Manuscript accepted on :26-12-2020
Published online on: 01-02-2021
Plagiarism Check: Yes
Reviewed by: Dr.Aman Gupta
Second Review by: Dr. Mandal, Amritlal
Final Approval by: Dr. Ian James Martin
Grisilda Vidya Bernhardt1, Malay Jhancy2, Pooja Shivappa3, Kavitha Bernhardt4 and Janita R. T. Pinto5*
1Department of Biochemistry, RAK College of Medical Sciences, RAK, UAE.
2Department of Pediatrics, RAK College of Medical Sciences, RAK, UAE
3Department of Basic Sciences, RAK University, RAK, UAE.
4Department of Physiology, Manipal Academy of Higher Education, Manipal, Karnataka, India.
5Department of Biomedical Sciences, College of Medicine, Gulf Medical University, Ajman, UAE.
Corresponding Author E-mail: janitap2010@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2128
Abstract
Maintaining iron homeostasis is of great importance to the growing fetus and neonates. There is no consensus as to whether the neonates iron status is determined by fetal demands or by maternal iron status. There is no conclusive data confirming the likelihood of neonates born to anemic mothers suffering from compromised iron status. Therefore the aim of our study was to evaluate the relationship of iron indices between maternal and cord blood collected from mother and infant pairs and explore the association of maternal anemia on the iron status indicators of the cord blood.
This prospective study included 60 mother and newborn pairs. The study population was subdivided as anemic and non-anemic groups based on hemoglobin (Hb) levels. The maternal venous blood samples were collected 1.5 h ± 20 min before the delivery. 5 ml of cord blood was collected soon after child birth. Samples were analyzed for hemoglobin (Hb), serum ferritin and iron.
Significantly lower values of Hb, Ferritin, iron (p < 0.05) was observed in neonates born to anemic mothers when compared to the indices of neonates born to non-anemic mothers. On multivariate linear regression analysis , maternal Hb showed positive linear correlation with cord Hb and ferritin (r =0.87, p<0.05). However, correlation between maternal Hb and cord iron was not significant.
Maternal anemia can effect neonatal iron stores. Lowered concentration of iron status indicators in cord blood of neonates born to anemic mothers indicates that fetal iron transfer may be dependent on that of the iron stores of the mothers. However, this process may involve complex factors.
Keywords
Anemic Pregnant Women; Cord Blood Iron; Cord Blood Ferritin; Cord Blood Hemoglobin; Ferritin; Hemoglobin; Iron
Download this article as:| Copy the following to cite this article: Bernhardt G. V, Jhancy M, Shivappa P, Bernhard K, Pinto J. R. T. Relationship between Maternal and Cord Blood Iron Status in Women and their New Born Pairs. Biomed Pharmacol J 2021;14(1). |
| Copy the following to cite this URL: Bernhardt G. V, Jhancy M, Shivappa P, Bernhard K, Pinto J. R. T. Relationship between Maternal and Cord Blood Iron Status in Women and their New Born Pairs. Biomed Pharmacol J 2021;14(1). Available from: https://bit.ly/3tdQMD9 |
Introduction
Maternal micronutrient deficiency and more often iron deficiency are matters of concern in most of the developing countries [1]. Iron is thought to have a role in tuning child immune mechanisms. Maternal iron is said to have a direct effect on infants T lymphocyte and natural cell populations [2]. In addition to the need of well-regulated fetal iron stores for immune system, normal level is also essential to provide iron to developing fetal organ systems [1,3] , both of which would be disturbed in maternal iron deficiency anemia. Anemia is common problem in pregnancy, occurrence rate ranging from 5.4% in developed countries to more than 80% in developing countries [4]. Several studies indicate that iron stores in the neonates born to mothers with deficient iron stores may be compromised [5]. Maternal iron deficiency anemia is significantly associated with the risk of preterm labor and low birth weight .Preterm labor and low birth weight are associated with fetal and neonatal morbidity and mortality, impairment of growth and development and chronic diseases later in life. Neonates born less than 38 weeks gestation may be at risk of insufficient iron endowment [6,7]. Iron-deficiency may also place neonates at high risk for cognitive, motor, social-emotional, and neuro developmental impairment [8]. Moreover, requirement of iron is particularly high for rapid growth and differentiation of the fetus and thus a well-balanced iron homeostasis is required. This balance can be threatened in neonates born to iron compromised mothers [9,10].
