Manuscript accepted on :04-03-2021
Published online on: 23-03-2021
Plagiarism Check: Yes
Reviewed by: Dr. B.Surendiran
Second Review by: Dr. Bhanu Lakhani
Final Approval by: Dr. Francesca Gorini
Doha M. Beltagy1 , Khloud Gamal Abdelsalam1
, Khloud Gamal Abdelsalam1 , Tarek M Mohamed2
, Tarek M Mohamed2 and Mai M. El-Keey2
and Mai M. El-Keey2
1Department of Chemistry, Biochemistry Division, Faculty of Science, Damanhour University, Egypt.
2Department of Chemistry, Biochemistry Division, Faculty of Science, Tanta University, Egypt.
Corresponding Author E-mail: dohabel4@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2106
Abstract
Liver cirrhosis is currently the 11th most common cause of death which includes inflammatory, oxidative damage, and immune response. Harmaline has antioxidant and anti-inflammatory mechanisms which can defeat against hepatic cirrhosis pathways. The present work aimed to evaluate the ameliorating effect of harmaline against liver cirrhosis induced by thioacetamide in mice. The study was carried out on sixty male mice divided into three main groups. Control and harmaline groups (GIa and GIb), thioacetamide-group (GII) and harmaline co-treated and treated groups (GIIIa and GIIIb). By the end of the experiment, adiponectin concentrations were measured in serum and liver tissue. Gene expression of adiponectin, transforming growth factor beta-1 (TGF-β1), tissue inhibitor metalloprotease-1(TIMP-1) and peroxisome proliferator activated receptor-gamma (PPAR-γ) were assessed. Some oxidative stress biomarkers as malondialdehyde, reduced glutathione, catalase, superoxide dismutase and nitric oxide were determined. The results indicated that harmaline administration cause significant suppression of oxidative stress and inflammatory response.Inhibition of hepatic stellate cell activation and extracellular matrix deposition were also noticed with a significant decrease in the expression of the profibrotic markers(TGF-β1 and TIMP-1) which have direct effects on adiponectin activation. These results were confirmed by the histological studies in liver tissue. In Conclusion,Harmaline has excellent protective role against liver cirrhosis induced by thioacetamide in mice via its antioxidant and anti-inflammatory properties.It can be therapeutically used as a safe liver support by a dose of 10 mg/kg after furtherin vivo studies.
Keywords
Adiponectin; Cirrhosis; Harmaline; Malondialdehyde; Thioacetamide
Download this article as:| Copy the following to cite this article: Beltagy D. M, Abdelsalam K. G, Mohamed T. M, El-Keey M. M. Role of Harmaline as Adiponectin Modulator in Defeating Liver Cirrhosis Induced By Thioacetamide in Mice. Biomed Pharmacol J 2021;14(1). |
| Copy the following to cite this URL: Beltagy D. M, Abdelsalam K. G, Mohamed T. M, El-Keey M. M. Role of Harmaline as Adiponectin Modulator in Defeating Liver Cirrhosis Induced By Thioacetamide in Mice. Biomed Pharmacol J 2021;14(1). Available from: https://bit.ly/2PdVe5j |
Introduction
Liver is an organ that performs a vital metabolic detoxification of endogenous and exogenous compounds.1 Liver diseases account approximately two million deaths each year worldwide, one million due to complication of cirrhosis which is currently the 11th most common cause of death representing 3.5% of all deaths worldwide.2
The main pathogenic mechanisms for liver damages are oxidative stress,dysfunction of cytochrome P450, inflammation and mitochondrial dysfunction. At the molecular level, liver cirrhosis results from the activation of hepatic stellate cells (HSCs), which differentiate into proliferative migratory myofibroblasts which accumulate in areas of hepatocyte apoptosis and necrosis. These cells secrete extracellular matrix (ECM) proteins including collagens and inhibit ECM degradation by the secretion of inhibitors, leading to formation of scar tissue within the liverand then to chronic cirrhosis.1
Thioacetamide is a potent hepatotoxin. Oxidative injury is the main mechanism in thioacetamide induced liver damage, by metabolizing through the hepatic flavin containing monooxygenase system and cytochrome P-450 monooxygenase system produces reactive oxidative agents especially the very reactive compound thioacetamide-S-dioxide which targets tissue macromolecules as lipids, protein, and DNA leading to tissue oxidative injury and necrosis.3
Adiponectin (ADN) is one of the several hormones secreted mainly by adipose tissue, and several others as bone, placenta cardiomyocytes, pituitary gland, and skeletal muscle, thus generating a local high concentration of the hormone as autocrine secretion. In the liver, adiponectin has a specific predominantly expressed receptor. It regulates both glucose and lipid metabolism and exerts an insulin-sensitizing effect.4 Adiponectin protects against liver injuries via its anti-inflammatory activity and improves hepatic liver accumulation via the antagonism of tumor necrosis factor (TNF). 5
Harmaline (HAL) is the abundant pharmacological β-carboline alkaloids of Peganumharmala L. It exhibits numerous clinical effects, includingreduction of inflammation, protection from radiation, analgesia, immunosuppression, antipruritic effects, relief from psoriasis and antitumor activitiesagainst human liver carcinoma.6
The present work aimed to evaluate the ameliorating effect of harmaline against liver cirrhosis induced by thioacetamide (TAA) in mice via studying the expression of some genes and their effects on adiponectin secretion, determination of different oxidative stress biomarkers and histopathological analysis.
