Manuscript accepted on :17-05-2021
Published online on: 08-05-2021
Plagiarism Check: Yes
Reviewed by: Dr. B.Surendiran
Second Review by: Dr. Malika Alili
Final Approval by: Dr. Ayush Dogra
Kirthika C P1 , Siva T1*
, Siva T1* , Rajeswaran R2
, Rajeswaran R2 , Kalpana R1
, Kalpana R1  and Yuvaraj Maria Francis3
and Yuvaraj Maria Francis3
1Department of Anatomy, Sri Ramachandra Institute of Higher Education and Research, Porur, Chennai, India.
2Department of Radiology, Sri Ramachandra Institute of Higher Education and Research, Porur, Chennai, India
3Department of Anatomy, Saveetha Medical College and Hospital, Thandalam, Chennai, India
Corresponding Author E-mail: siva17187@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2168
Abstract
Introduction: Corpus callosum (CC) is the largest commissural white fibres interconnecting cerebral hemispheres. The corpus callosum is responsible for interhemispheric transfer of information which is essential for cognitive function. The foetal corpus callosum serves as sensitive indicator for normal brain development and maturation. As the corpus callosum is a part of the highest order latest maturing mental network of the brain, its measurements are important to assess normal brain development and to locate structural changes. A comprehensive evaluation of normal human foetal corpus callosal development is essential to detect and understand the congenital anomalies of the brain. Thus, the prenatal diagnosis of partial or complete agenesis of the corpus callosum is important for predicting the normal development of the foetus. Foetal neural anomalies that are suspected on prenatal ultrasonography (USG) can be detected in early stage using foetal MRI. This imaging technique is highly useful for detailed visualization of normal neural development. Certain conditions like colpocephaly and widening of interhemispheric fissure can be clearly visualized using foetal MRI when compared to prenatal ultrasonography. Aim and objective: Was to establish the normal reference values for the measurement of foetal corpus callosum. The length and thickness of the foetal CC was measured corresponding to gestational age (GA) between 18-36weeks. Materials and methods: A retrospective MRI study was carried out in Radiology department of Sri Ramachandra Hospital. The study was conducted on 50 pregnant women with GA of 18-32 weeks and morphology of foetal corpus callosum was measured using MRI. The corpus callosum was visualized in a mid-sagittal plane as an anechoic structure, delimited by two echogenic lines superiorly by sulcus of the corpus callosum and inferiorly by the septum pellucidum. The length of corpus callosum was measured from the anterior most aspect of genu to the posterior most aspect of the splenium and the width of individual parts were measured and correlated with gestational age. The values obtained from the study were statistically calculated using regression coefficient method. Results: In the present study following parameters were observed such as length and width of diverse parts of Corpus callosum. The length of foetal CC ranged from 25.96 to 47.2 mm in 18 to 32 weeks of gestational age. The range of width of rostrum, genu, body and splenium were 1.2 to 2.2 mm, 1.2-2.8mm, 1.3-3.1mm and 1.36-3.2mm respectively. Conclusion: The periodic development of nervous system can be calculated more effectively with the morphometric measurement of foetal CC and its correlation with BPD. It is considered to be accurate than using BPD measurement of head circumference in USG. Hence, with the normative data of foetal CC measurements correlated with gestational age would give us accurate details of neuronal growth rather than measuring biparietal diameter (BPD) alone using USG. This knowledge will be highly helpful for the gynaecologists to predict the abnormal development of the foetus and it is advised to include foetal CC parameters as a one of the tools for early detection of CNS anomalies.
