafez HF. The antioxidant status of the plasma in patients with breast cancer undergoing chemotherapy. Open Journal of Molecular and Integrative Physiology.; 1, 29-35 (2011).
CrossRef - Saintotj M, Astre C, Pujol H, Gerber M. Ttimor progression and oxidant-antioxidant status. Carcinogenesis,; 17 (6): 1267—1271 (1996).
CrossRef - Gönenç A, Erten D, Aslan S, Akinci M, Simşek B, Torun M.Sener, D.E., Gönenç, A., Akinci, M. Lipid peroxidation and antioxidant status in blood and tissue of malignant breast tumor and benign breast disease. Cell Biol Int.; 30(4):376-80 (2006).
CrossRef - Sener DE, Gönenç A, Akinci M, Torun M. Lipid peroxidation and total antioxidant status in patients with breast cancer. Cell Biochem Funct. 25(4):377-82 (2007).
CrossRef - Carneiro Do Val JL, Nixdorf SL, Mantovani MS, Da Silva Do Amaral Herrera AC, Aoki MN, Ama-rante MK, Fabris BA, Pelegrinelli Fungaro MH, Ehara Watanabe MA. Plasma malondialdehyde levels and CXCR4 expression in peripheral blood cells of breast cancer patients. Journal of Cancer Research and Clinical Oncology.; 135, 997-1004 (2009).
CrossRef - Tas F, Hansel H, Belce A, Ilvan S, Argon A, Camlica H, Topuz E. Oxidative stress in breast cancer. Med Oncol.; 22(1):11-5(2005).
CrossRef - Serafini, M, and Del Rio, D. Understanding the association between dietary antioxidants, redox status and disease: Is the total antioxidant capacity the right tool? Redox Report, 9, 145-152 (2004).
CrossRef - Sies H. Total antioxidant capacity: Appraisal of a concept. J. Nutr. 137: 1493–1495 (2007).
CrossRef - Rashad YA, Elkhodary TR, El-Gayar AM, Eissa LA. Evaluation of serum levels of HER2, MMP-9, nitric oxide, and total antioxidant capacity in Egyptian breast cancer patients: Correlation with clinico-pathological parameters. Sci Pharm.; 82(1):129-45 (2013).
CrossRef - Feng JF, Lu L, Zeng P, Yang YH, Luo J, Yang YW, Wang D. Serum total oxidant/antioxidant status and trace element levels in breast cancer patients. Int J Clin Oncol.; 17:575–583 (2012).
CrossRef - Papageorgiou, M., Stiakaki, E., Dimitriou, H., Malliaraki, N., Notas, G., Castanas, E. and Kalmanti, M. Can-cer chemotherapy reduces plasma total antioxidant ca-pacity in children with malignancies. Leukemia Research.; 29, 11-16 (2005).
CrossRef - Güler E., Balat, A, Çekmen M, Kılınç M, Sivasli E, Yürekli M. and Duman C. The effects of anti-cancer drugs on levels of nitric oxide and adrenomedullin. The Turkish Journal of Pediatrics.; 48, 202-208 (2006).
- Thomas DD, Heinecke JL, Ridnour LA, Cheng RY, Kesarwala AH, Switzer CH, McVicar DW, Roberts DD, Glynn S, Fukuto JM, Wink DA, Miranda KM. Signaling and stress: The redox landscape in NOS2 biology. Free Radic Biol Med. 87:204-25 (2015).
CrossRef - Loibl S, von Minckwitz G, Weber S, Sinn H-P, Schini-Kerth VB, Lobysheva I, Nepveu F, Wolf G, Strebhardt K, Kaufmann M. Expression of endothelial and inducible nitric oxide synthase in benign and malignant lesions of the breast and measurement of nitric oxide using electron paramagnetic resonance spectroscopy. Cancer.; 95:1191–8 (2002).
CrossRef - Cheng H, Wang L, Mollica M, Re AT, Wu S, Zuo L. Nitric oxide in cancer metastasis. Cancer Lett.; 353(1):1-7 (2014).
CrossRef - Chang CF, Diers AR, Hogg N. Cancer cell metabolism and the modulating effects of nitric oxide. Free Radic Biol Med.; 79:324-36 (2015).
CrossRef - Heinecke JL, Ridnour LA, Cheng RY, Switzer CH, Lizardo MM, Khanna C, Glynn SA, Hussain SP, Young HA, Ambs S, Wink DA. Tumor microenvironment-based feed-forward regulation of NOS2 in breast cancer progression. Proc Natl Acad Sci U S A.; 111(17):6323-8 (2014).