Materials and Methods
Chemicals
Adiponectein Kit was purchased from Elabscience Biotechnology (Cat No. E-EL-M0002). Harmaline (HAL), thioacetamide (TAA), Phosphate buffer (PBS, pH 7.4), thiobarbituric acid (TBA), trichloroacetic acid (TCA) and n-butanol, Disodium hydrogen phosphate solution and 5,5′-dithiobis-2-nitrobenzoic acid (DTNB), Folin Lowry reagent, Bovine serum albumin (BSA), Cadmium powder, Sodium nitrite, zinc sulfate, sulphanilamide, phosphoric acid, N-1-naphthyle ethylene diamine and all other chemicals used were purchased from Sigma-Aldrich, Co., Ltd. (St.Louis, MO, USA). Kits for assessment of liver function (γ-GT, ALT, AST, Albumin and T.P) and kidney function (urea, creatinine) profiles, superoxide dismutase (SOD), and malondialdehyde (MDA) kits were purchased from Bio-Diagnostics, Egypt.
Animal grouping
The study employed 60 albino male mice (C57BL/6J strain (weighing 25-30g, kept in breeding cages and receiveda similar basic care with standard diet (Egyptian Company of Oils and Soap, Kafr-Elzayat, Egypt) and water ad libitum. Animal maintenance and treatments were conducted in accordance with Faculty of Science, Tanta University guide for animals, as approved by the Institutional Animal Care and Use Committee (REC-SCI-TU-00177). Mice weregrouped into three main groups which in turn divided into five subgroups each one contains 12 mice as follow:
Group Ia
(Normal control Group): mice fed with ordinary diet only without receiving any treatment during the entire experimental period of 10 weeks.
Group Ib
(HAL Group): mice were intraperitoneally injected with harmaline by a dose of (10 mg/kg b. wt.) twice a week for 6 weeks.7
Group II
(TAA Group): mice were intraperitoneally injected with Thioacetamide by a dose of (150 mg/kg b. wt.) twice a week for 4 week.8
Group IIIa
(Co-treated Group): mice were administered with Thioacetamide and Harmaline concurrently twice a week (by their recommended doses).
Group IIIb
(Treated Group): mice were firstly injected with TAA for successive 4 weeks then then it treated with Harmaline for other successive 6 weeks.
During the experimental period, all doses were adjusted every week according to any change in body weight to maintain similar dose per kg body weight of mice over the entire period of study for each group.
Samples Collection
At the end of the experiment, blood samples were gathered from the orbital sinus in plain tubes after anesthetized mice by utilizing diethyl ether. The specimens were centrifuged at 5000 rpm for 10 minutes to obtain serum. The liver tissues were instantly removed and partitioned into two parts. Each part is washed with ice-cold saline solution. The first part was fixed in 10% formalin to be used in histopathological analysis, while, the other part and the obtained serum samples was stored at -80 °C until used in biochemical investigations.
Preparation of liver homogenate
One gram of liver tissue was cut into small pieces and immersed into ice-cold 0.1M phosphate buffer (PBS pH 7.4), and then it was homogenized to obtain 10% (w/v) homogenates which were centrifuged at 4oC at 12,000 rpm for 10 min, and the supernatants were separated and then stored at -80o C until use.