Keywords
Biparietal Diameter; Congenital Anomalies Foetal Corpus Callosum; Gestational Age; Magnetic Resonance Imaging
Download this article as:| Copy the following to cite this article: Kirthika C. P, Siva T, Rajeswaran R, Kalpana R, Francis Y. M. Morphology and Morphometry of Foetal Corpus Callosum Using MRI – A Retrospective Study. Biomed Pharmacol J 2021;14(2). |
| Copy the following to cite this URL: Kirthika C. P, Siva T, Rajeswaran R, Kalpana R, Francis Y. M. Morphology and Morphometry of Foetal Corpus Callosum Using MRI – A Retrospective Study. Biomed Pharmacol J 2021;14(2). Available from: https://bit.ly/3f6zkdk |
Introduction
Corpus Callosum is the largest commissural white fibres interconnecting the cerebral hemispheres. In sagittal section of cerebrum, it is seen as C shaped mass of white fibres on the medial surface of the hemisphere forming the roof of the lateral ventricle. The corpus callosum (CC) is divided from anterior to posterior into four parts namely rostrum, genu, trunk, splenium. Genu is thick curved anterior extremity of corpus callosum which lies 4cm behind the frontal pole. Rostrum extends downwards and backwards from genu as a thin prolongation to join the lamina terminalis. Trunk is main middle part of the corpus callosum and it lies between genu anteriorly and splenium posteriorly. Splenium is the posterior extremity of the corpus callosum situated 6 cm anterior to the occipital pole [1]. The corpus callosum is responsible for interhemispheric transfer of information which is essential for cognitive function. At 11-12 weeks of gestation, the corpus callosum begins to develop, which grows caudally along with the cerebral hemisphere [2]. By 18 weeks of gestation all the components of the corpus callosum are present and can be visualized on ultrasonography. Because of its relatively late development various brain anomalies are often associated with abnormalities of corpus callosum [3]. The foetal corpus callosum serves as sensitive indicator for normal brain development & maturation. As the corpus callosum is a part of the highest order latest maturing mental network of the brain, its measurements are important to assess normal brain development and to locate structural changes [4]. A Comprehensive evaluation of normal human foetal corpus callosal development is essential to detect and understand the congenital abnormalities of the brain. Thus, the prenatal diagnosis of partial or complete agenesis of the corpus callosum is important in predicting the development of the foetus [5]. Although prenatal detection of corpus callosum abnormality has been widely reported, its normal in-utero growth and development are scarcely documented. The advanced high-resolution MRI imaging technology of the foetal corpus callosum will be helpful in detecting the anomalies at the early stages of gestation [6]. As it is indicated by Harreld, understanding the normal growth pattern of corpus callosum will surely enhance the detection of abnormal growth [7]. The corpus callosal growth is an indicator for mental maturity of the developing foetus. It is uncertain at what stage the foetal brain becomes adversely affected by callosal abnormalities [8]. Foetal neural anomalies that are suspected on prenatal ultrasonography (USG) can be detected in early stage using foetal MRI [9]. Foetal MRI is highly useful for detailed visualization of normal neural development [10, 11]. Certain conditions like colpocephaly and widening of interhemispheric fissure can be clearly visualized using foetal MRI when compared to prenatal USG. Thus, according to this study aims foetal MRI is considered as a better tool to assess the morphology of foetal corpus callosum. This study also aims to establish the normal reference values for the foetal corpus callosum by measuring its length and thickness corresponding to gestational age (GA) between 18-36weeks.