CrossRef - Thomas DD, Wink DA. NOS2 as an emergent player in progression of cancer. Antioxid Redox Signal.; 26(17):963-965 (2017).
CrossRef - Rabender CS, Alam A, Sundaresan G, Cardnell RJ, Yakovlev VA, Mukhopadhyay ND, Graves P, Zweit J, Mikkelsen RB. The role of nitric oxide synthase uncoupling in tumor progression. Mol Cancer Res.; 13(6):1034-43 (2015).
CrossRef - Jespersen C, Doller A, Akool E-S, Bachmann M, Muller R, Gutwein P, Muhl H, Pfeilschifter J, Eberhardt W. Molecular mechanisms of nitric oxide-dependent inhibition of TPA-induced matrix metalloproteinase-9 (MMP-9) in MCF-7 cells. J. Cell. Physiol.; 219: 276–287 (2009).
CrossRef - Monteiro HP, Costa PE, Reis AKCA, Stern A. Nitric oxide: protein tyrosine phosphorylation and protein S-nitrosylation in cancer. Biomed J.; 38:380-388 (2015).
CrossRef - Muraoka, S. and Miura, T. Inhibition by uric acid of free radicals that damage biological molecules. Toxicology and Pharmacology.; 93, 284-289 (2003).
CrossRef - Stinefelt B, Leonard, SS, Blemings KP, Shi, X. and Klandorf H. Free radical scavenging, DNA pro-tection, and inhibition of lipid peroxidation mediated by uric acid. Annals of Clinical & Laboratory Science.; 35, 37-45 (2005).
- Ame, B.N, Cathcar, R, Schwiers E. and Hochstein, P. Uric acid provides an antioxidant defense in hu-mans against oxidant- and radical-caused aging and can-cer: A hypothesis (lipid peroxidation/ascorbic acid/pri- mate evolution/erythrocyte aging). Proceedings of the National Academy of Sciences.; 78, 6858-6862 (1981).
CrossRef - Waring WS, Convery A, Mishra V, Shenkin A, Webb DJ. and Maxwell SRJ. Uric acid reduces exercise-induced oxidative stress in healthy adults. Clinical Science.; 105, 425-430 (2003).
CrossRef - Spina CS, Tangpricha V, Uskokovic M, et al. Vitamin D and cancer. Anticancer Res.; 26: 2515–2524 (2006).
- Feldman D, Krishnan AV, Swami S, et al.: The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer,; 14: 342–357 (2014).
CrossRef - Kim JS, Haule CC, Kim JH, Lim SM, Yoon KH, Kim JY, Park HS, Park S, Kim SIl, Cho YU, Park BW . Association between changes in serum 25-hydroxyvitamin d levels and survival in patients with breast cancer receiving neoadjuvant chemotherapy. J Breast Cancer.; 21(2): 134-141 (2018).
CrossRef - Dieuwertje E. K, Maaike MGA, Berg V D, Posthuma L, Iris van’t Erve, Fränzel JBD, Wilfred K. de Roos, Grosfeld S, Los M, Dirkje W. Sommeijer, Hanneke W. M. van Laarhoven, Winkels R M. and Kampman E. Changes in circulating levels of 25-hydroxyvitamin d3 in breast cancer patients receiving chemotherapy. Nutrition and Cancer, 71:5, 756-766 (2019).
CrossRef - Li M, Chen P, Li J, Chu R, Xie D, Wang H. Review: the impacts of circulating 25-hydroxyvitamin d levels on cancer patient outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab.; 99(7):2327–2336 (2014).
CrossRef - Almeida-Filho de Sousa, Vespoli H. De Luca , Pessoa E.C., Machado M., Nahas-Neto J., Petri Nahas E. A. Vitamin D deficiency is associated with poor breast cancer prognostic features in postmenopausal women. J Steroid Biochem Mol Biol. 174: 284-289 (2017).
CrossRef - Cheney C.P. Thorand B. Huth C. Berger K. Peters A. Seifert-Klauss V. Kiechle M. Strauch K. Quante A.S. The Association betweenSerum 25-Hydroxyvitamin D and Cancer Risk: Results from the Prospective KORA F4 Study. Oncol Res Treat.; 41:117-121 (2018).