Liver and kidney tests in serum
Activities of serum aspartate aminotransferase (AST, EC 2.6.1.1.), alanine aminotransferase (ALT, EC 2.6.1.2), Gamma glutamyl transferase (γ-GT, EC 2.3.2.2) and concentrations of albumin (ALB), total proteins, urea and creatinine were determined following the recommended procedures of the commercial kits (Bio-diagnostic, Egypt, Cat no.# AT1034, AT1045, AB-1010, TP-2020, UR-2110, CR-1250, respectively).
Adiponectin and Oxidative stress markers
Adiponectin concentrations were assayed in serum and liver homogenate using ELISA-kit (Elabscience Biotechnology Co., Ltd, USA; Cat.no.#E-EL-M0002). Superoxide dismutase (SOD, EC 1.15.1.1) and malondialdehyde (MDA) levels were measured following the instructions of the used commercial kits (Bio-diagnostic, Egypt, CAT no.SD-2521 and MD-2529, respectively). Reduced glutathione)GSH) was analyzed using Disodium hydrogen phosphate solution and 5,5′-dithiobis-2-nitrobenzoic acid (DTNB).9 Nitric oxide (NO) and catalase (CAT, E.C. 1.11.1.6) were determined following recommended assays.10-11 Total protein (TP) contents were assessed by Folin-Lowry method using bovine serum albumin as a standard.12
RNA Isolation and Quantitative Real-time PCR
Total mRNA from liver tissues was isolated by using total RNA Purification Kit following the manufacturer protocol (Thermo Scientific, Fermentas, #K0731).A260/280 ratios and RNA quantity were determined by a NanoDrop (ND-1000 spectrophotometer). 1000 ng of total RNA was reverse-transcribed into cDNA usingRevert Aid-H minus Reverse Transcriptase which is a genetically modified M-MuLVRT, to convert RNA into cDNA (Thermo Scientific, Fermentas, #EP0451). Specific primersused in the amplification of the measured genes (adiponectin, ADN; Tumor growth factor beta, TGF-β1; peroxisome proliferator-activated
receptor gamma, PPARγ, tissue inhibitor metalloprorease, TIMP-1and the housekeeping gene Glyceraldehyde 3-phosphate dehydrogenase, GAPDH-EC 1.2.1.12) obtained from the gene bank of web based tool, ncbi(https://www.ncbi.nlm.nih.gov)are shown in Table 1.
Table 1: Forward and reverse primers sequence for ADN, TGF-β1, PPARγ and TIMP-1 and housekeeping (GAPDH) genes.
|
Gene |
Forward primer
(/5 —— /3) |
Reverse primer (/5 —— /3) |
| ADN | GGTCCTGATTGGATGTGCCA | ACTGGACTCACCCTGCAAAG |
| TGF-β1 | AAGGGCTACCATGCCAACTT | CTGACTCCCCACTGCTCTAA |
| PPARγ | GGCTTGAACTGCATTGTCCC | AGGGAAACCCACGAAGACAC |
| TIMP-1 | CTTCTTGGTTCCCTGGCGTA | GTGATTGGGTTTGGGCAGC |
| GAPDH | TCACCACCATGGAGAAGGC | GCTAAGCAGTTGGTGGTGCA |
Table 2: Serum levels of liver and kidney functions.
| Group | ALT
U/L |
AST
U/L |
γ-GT
U/L |
TP
g/dl |
ALB
g/dL |
Urea
mg/dL |
Creatinine
mg/dL |
| GIa | 99.8±3.6 | 121.4±2.2 | 1.60±0.72 | 5.8±0.87 | 3.3±0.51 | 31.2 ±1.8 | 0.59±0.02 |
| GIb | 96.5±1.2b | 114.7±3.4b | 2.07±0.47b | 6.2±0.37b | 3.1±0.13b | 32.2±1.7b | 0.61±0.02b |
| GII | 155.1±3.2a | 256.3±3.9a | 15.2±3.1a | 3.9±1.2a | 2.3±0.26a | 65.2±1.1a | 1.05±0.04a |
| GIIIa | 125.4±3.7ab | 172 ±4.4ab | 3.2±1.3ab | 6.0±0.41b | 2.8±0.02ab | 54.1±3.2a | 0.73±0.01ab |
| GIIIb | 101.1±3.3b | 122.1±7.1b | 1.67±0.81b | 5.6±0.75b | 3.08±0.22b | 32.1±1.7b | 0.61±0.02b |
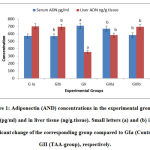
Small letters (a) and (b) indicate a significant change of the corresponding group compared to GIa (Control) and GII (TAA-group), respectively.