Materials and methods
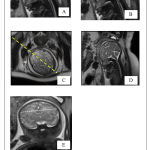
A retrospective study was conducted in Department of Radiology in Sri Ramachandra Hospital. About 50 pregnant women with GA of 18-36 weeks who underwent foetal MRI were selected. Gestational age of the foetus was estimated by the combination of early sonographic examination and last menstrual period and ranged from 18 to 36 Weeks. Morphology and morphometry of foetal CC was measured using MRI. Foetal images with normal CNS anatomy were only included in the study. Exclusion criteria: Foetus images with CNS anomalies, including ventriculomegaly, Arnold Chiari malformation and abnormal head circumference were excluded. The study was carried after obtaining Institutional Ethical Committee clearance from Sri Ramachandra Institute of Higher Education & Research (NI/17/APR/ 59/43). The foetal MRI was done on 1.5 Tesla GE SignaHDxt scanner. Body torso / wrap around coil (phased array coil) was placed around the mother’s pelvis for imaging the foetus. An 18 sec, 3 plane localizers were initially obtained in which the foetal imaging planes were planned. Foetal imaging parameters included TE 95-110ms, TR 1300-1500 ms, FOV – 24-30 cm, matrix 256×160, bandwidth 31.2 kHz, NEX – 4, slice thickness – 3.5 – 5 mm and spacing – 0.2 mm. The foetal brain was imaged in axial, coronal and sagittal planes and were analysed in the ADW functool 4 workstation. The morphology of the CC was analysed in the sagittal plane of HASTE (Half – Fourier Single Shot Turbo Spin Echo) sequence. Length of CC was measured in the midsagittal image, from the anterior most aspect of the genu to posterior most aspect of splenium. The body was found to be linear, horizontal structure with low signal intensity just above the lateral ventricle. When an imaginary line is drawn connecting anterior commissure and mamillary body, the genu was found to be the curved anterior portion of the CC projecting anterior to the line (Kier and Truwit). The rostrum is seen as a beak – shaped segment seen postero inferior to the genu. The splenium is caudally oriented round structure. The data obtained in this study were analysed statistically analysed using Pearson correlation test (2tailed).
Results
Length of Corpus callosum
The length of foetal CC ranged from 25.96 – 47.2 mm for 18 – 32 weeks of gestational age. The regression co efficient analysis done between the length of CC and the gestational age showed Y = -7.01+1.5(mm) *X. where Y= length of CC and X= gestational age (weeks). When correlating the length of CC with corresponding week of gestation, the value was 0.975(mm), which is statistically significant P˂0. 001.It was clearly seen that the length of foetal CC increases in an average of 1.5mm per week of gestation and the regression coefficient was statistically significant (P≤0.0001). Similarly, the individual width of parts of CC such as rostrum, genu, body and splenium also showed a highly significant P value when compared with corresponding gestational age.
Table 1: Shows the Morphometric parameters of Foetal corpus callosum
| Variables | Intercept | Beta | 95.0% Confidence Interval for B | R square | P value | |
| Lower bound | Upper bound | |||||
| Length of CC (mm) | 1.568 | 0.975 | 1.463 | 1.672 | 0.950 | ˂0.001 |
| Width of rostrum(mm) | 0.081 | 0.921 | 0.071 | 0.091 | 0.848 | ˂0.001 |
| Width of genu (mm) | 0.107 | 0.850 | 0.088 | 0.127 | 0.723 | ˂0.001 |
| Width of body(mm) | 0.103 | 0.797 | 0.080 | 0126 | 0.635 | ˂0.001 |
| Width of splenium (mm) | 0.102 | 0.874 | 0.086 | 0.119 | 0.764 | ˂0.001 |
| Frontal pole to genu(mm) | 0.530 | 0.884 | 0.449 | 0.611 | 0.782 | ˂0.001 |
| Occipital to splenium (mm) | 0.807 | 0.917 | 0.705 | 0.909 | 0.841 | ˂0.001 |
Width of rostrum
The width of rostrum ranged from 1.2 – 2.2 mm for 18 – 36 weeks of GA and the regression coefficient analysis showed width of rostrum = -0.576+0.08 X weeks and the R2 value was 0.848 mm which showed a statistically significant P value.
Width of genu
The width of genu ranged from 1.2 – 2.8mm for 18 – 36 weeks of gestational age and the regression coefficient for width of genu was =-0.701+0.10*X weeks and the R2value was 0.723mm which was statistically significant (P˂0.001).
Width of body
The width of body ranged from 1.3-3.1mm for 18-36 weeks of gestational age and the regression coefficient for width of body =-0.658+ 10*X weeks and the R2 value was 0.635mm which was again statistically significant (P˂0.001).
Width of splenium
The width of splenium ranged from 1.36-3.2mm for 18-36 weeks of gestational age and the regression coefficient for width of splenium = -0.359+0.10*X weeks and the R2 value was 0.764mm which was statistically significant (P˂0.001).
