Manuscript accepted on :17-04-2021
Published online on: 17-05-2021
Plagiarism Check: Yes
Reviewed by: Dr. Y. Chen
Second Review by: Dr. Tejaswi Chavan
Final Approval by: Dr Anton R Kiselev
Sachin A. Patharkar , Uzma M J Shaikh, Neelam J. Patil*, Alka V. Nerurkar and Umesh- Shinde.
, Uzma M J Shaikh, Neelam J. Patil*, Alka V. Nerurkar and Umesh- Shinde.
Department of Biochemistry, Topiwala National Medical College and BYL Nair Charitable Hospital, Mumbai, India.400008.
Corresponding Author E- mail: neelamb99@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2185
Abstract
Lead is a toxic heavy metal whose widespread use has caused extensive environmental contamination and health problems particularly in children and women of reproductive age. It affects multiple body systems, including the neurologic, hematologic, gastrointestinal, cardiovascular, and renal systems. Recent studies have shown that lead, has the potential to harm not only the young and the occupationally-exposed, but also older people and females with in the reproductive age. They may have been exposed to lead while working in unregulated occupations, or they may have encountered more lead in the environment on a daily basis. The Aim of the present study is to estimate urinary δ-ALA levels in heterogenous population of females in the reproductive age as an index of lead exposure. This cross- sectional study was conducted on randomly chosen 80 females of reproductive age from different areas of Mumbai with brief history related lead exposure. The morning first mid stream urine samples were collected with all standard precautions and analyzed for δ-ALA using modified Ehrlich’s reagent method. Out of the 80 samples – 69 samples (86.25%) urinary δ-ALA had levels above reference level i.e. (< 5mg/l). This is alarming. The reasons for this exposure may vary and we could not find a single cause for such a high level exposure, but we can say that it is closely related to the duration of exposure. Hence it is to conclude that it is difficult to stop lead exposure completely but by taking necessary precautions and providing health education we can reduce the lead exposure and its ill effects on health.
Download this article as:| Copy the following to cite this article: Patharkar S. A, Shaikh U. M. J, Patil N. J, Nerurkar A. V, Shinde U. Estimation of Urinary Delta Aminolevulinic Acid Levels in Females of Reproductive Age as an Index of Lead Exposure. Biomed Pharmacol J 2021;14(2). |
| Copy the following to cite this URL: Patharkar S. A, Shaikh U. M. J, Patil N. J, Nerurkar A. V, Shinde U. Estimation of Urinary Delta Aminolevulinic Acid Levels in Females of Reproductive Age as an Index of Lead Exposure. Biomed Pharmacol J 2021;14(2). Available from: https://bit.ly/3tSvkTl |
Introduction
Lead is a toxic heavy metal whose widespread use has caused extensive environmental contamination and health problems particularly in children and women of reproductive age. They may have been exposed to lead while working in unregulated occupations or they may have encountered more lead in the environment on a daily basis. Additionally young female population get exposed to lead by use of Kohl or Kajal eyeliners, some traditional ethnic medicines, time spent at firing ranges and some hobbies like wall paintings or jobs related to plumbing and soldering. Young population also gets exposed to lead by eating junk foods wrapped in newspaper, licking of fingers for turning the photocopies, book pages, printout etc and use of same fingers for eating purpose 1. Lead poisoning among exposed persons is known to pose serious health problems on the nervous system, heme biosynthesis, kidneys, reproductive system and cardiovascular system 2. In young women of reproductive age lead exposure reduce fertility potential and increases chances of miscarriages, preterm birth, and so on. Lead affects female reproduction by impairing menstruation, delaying conception time, altering the hormonal production and circulation 3. Recent studies have shown that lead, has the potential to harm not only the young and the occupationally-exposed, but also older people and females within the reproductive age 4. The effect of lead on heme synthesis is attributed to the inhibition of enzymes involved in heme synthesis, resulting in abnormal concentrations of heme precursors in blood and urine. Essentially, lead interferes with the activity of three enzymes: it indirectly stimulates the mitochondrial enzyme aminolevulinic acid synthetase; it directly inhibits the activity of the cytoplasmic enzyme aminolevulinic acid dehydratase (ALAD); and it interferes with the normal functioning of intramitochondrial ferrochelatase. This results in increased levels of delta aminolevulinic acid (δ-ALA) excretion through urine 5, 6, 7. Hence we planned to estimate urinary δ-ALA as a marker of lead exposure in study population.
The aim of the present study is to find out the prevalence of lead exposure among the females of reproductive age, in sub urban Mumbai, by estimating the urinary δ-ALA levels as an index of lead exposure and educate the community of major health problems associated with elevated lead levels, the paths of lead exposure in their environment and ways they can protect themselves and their families from lead exposures.