Table 3: Oxidative stress markers levels in the experimental groups.
| Group | SOD
U/mg.protein |
CAT
U/mg.protein |
GSH
mg/g.tissue |
MDA
nmol/g.tissue |
NO
μmol/g.tissue |
| GIa | 1.41±0.1 | 13.1±0.35 | 17.3±0.52 | 0.86±0.03 | 6.8±0.31 |
| GIb | 1.44±0.14b | 11.2±0.79b | 16.0 ±1.3b | 0.81±0.07b | 7.2±0.35b |
| GII | 1.04±0.05a | 8.0±0.05a | 9.8±0.76a | 3.1±0.02a | 35.1±0.25a |
| GIIIa | 1.36±0.04b | 11.1±0.54b | 15.7±0.08b | 1.1±0.014ab | 26±0.28ab |
| GIIIb | 1.41±0.20b | 12.3±0.48b | 15.8±0.65b | 0.94±0.025b | 9.1±0.93b |
Small letters (a) and (b) indicate a significant change of the corresponding group compared to GIa (Control) and GII (TAA-group), respectively.
The final reaction mixture was placed in a Step One plus real time thermal cycler (Applied Biosystems, Life technology, USA) and the PCR program was carried out with the PCR conditions.
Histopathological Examination
The standard protocol of tissue staining by hematoxyline and eosin13was applied and slides were examined and reviewed.
Statistical Analysis
The obtained data was statistically analyzed using SPSS- software (version 25.0), and the data were expressed as mean ± standard deviations (SD) and it statistically analyzed by one-way ANOVA (Analysis of Variance) for multiple comparisons. P values less than 0.05 were considered significant.
Results and Discussion
In the present study, liver cirrhosis in mice was induced by thioacetamide which cause cirrhosis resembling that in humans.14The metabolite thioacetamide-S-dioxide is more toxic than TAA itself and leads to hepatotoxicity by targeting all tissue macromolecules as lipids, protein, and DNA leading to liver oxidative injury, necrosis and cirrhosis.15-16
The obtained results from this study indicated the cirrhotic effects and liver dysfunction induced by TAA appeared in the highly significant elevation in all liver enzymes as AST, ALT and γ-GT and significant decrease in TP and albumin in mice injected with TAA (GII) if compared with control groups (G1a, mice did not receive any treatment and GIb, mice administered with only harmaline). Kidney function tests as urea and creatinine also showed significantly increase reached to about 2 folds by TAA injection. Harmaline treatment showed excellent improvement in all these serum biomarkers especially in treated group (GIIIb). All these results are in line with many previous studies.17-18
Oxidative stress markers results in the liver tissues of TAA-injected mice (GII) showed highly significant increase in MDA and NO reached to about 3.6 and 5.16 folds, respectively if compared to control mice (GIa). In contrast, SOD, CAT and GSH results showed significant decrease. Harmaline treatment improved these parameters to their nearly normal values especially in GIIIb. It can be explained by the confirmed strong antioxidant effects of harmaline.7
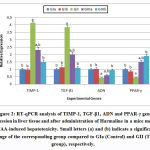
To explain the anti-cirrhotic properties of harmaline, adiponectin concentrations in serum and liver tissue were determined in addition to the relative concentrations of ADN, TGF-β1, PPAR-γ and TIMP-1 gene exressions. Adiponectin is a well-known anti-cirrhotic hormone. It is a potent inhibitor of HSCs activation. It reduces pro-fibrogenic transformation and proliferation, prevent apoptosis, mediate collagen secretion, and increase secretion of liver anti-inflammatory cytokines and down-regulates the expression of proinflammatory cytokines as TGF-β1and TIMP-1.19Numerous studies in liver injury have demonstrated that, fibrosis is stimulated in mice lacking adiponectin.20-21 Adiponectin also plays role in recovery of liver injury, by regulating hepatocyte proliferation.22 Lower serum adiponectin concentrations are associated with the development of steatosis, inflammation and fibrosis in the liver. Hypo-adiponectinemia is associated with severity of hepatic fibrosis.23However, high adiponectin levels in serum were associated with an increased risk to develop HCC.24 Therefore, plasma adiponectin concentrations are considered a good biomarker for the development of various liver diseases.25
Adiponectin binding with its specific receptors (AdipoR1 and AdipoR2) induces the activation of a proper signaling cascade that becomes altered in liver pathologies and play important roles in adiponectin anti-fibrotic properties. HSCs and Kupffer cells constitutively express the same amount of AdipoR1 and AdipoR2.26 Increased expression of AdipoR2 results in suppression of the proinflammatory cytokines as TGF-β1 and TIM-1 induced ROS production by stimulating PPAR-γ (peroxisome-proliferator-activated receptor-α) activation.25Adiponectin signal transduction in the liver is conducted primarily through activation of AMPK by phosphorylation at threonine (Thr172), although there is also evidence of a role for PPARα mediated signaling in response to adiponectin.27