CrossRef - Charehbili A, Hamdy NAT , Smit VTHBM , Kessels L , van Bochove A , van Laarhoven HW , Putter H , Meershoek-Klein Kranenbarg E , van Leeuwen-Stok AE , van der Hoeven JJM , van de Velde CJH, Nortier JWR , Kroep JR , Dutch Breast Cancer Research Group (BOOG). Vitamin D (25-0H D3) status and pathological response to neoadjuvant chemotherapy in stage II/III breast cancer: Data from the NEOZOTAC trial (BOOG 10-01) Breast.; 25:69-74 (2016).
CrossRef - Ben-Baruch A. Host microenvironment in breast cancer development Inflammatory cells, cytokines and chemokines in breast cancer progression: reciprocal tumor–microenvironment interactions. Breast Cancer Res.; 5:31-36 (2003).
CrossRef - Balkwill F. Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev 13:135-141(2002).
CrossRef - Martínez-Reza I, Díaz L, García-Becerra R. Preclinical and clinical aspects of TNF-α and its receptors TNFR1 and TNFR2 in breast cancer. J Biomed Sci .; 24:90 (2017).
CrossRef - Saglam S, Suzme R, Gurdol F. Serum tumor necrosis factor-alpha and interleukin-2 concentrations in newly diagnosed ERBB2 (HER2/neu) positive breast cancer patients. Int J Biol Markers.; 24: 142-6 (2009).
CrossRef - Ma Y, Ren Y, Dai ZJ, Wu CJ, Ji YH, Xu J. IL-6, IL-8 and TNF-α levels correlate with disease stage in breast cancer patients. Adv Clin Exp Med.; 26(3):421–426 (2017.
CrossRef - Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer.; 2: 161–174 (2002).
CrossRef - Fingleton B. Matrix metalloproteinases: roles in cancer and metastasis. Front Biosci.; 11: 479–491 (2006).
CrossRef - McGowan PM, Duffy MJ. Matrix metalloproteinase expression and outcome in patients with breast cancer: analysis of a published database. Annals of Oncology.; 19: 1566–1572 (2008).
CrossRef - Brown LF, Berse B, Jackman RW et al: Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum Pathol.; 26: 86-91 (1995).
CrossRef - Obermair A, Kucera E, Mayerhofer K et al: Vascular endothelial growth factor (VEGF) in human breast cancer: correlation with disease-free survival. Int J Cancer.; 74: 455-458 (1997).
CrossRef - Adams J, Carder PJ, Downey S, Forbes MA, MacLennan K, Allgar V, Kaufman S, Hallam S et al. Vascular endothelial growth factor (VEGF) in breast cancer: comparison of plasma, serum, and tissue VEGF and microvessel density and effects of tamoxifen. Cancer Research.; 60, 2898–2905 (2000).
- Samy N, Afify M, Abd El Maksoud N, Shalaan M. Serum vascular endothelial growth factor as prognostic biomarker in Egyptian breast cancer patients. Int J Pharmaceut Clin Res., (IJPCR ) 8(9): 1339-1342 (2016).
- Byrne GJ, MCdowell G., Agarawal R, Sinha G, Kumar S , Bundred NJ. Serum vascular endothelial growth factor in breast cancer. Anticancer Research 27: 3481-3488 (2007).
- Bachelot T, Ray-Coquard I , Menetrier-Cauxl C, Rastkha M, Duc A, Blay J-Y. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. British Journal of Cancer.; 88, 1721 – 1726 (2003).
CrossRef - Owusu BY, Galemmo R, Janetka J, Klampfer L. Hepatocyte growth factor, a key tumor-promoting factor in the tumor microenvironment. Cancers.; 17;9(4):35 (2017).
CrossRef - Yang H, Zhang C, Cui S. Expression of hepatocyte growth factor in breast cancer and its effect on prognosis and sensitivity to chemotherapy. Mol Med Rep 11: 1037-1042 (2015).
CrossRef - El-Attar HA, Ragab MS , Sheta MI , Ahmed AS. Hepatocyte growth factor in egyptian females with breast benign lumps and cancers. Asian Pacific J Cancer Prev.; 11, 893-896(2010).
- Nadji M,Gomez-Fernandez C,Ganjei-Azar P, Morales AR, Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. The American Journal of Clinical Pathology.; 123 (1): 21–27 (2005).
CrossRef - Deblois G, Giguere V. Oestrogen-related receptors in breast cancer: control of cellular metabolism and beyond. Nature Reviews Cancer.; 13: 27–36 (2013).
CrossRef