Similarly, the distance between the frontal pole and genu ranged between 8.47-19.6mm and also the distance between occipital pole and splenium ranged between 23.04-34.98mm at 18-36 weeks of gestational age and when correlated with corresponding gestational age the R2 value was 0.782mm and 0.841mm respectively and which was statistically significant (P˂0.001). The BPD, head circumference of the foetus ranged between 45.5-84.2mm for 18-36 weeks of gestational age and when correlated with length of CC corresponding to gestational age, it showed significant P Value P˂0.001.
A regression line between BPD and gestational age was derived in the present study to find out the BPD with known GA. Y = – 7.91+2.74 X where Y= BPD and X= GA (weeks).
In this study the regression analysis showed that the BPD increased in an average of 2.74mm per week of gestation and the regression co efficient was statistically significant (p≤0.0001). Using this equation, with known gestational age, the BPD of the foetus can be estimated. The results obtained in this study were correlated with the mean length of foetal CC corresponding with gestational age. Statistical analysis using Simple linear regression shows P ˂0.001. There was significant difference between all the variables with corresponding gestational age.
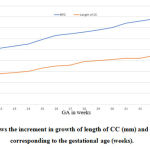 |
Figure 2: Shows the increment in growth of length of CC (mm) and BPD (mm), corresponding to the gestational age (weeks). |
Discussion
The present study found that the length of foetal CC increases in an average of 1.5mm per week of gestational age. These normative data of measurement of foetal corpus callosum indicates a normal CNS development and the knowledge of normal morphology and morphometry of foetal CC is highly useful to correlate or diagnose hypoplasia of foetal CC. With an advent of advanced MRI technology, visualization of foetal brain structures especially the corpus callosum is more effective detectable when compared to USG. Most of the gestational abnormalities are commonly diagnosed by prenatal ultrasonography using trans-abdominal or trans-vaginal scanning. But in few cases, such as ventriculomegaly or posterior cranial abnormalities it is difficult to evaluate the clear morphology of foetal CC. In certain conditions like maternal hypothyroidism or insufficient maternal thyroid hormone (TH) during pregnancy affect the development of foetal CC [12]. In TH deficiency the foetal CC development varies in size such as reduced number of fibres, abnormal maturation and myelination of axons [13,14]. Previous studies showed significant difference in size and shape of corpus callosum and the difference in the thickness of CC indicated a greater number of corticocortical fibres between the hemispheres [15]. In few studies larger CC (posterior splenial part) in humans were reported in left-handed males with dyslexia [5]. In few studies not only, the size but also changes in the shape of the CC were reported mainly in the population suffering from psychiatric disorders [16, 17]. In this study it is very clear that as the gestational age increases, the length of the corpus callosum also increases. Similar study was conducted by (Araujo et al, 2012) [4] and found that the length of corpus callosum was 19.52 ± 2.24mm at 20 weeks of gestation and 40.36 ± 2.87mm at 33weeks of gestation. The regression equation between corpus callosal length and gestational age was Y = –52.41 + 4.71 × gestational age – 0.06 × gestational age2 (R2 = 0.868). Wherein this equation highly correlated with length and gestational age of the present study. (Zhung et al, 2009) evaluated 622 Chinese foetuses between 16-39 weeks gestation using 2D trans abdominal sonographic acquisition in a sagittal plane [18]. The results showed a significant increase in the length of CC in a linear fashion corresponding to the gestational age (r=0.93; P≤0.001) and they found a regression line between length of corpus callosum and gestational age, where Y=-9.561 + 1.495* gestational age (weeks). In the present study the length of foetal CC slightly increased i.e. 1.51mm whereas in Chinese population it increased about 1.495mm this may be due to geographical variation. In 1993 Gustavo