TGF-β1 is a main profibrotic marker that triggers HSC differentiation and ECM synthesis. TGF-β1 elevated expression in liver is correlated with liver cirrhosis of different etiologies.28 Harmaline affects directly on increase in TGF-β1 expression induced by TAA or indirectly through activation of adiponectin.The activation and proliferation of HSCs-induced by TGF-β1, leptin and others can be lowered by the pharmacological activation of Adenosine monophosphate-activated protein kinase(AMPK) stimulated by adiponectin, subsequently blocking the secretion of TIMP-1 and significantly increasing MMP-1 activity.29
Matrix metalloproteases (MMPs) regulate ECM homoeostasis by catalyzing the degradation of various ECM components. MMP-1, or collagenase, is produced by activated HSCs and catalyses proteolysis of fibrillar collagens. TIMPs, on the other hand, regulate ECM homoeostasis by binding a particular MMP to prevent its activity.The findings suggest that the anti-fibrogenic inhibition signalling by adiponectin may reduce stimulated formation of extracellular TIMP-1–MMP-1 complexes.30
Activated HSCs (myofibroblasts) are key effectors of the fibrogenic response in the liver. Recent evidence has emphasized an adipogenic transcriptional program in stellate cells that is regulated by typical transcription factors in this pathway, including peroxisome proliferator-activated receptor-γ (PPARγ).31-32
Due to the significant role of PPARγ in defeating against liver diseases, the identification of PPARγ agonists or activators is regarded as targets of numerous drug development works. From the obtained results, harmaline can activate PPARγ. PPARγ was up-regulated after overexpression of adiponectin in stellate cells,the similarities between PPARγ and adiponectin effects, showed that adiponectin may have acted in a PPARγ-dependent manner.32
From the obtained results, when the concentrations of adiponectin in liver tissue decreased, it increased in the blood. The possible explanation might be attributed to a reduction in adiponectin clearance where it is cleared from the circulation primarily by the liver. In liver cirrhosis, declined adiponectin clearance could result from its reduced uptake by liver sinusoidal endothelial cells (LSECs), which lead to elevated adiponectin levels in the circulation.It is widely known that the dysfunction of LSECs is one of the pathologic events in liver fibrogenesis. Generally, in the healthy liver LSECs promote the quiescence of HSCs. During the cirrhosis process, LSECs undergo phenotypic changes with the loss of several receptors and LSECs fenestration, leading to the capillarization of liver sinusoids and the abnormality of various substances uptake.21
It was also reported the increased systemic adiponectin in liver cirrhosis. Importantly, it is found that elevated adiponectin is independent of disease etiology. High adiponectin even increases the risk to develop hepatocellular carcinoma suggesting that the well-characterized hepatoprotective and anti-carcinogenic effects of this adipokine are blocked. All this finding principally indicates that high adiponectin is indeed related to liver cirrhosis and further suggested the central function of the liver in the adiponectin excretion.33
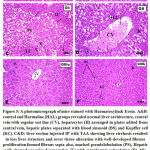 |
Figure 3: A photomicrograph of mice stained with Haematoxylin& Eosin. |
A&B: control and Harmaline (HAL) groups revealed normal liver architecture, central vein with regular out line (CV), hepatocytes (H) arranged in plates radited from central vein, hepatic plates separated with blood sinusoid (BS) and Kupffer cell (KC). C&D: liver section injected IP with TAA showing liver cirrhosis resulted in loss liver structure and sever tissue alteration with well-developed fibrous proliferation formed fibrous septa also, marked pseudolobulation (PS), Hepatic cells showed vascular degeneration (V) with cytoplasmic reticulation (R), bile ductules proliferation indicated by stars necrotic nuclei (N) and leucocyte infiltration (LI).E: GIIIa liver section showing partially preserved hepatocytes (H)with thinner incomplete hepatic septa, small area of leucocytes infiltration. F:GIIIb liver section showing liver tissue preserved it’s nearly normal hepatic architecture, marked degree of recovery with hepatocytes (H), central vein (CV) and radiating hepatic strands separated with normal blood sinusoids (BS) and Kupffer cells (KC).