Materials and Methods
This is a cross-sectional pilot observational study in suburban Mumbai. Urine samples of 80 females of reproductive age with due informed written consent were collected, by random sampling with due ethical considerations. Urine samples were collected in 15-mL plastic containers, covered with brown paper, exercising standard precautions. First morning midstream urine samples were collected after cleaning local area. Sample size was decided on the basis of formula:
n = 4pq/l2
where l is permissible error in the estimation of new statistics, p is positive character, and q is 1 − p. Prevalence to estimate sample size found from previous studies of own institute, and National journals.
Urine samples were analyzed for δ-ALA by using Ehrlich’s reagent in which acidic urine reacts with n-butanol and δ-ALA was converted to its pyrrole at pH 6.8. The pyrrole reacted with Ehrlich’s reagent to form red color, which was extracted with chloroform and read colorimetrically 8. Comparing this method with other methods like ion exchange chromatography, the method discussed by Tomokuni et al., it is found that this method being colorimetric is easy, rapid, and accurate as all interfering substances are removed by butanol extraction 9,10. The procedure is standardized, and graph is plotted prior to use on subjects.
Results and Discussion
In the present study, 80 randomly collected urine samples from females of reproductive age group were analyzed for δ-ALA levels. The analyzed samples were categorized into δ-ALA levels below reference value and δ-ALA levels above reference value. δ-ALA concentration equal to or more than 5mg/litre is considered as above reference value and δ-ALA concentration less than 5 mg/litre is considered as below reference value 11, 12.Out of 80 samples 46 (65.71%) were categorized;69 samples (86.25%) were categorized as above reference level and remaining 11 samples (13.75%) as below reference level i.e. (< 5mg/l).
Statistical Analysis: The main outcome parameter urinary δ-ALA level is a continuous scaled data, to find out the prevalence of lead exposure we converted this data into categorical data depending upon the reference range of urinary δ-ALA levels. Hence we calculated only percentage of high exposed individuals. No other statistical test is required.
Standard error of proportion S.E.P =
 So, 95% confidence interval 78.51–93.99
So, 95% confidence interval 78.51–93.99
In this study, we estimated the lead exposure in study group by measuring urinary marker, i.e., δ-ALA levels. The activity of δ-ALA dehydratase is markedly decreased by lead, by which there is an increase in δ-ALA excretion in urine 13. By measuring the urinary δ-ALA, we can estimate the lead exposure 14, 15. The quantitative estimation of δ-ALA is basically based on the well-known reactivity of pyrroles with p-dimethylamino benzaldehyde. Elevated δ-ALA concentrations were indicated by a reddish color in chloroform, while normal concentrations usually gave only faint yellow or faint red colours. Urine usually contains many substances which react with Ehrlich reagent to form red colour and also contains some substances which interferes with the formation of pyrroles and aldehydes. These substances are removed by n-butanol extraction. Small amounts of Ehrlich positive substances which escaped the n-butanol extraction formed a red colour on addition of Ehrlich’s reagent, but this never entered the chloroform phase. The only substance that behaved like δ-ALA was aminoacetone. Urinary aminoacetone levels in lead poisoning have been reported to be entirely normal. The specificity is good enough to use this method for screening for lead exposure 16. Normal urinary δ-ALA excretion by adult healthy men averaged 1.6 mg/l 17.
Table 1: Statistical parameters
| Statistical parameter | Urinary δ-ALA below reference
level (≤5 mg/l |
Urinary δ-ALA above reference
level (>5 mg/l |
| *No. of samples | 11 | 69 |
| Percentage of exposure | 13.75% | 86.25% |
| Mean | 2.63 | 17.13 |
| Standard deviation) | ±1.32 | ±5.58 |
*urine from study population (students).
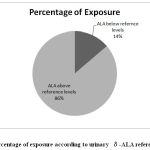 |
Figure 1: Percentage of exposure according to urinary δ-ALA reference value. |
Table 2: Age distribution of females.
| Age in years | Urinary δ-ALA below reference
level (≤5 mg/l |
Urinary δ-ALA above reference
level (>5 mg/l |
| 18-25 | 11 | 22 |
| 26-33 | – | 20 |
| 34-41 | – | 18 |
| 42-49 | – | 9 |
Out of the above categorized 69 females
64 were categorized as Acceptable – High exposure level.
5 were categorized as Dangerous – Very High exposure level.
Table 3: Categorization of subjects based on acceptable and dangerous urinary δ-ALA.
| Statistical Parameter | Acceptable | Dangerous |
| Number of females | 64 | 5 |
| Percentage | 92.75% | 7.25% |
The highest level of urinary δ-ALA found in this study was 29 mg/l. This clearly indicates that the individuals are already at a high risk of lead exposure. Further 11 samples in this study have δ-ALA below reference level. This indicates we can prevent these individuals from detrimental effects of lead exposure by explaining necessary precautions. These includes avoiding the use of canned foodstuffs and eating food wrapped in newspaper, minimizing the uses of Xerox/ printout and avoiding the habit of licking the fingers to turn Xerox/ printout pages, regularly checking the blood lead levels, washing hands, drinking clean and filter water. Avoiding use of lead based eyeliners and nail paints, lipsticks etc.