et al, [2] studied the length of foetal corpus callosum in 101 foetuses belonging to 18-42 weeks of gestation. The mean corpus callosal length was found to be 17mm at 18 weeks of gestation to 44mm at the term. The study reported that ratios of the length of corpus callosum to antero-posterior diameter of the brain remained relatively constant from 20 to 21 weeks of gestation. Apart from the ultrasonography the length of foetal corpus callosum was measured using foetal MRI by (Harrled et al, 2010) they studied 68 foetuses, between 18.5 to 37.7 weeks of gestation [19]. The results showed that the mean length of foetal corpus callosum was 28.2mm at 22 weeks of gestation and the r=0.828 showed high significance. A similar regression equation was calculated between length and gestational age, where Y=-40.37+4.017*gestational age-0.048(gestational age) which showed a high statistical significance (P≤0.001). The result of the above study correlated with the present study and this shows that the length of corpus callosum remains the same. By measuring the development of axonal fibres by means of corpus callosum will clearly indicate the direct development of CNS when compared to measuring of head circumference (BPD). In the present study, the length of CC was correlated with BPD with corresponding gestational age. Similar study was done by (Visentainer et al, 2010) in which the length and area of foetal corpus callosum and BPD were measured [20]. They correlated the mean foetal corpus callosal length with BPD and gestational age. The present study was carried out to set a reference range for an early deduction of abnormal callosal development and to set a valuable guide for pregnancy management. Even though agenesis of CC can be easily identified or visualized, hypogenesis of foetal CC required adequate knowledge of normative reference values of foetal CC morphology and morphometry. Throughout the MRI foetal study, the genu, body and splenium were clearly visualised and measured without any error for the entire gestational age between 18-36 weeks. But the rostrum, caudal portion of CC were difficult to visualise at the early gestational age. According to some authors, rostrum is visualized only at 20-22 weeks of GA [21]. Even though rostrum is not included in measuring the length of CC; absence of rostrum at early GA does not imply abnormality. This is because the corpus callosum develops from anterior to posterior. The direction of development is from genu, body, splenium followed by flexion of genu anteriorly forming the rostrum [22].
Conclusion
The periodic development of nervous system can be calculated more effectively with the morphometric measurements of foetal CC and correlating it with BPD. This is more accurate in comparison with BPD measurement of head circumference in USG. This knowledge will be highly helpful for the gynaecologist to predict the abnormal development of the foetus and it is advised to include foetal CC parameters as one of the tools for early detection of CNS anomalies.
Conflict of interest
There are no conflict of interest
Funding sourse
None
References
- Susan Standring,Gray’s Anatomy: The Anatomical Basis of Clinical Practice, Elsevier, London, UK, 40th edition. 2008;1086-1089.
- Gustavo Malinger, Haim Zakut. The Corpus Callosum: Normal Fetal Development as Shown by Transvaginal Sonography. AJR 1993; 161:1041-1043.
CrossRef - Gustavo Malinger, Dorit Lev, Tally Lerman-Sagie. The fetal corpus callosum. ‘The truth is out there’ – Opinion.Ultrasound in Obstetrics and Gynecology. 2007. 30(2):140-1.
CrossRef - Edward Araujo Júnior, Milena Visentainer, Christiane Simioni, Rodrigo Ruano, Antonio Fernandes Moron. Reference Values for the Length and Area of the Fetal Corpus Callosum on 3‐Dimensional Sonography Using the Transfrontal View.2012. J Ultrasound Med 2012; 31:205–212.
CrossRef - Goldstein I, Tamir A, Reece AE, Weiner Z. Corpus callosum growth in normal and growth restricted fetuses. Prenatal diagnosis. 2011; 31(12):1115-9.
CrossRef - Ana Monteagudo, Ilan E Timor-Tritsch. Normal sonographic development of the central nervous system from the second trimester onwards using 2D, 3D and transvaginalsonography. Prenat Diagn. 2009 Apr; 29(4):326-39.