Conclusion
In order to development of anti-cirrhotic therapies, considerable previous researches demonstrated the potentially contradicting effects of adiponectin as a potent novel regulator of liver fibrogenesis. The present study demonstrates for the first time that harmaline has anti-cirrhotic effects by significantly increase the expression of ADN andPPAR-γ genes in livers of TAA-induced cirrhotic mice. Harmaline decrease the oxidative stress markers in serum and liver tissue of treated mice with markedly significant decrease in TGF-β1 and TIMP-1 gene expressions. Harmaline could be a novel therapeutic approach for the treatment of liver cirrhosis.
References
- Marcellin P, Kutala BK. Liver diseases: A major, neglected global public health problem requiring urgent actions and large‐scale screening. Liver Int. 2018;38:2-6.
CrossRef - Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J hepatol. 2019;70(1):151-71.
CrossRef - Bruck R, Shirin H, Aeed H, Matas Z, Hochman A, Pines M, Avni Y. Prevention of hepatic cirrhosis in rats by hydroxyl radical scavengers. J hepatol. 2001;35(4):457-64.
CrossRef - Gamberi T, Magherini F, Modesti A, Fiaschi T. Adiponectin signaling pathways in liver diseases. Biomedicines. 2018;6(2):52.
CrossRef - Erotides da Silva T, Costa-Silva M, Correa CG, Denardin G, Ayres Alencar ML, Pacheco Honório Coelho MS, muraro-wildner L, luiza-bazzo M, gonzalez-chica DA, dantas-correa EB. Clinical significance of serum adiponectin and resistin levels in liver cirrhosis. Ann hepatol. 2018;17(2):286-99.
CrossRef - Xu B, Li M, Yu Y, He J, Hu S, Pan M, Lu S, Liao K, Pan Z, Zhou Y. Effects of harmaline on cell growth of human liver cancer through the p53/p21 and Fas/FasL signaling pathways. Oncol lett. 2018;15(2):1931-6.
CrossRef - Bourogaa E, Jarraya RM, Damak M, Elfeki A. Hepatoprotective activity of Peganum harmala against ethanol-induced liver damages in rats. Arch physiol biochem. 2015;121(2):62-7.
CrossRef - Algandaby MM, Breikaa RM, Eid BG, Neamatallah TA, Abdel-Naim AB, Ashour OM. Icariin protects against thioacetamide-induced liver fibrosis in rats: Implication of anti-angiogenic and anti-autophagic properties. Pharmacol Rep. 2017;69(4):616-24.
CrossRef - Ellman GL. Tissue sulfhydryl groups. Arch physiol biochem. 1959;82(1):70-7.
CrossRef - Vodovotz Y, Lucia MS, Flanders KC, Chesler L, Xie QW, Smith TW, Weidner J, Mumford R, Webber R, Nathan C. Inducible nitric oxide synthase in tangle-bearing neurons of patients with Alzheimer’s disease. J Exp Med. 1996;184(4):1425-33.
CrossRef - Xu J, Yuan X, Lang P. The determination of enzymic activity and its inhibition on catalase by ultraviolet spectrophotometry. Environ chem. 1997;16(1):73-6.
- Ohnishi ST, Barr JK. A simplified method of quantitating protein using the biuret and phenol reagents. Anal biochem. 1978;86(1):193-200.
CrossRef - Scheuer P, Chalk B. Staning methods. In “clinical tests of histopathology” Scheuer PJ, Chalk BT, Wolf Medical Publication Ltd(London). 1986:84-5.
- Liedtke C, Luedde T, Sauerbruch T, Scholten D, Streetz K, Tacke F, Tolba R, Trautwein C, TrebickaJ, Weiskirchen R. Experimental liver fibrosis research: update on animal models, legal issues and translational aspects. Fibrogenesis & tissue repair (FTR). 2013;6(1):19.