To reduce this exposure we should eliminate non-essential uses of lead such as lead in paint, ensuring the safe recycling of lead-containing waste, educating the public about the importance of safe disposal of lead-acid batteries and computers, and monitoring of blood lead levels in children, women of child-bearing age and workers4.We can reduce the lead exposure to an extent by taking necessary precautions.
Conclusion
The study sample represents females of reproductive age,who are not only the backbone of our society but also the health of future generations depend upon their health. It is important to make them aware about lead exposure and its detrimental effects in their daily routine. The results obtained from the study shows that more than half of the study population have already been exposed to lead. It is now our duty to save the rest of the students from ill effects of lead exposure and therefore, it is important to educate them regarding standard precautions to avoid lead exposure.
Acknowledgement
We acknowledge Department of Biochemistry,TNMC and BYL Nair hospital for funding thisproject.
Conflict of Interest
Authors have declare that no conflict of interest exist.
Funding Source
Departmental fund, Department of Biochemistry, T.N.M.C and B.Y.L Nair Charitable Hospital.
Reference
- Patharkar SA, Jain SH, Nerurkar AV, Patil NJ, Surve PN. Estimation of Urinary Delta Aminolevulinic Acid (δ-ALA) Levels in Students of Age Group 15 to 25 Years as an Index of Lead Exposure. International Journal of Biochemistry Research & Review (IJBcRR). 29, 4 (May 2020), 12-16.
CrossRef - World Health Organization Regional Office for Europe Air Quality Guidelines. Copenhagen; 2001.
- Winder C. Lead, reproduction and development. Neurotoxicology.1993; 14:303–17.
- Definition of Lead by World Health Organization.www.who.int/ipcs/assessment/public_health/lead/en
- Patharkar SA, Benwal SJ, Nerurkar AV, et al.Estimation of Urinary Delta Aminolevulinic Acid Levels in Garage Workers as an Index of Lead Exposure. Indian J Med Biochem 2019;23(3):312–315..
CrossRef - Kappas A, Sassa S, Galbraith RA, Nordmann Y. In: Scriver CR,Beaudet AL, Sly WS, Valle D, editors. The metabolic basis ofinherited diseases. 7th Ed. USA:McGraw Hill. 1995;2103–59
- DM Vasudevan, Sreekumari S, KananVaidyanathan. Textbook of Biochemistry for medical students 9th Edition, New Delhi India: Jaypee Brothers Medical Publishers; 2019. p. 363.
- Osamu Wada, KoheiToyokawa,Gumpei Urata, Yuzo Yano, Kiku Nakao. A simple method for the quantitative analysis of urinary delta-aminolevulinic acid to evaluate lead absorption, Brit. J. industr. Med., 1969, 26: 240-243
CrossRef - Cramer, K. and Selander, S. Studies in lead poisoning: Comparison between different laboratory tests. Brit. J. Industr. Med. 1965, 22: 311-314.
CrossRef - Davis, J. R. and Andelman, S. L.: Urinary delta-aminolevulinic acid (ALA) levels in lead poisoning. A modified method for the rapid determination of urinary delta- aminolevulinic acid using disposable ion-exchange chromatography columns. Arch. Environ. Health. 1967, 15: 53-59.
CrossRef - Tomokuni K, Ogata M. Simple method for determination of urinary deltaaminolevulinic acid as an index of lead exposure. Clin Chem. 1972; 18(12):1534–1536.
CrossRef - KatsumaroTomokuni, Masayoshi Ichiba and Yukio Hirai. Measurement of urinary δ-aminolevulinic acid (ALA) by fluorometric HPLC and colorimetric methods. Industrial Health. 1992;30:119-128.
CrossRef - Tanabe Y. Metabolism of deltaaminolevulinic acid (δ-ALA) andporphobilinogen in lead poisoning.Amounts of δ-ALA and PBP in the urine and blood. Jap. J. Nat.Health. 1959; 28:386-397.
- Masana Ogata, Toyohiro Taguchi. Quantitative determination of urinary deltaaminolevulinic acid as an index of lead exposure by high performance LiquidChromatography. Industrial Health. 1986; 24:259-264.
CrossRef - Patharkar S, Chavan S, Phadke MS, Patil NJ, Bokankar DK. Estimation of urinary δ aminolevulinic acid levels (δ-ALA) in children of age group 1 to 5 years as an index of lead exposure. MRIMS J Health Sciences. 2018; 6(1):16-19.
CrossRef - KatsumaroToniokuni, YukloHiral. Factors affecting determination of δ-aminolevulinate by use of ehrlich’s reagent. Clin. Chem. 1986; 32/1:192-193.
CrossRef - Wada O, Toyokawa K, Urata G, Yano Y, Nakano K. A simple method for the quantitative analysis of urinary deltaaminolevulinic acid to evaluate lead absorption, Br. J. Ind. Med. 1969; 26:240.
CrossRef