CrossRef - Harreld JH, Bhore R, Chason DP, Twickler DM. Corpus callosum length by gestational age as evaluated by fetal MR imaging. American journal of neuroradiology. 2011 Mar 1; 32(3):490-4.
CrossRef - Ghi T, A Carletti, E Contro, E Cera, P Falco, G Tagliavini, L Michelacci, G Tani, A Youssef, P Bonasoni, N Rizzo, G Pelusi, G Pilu. Prenatal diagnosis and outcome of partial agenesis and hypoplasia of the corpus callosum. Ultrasound Obstet Gynecol. 2010 Jan; 35(1):35-41.
CrossRef - Glenn OA, Goldstein RB, Li KC, Young SJ, Norton ME, Busse RF, Goldberg JD, Barkovich AJ. Fetal magnetic resonance imaging in the evaluation of fetuses referred for sonographically suspected abnormalities of the corpus callosum. Journal of ultrasound in medicine. 2005 Jun; 24(6):791-804.
CrossRef - Girard NJ, Raybaud CA. In vivo MRI of fetal brain cellular migration. Journal of computer assisted tomography. 1992 Mar 1; 16(2):265-7.
CrossRef - Abe S, Takagi K, Yamamoto T, Okuhata Y, Kato T. Assessment of cortical gyrus and sulcus formation using MR images in normal fetuses. Prenatal Diagnosis: Published in Affiliation With the International Society for Prenatal Diagnosis. 2003 Mar; 23(3):225-31.
CrossRef - Samadi A, Skocic J, Rovet JF. Children born to women treated for hypothyroidism during pregnancy show abnormal corpus callosum development. Thyroid. 2015 May 1;25(5):494-502
CrossRef - Ferreira AA, Pereira MJ, Manhaes AC, Barradas PC. Ultrastructural identification of oligodendrocyte/myelin proteins in corpus callosum of hypothyroid animals. International journal of developmental neuroscience. 2007 Apr 1; 25(2):87-94.
CrossRef - Berbel P, Guadan A, Angulo A. Role of thyroid hormones in the maturation of interhemispheric connections in rats. Behavioural brain research. 1994 Oct 20; 64(1-2):9-14.
CrossRef - Coe CL, Lulbach GR, Schneider ML. Prenatal disturbance alters the size of the corpus callosum in young monkeys. Developmental psychobiology. 2002 Sep; 41(2):178-85.
CrossRef - Denenberg VH, Kertesz A, Cowell PE. A factor analysis of the human’s corpus callosum. Brain research. 1991 May 10; 548(1-2):126-32.
CrossRef - Kubik-Huch RA, Huisman TA, Wisser J, Gottstein-Aalame N, Debatin JF, Seifert B, Ladd ME, Stallmach T, Marincek B. Ultrafast MR imaging of the fetus. American Journal of Roentgenology. 2000 Jun; 174(6):1599-606.
CrossRef - Zhang HC, Yang J, Chen ZP, Ma XY. Sonographic study of the development of fetal corpus callosum in a Chinese population. Journal of Clinical Ultrasound. 2009 Feb;37(2):75-7.
CrossRef - Harreld JH, Bhore R, Chason DP, Twickler DM. Corpus callosum length by gestational age as evaluated by fetal MR imaging. American journal of neuroradiology. 2011 Mar 1; 32(3):490-4.
CrossRef - Visentainer M, Araujo Júnior E, Rolo LC, Nardozza LM, Moron AF. Avaliação do comprimento e área do corpo caloso fetal por meio da ultrassonografia tridimensional. Revista Brasileira de Ginecologia e Obstetrícia. 2010 Dec; 32(12):573-8.
CrossRef - Rakic P, Yakovlev PI. Development of the corpus callosum and cavum septi in man. Journal of Comparative Neurology. 1968 Jan; 132(1):45-72.
CrossRef - Kier EL, Truwit CL. The normal and abnormal genu of the corpus callosum: an evolutionary, embryologic, anatomic, and MR analysis. American Journal of Neuroradiology. 1996 Oct 1; 17(9):1631-41.