CrossRef - Mehendale HM, Chilakapati J. Thioacetamide. In C. A. McQueen (Ed.), Comprehensive Toxicology (Second Edition), Oxford: Elsevier 2010;9: P. 627-38.
CrossRef - Takahashi Y, Fukusato T. Animal Models of Liver Diseases. Animal Models for the Study of Human Disease: Elsevier; 2017. p. 313-39.
CrossRef - Diwan SY. Effect of Peganum harmala methanol extract on liver and kidney of mice administered MTX drug. ANJS. 2013;16(4):161-6.
CrossRef - Hessien MH, El-Sharkawi IM, El-Barbary AA, El-Beltagy DM, Snyder N. Non-invasive index of liver fibrosis induced by alcohol, thioacetamide and schistosomal infection in mice. BMC gastroenterol. 2010;10(1):53.
CrossRef - Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr rev. 2012;33(4):547-94.
CrossRef - Kamada Y, Tamura S, Kiso S, Matsumoto H, Saji Y, Yoshida Y, Fukui K, Maeda N, Nishizawa H, Nagaretani H. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterol. 2003;125(6):1796-807.
CrossRef - Udomsinprasert W, Honsawek S, Poovorawan Y. Adiponectin as a novel biomarker for liver fibrosis. World J hepatol. 2018;10(10):708.
CrossRef - Correnti JM, Cook D, Aksamitiene E, Swarup A, Ogunnaike B, Vadigepalli R, Hoek JB. Adiponectin fine‐tuning of liver regeneration dynamics revealed through cellular network modelling. J physiol. 2015;593(2):365-83.
CrossRef - Yoneda M, Iwasaki T, Fujita K, Kirikoshi H, Inamori M, Nozaki Y, Maeyama S, Wada K, Saito S, Terauchi Y. Hypoadiponectinemia plays a crucial role in the development of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus independent of visceral adipose tissue. Alcohol clin exp res. 2007;31:15-21.
CrossRef - Feder S, Kandulski A, Schacherer D, Weiss TS, Buechler C. Serum Adiponectin Levels Do Not Distinguish Primary from Metastatic Liver Tumors. Anticancer Res. 2020;40(1):143-51.
CrossRef - Fang H, Judd RL. Adiponectin regulation and function. Compr Physiol. 2011;8(3):1031-63.
CrossRef - Yamauchi T, Iwabu M, Okada-Iwabu M, Kadowaki T. Adiponectin receptors: a review of their structure, function and how they work. Best Pract Res Clin Endocrinol Metab. 2014;28(1):15-23.
CrossRef - Yamauchi T, Kamon J, Minokoshi Ya, Ito Y, Waki H, Uchida S, Yamashita, S, Noda M, Kita S, Ueki K. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat med. 2002;8(11):1288-95.
CrossRef - Laurence J, Elhadad S, Robison T, Terry H, Varshney R, Woolington S, Ghafoory S, Choi M E & Ahamed J. HIV protease inhibitor-induced cardiac dysfunction and fibrosis is mediated by platelet-derived TGF-β1 and can be suppressed by exogenous carbon monoxide. PloS one. 2017;12(10):e0187185.
CrossRef - Wang H, Zhang H, Zhang Z, Huang B, Cheng X, Wang D, La Gahu Z, Xue Z, Da Y, Li D. Adiponectin-derived active peptide ADP355 exerts anti-inflammatory and anti-fibrotic activities in thioacetamide-induced liver injury. Sci rep. 2016;6(1):1-11.
CrossRef - Handy JA, Fu PP, Kumar P, Mells JE, Sharma S, Saxena NK, Anania F A. Adiponectin inhibits leptin signalling via multiple mechanisms to exert protective effects against hepatic fibrosis. Biochem J. 2011;440(3):385-95.
CrossRef - Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-κB activation and IL-6 production and increases PPARγ2 expression in adipocytes. Am J Physiol Regul Integr Comp Physiol.2005;288(5):1220-5.
CrossRef - Shafiei MS, Shetty S, Scherer PE, Rockey DC. Adiponectin regulation of stellate cell activation via PPARγ-dependent and-independent mechanisms. Am. J. Pathol. 2011;178(6):2690-9.
CrossRef - Buechler C, Haberl EM, Rein-Fischboeck L, Aslanidis C. Adipokines in liver cirrhosis. Int J Mol Sci. 2017;18(7):1392.
CrossRef