Simultaneously, since antioxidant capacity in preterm infants will not be fully developed and neither will they be fully equipped with sufficient iron binding proteins, if these neonates have increased iron they will be at risk of oxidative effect of free iron, and therefore, cautious treating with iron supplementation in neonates is imperative [11,12].
Some studies have suggested that the fetus reflects maternal iron status, but this concept is still being debated as few other studies show contrasting results [13]. To draw consensus regarding the same, more studies, that will look into the role of maternal iron status on fetal stores and factors involved in maternal and fetal iron transfer are required [14].
Proper understanding of the relationship between maternal and neonatal iron indices will aid in formulating protocols that is required to improve the maternal and neonatal outcome. Investigating the effects of maternal iron on fetal iron indices can be of great value, so that early intervention to prevent or reverse the detrimental effects of iron deficiency on development issues much before iron deficiency effects becomes severe can be instituted [15,16].
Therefore, this study was planned to evaluate the relationship of iron indices between maternal and cord blood collected from mother and infant pairs at the time of delivery irrespective of gestational age , and to explore the association of maternal anemia on the iron status indicators of cord blood.
Material and methods
Ethical issues
This prospective study was conducted in a tertiary care center in south India from July 2012 to October 2014. The protocol was approved by the Institutional Ethical Committee Reference ID:2012-01340. Sixty mothers ranging from the age group of 21-35 years and their infant pairs were recruited for the study after obtaining informed consent form the participants.
Inclusion criteria
All pregnant women and their newborn pairs who had given consent to participate ≥ 26 weeks gestation were recruited for the study.
Exclusion criteria
Pregnant women who presented with medical problems such as gestational diabetes, hypertension, hypothyroidism, epilepsy, renal diseases, cardiorespiratory diseases, immunodeficiency syndromes, antepartum hemorrhage history of blood transfusion in antenatal period were excluded from the study. Babies with major congenital anomalies, pathological jaundice and twin deliveries were excluded.
Methodology
Sixty mothers ranging from the age group of 21-35 years and their infant pairs were recruited for the study. Required information and pregnancy outcomes was recorded in a standard proforma. Maternal blood and cord blood samples were collected from mother and neonate pairs. Maternal venous blood samples were collected 1.5 h ± 20 min before the delivery. 5 ml of cord blood was collected following child birth after clamping the cord with two clamps placed 4-5 inches apart. Diamine Tetra acetic acid was used as anticoagulant and plasma obtained on centrifugation was used to estimate iron and ferritin and whole blood was used for the estimation of hemoglobin. The enrolled mother-infant pairs were grouped based on maternal hemoglobin levels. Mothers with 11g/dl and more were considered non-anemic [17].
The hemoglobin concentration was determined by the standard spectrophotometric cyanometh-haemaglobin method.
Plasma Iron was assayed using Abcam iron assay kit without deprotenisation of the sample. This assay kit is based one the principle were in ferric carrier protein will dissociate ferric into solution in the presence of an acid buffer. After reduction to the ferrous form, iron reacts with an iron chromogen to produce a stable colored complex with absorbance at 593 nm. Values are expressed as mM/L
Plasma ferritin was analysed using human Ferritin Enzyme linked immune assay( ELISA) kits from Abcam following manufacturers instruction. Assay was based on solid phase direct sandwich principle, the results were obtained from standard curve and expressed as ng/ml.
Statistical Analysis
All the values are expressed as mean ± standard deviation. Paired Students t test was used to analyse and compare the data and p-value of less than 0.05 was considered statistically significant.
Descriptive statistics was used for baseline variables. Correlation between maternal iron indices and cord blood iron indices was done by multivariate regression analysis, using SPSS software. r =0.87, p<0.05 was considered as significant as positive linear correlation.
Results
Sixty mother-infant pairs were recruited for the study and their data was included for the final analysis. Thirty of the women were anemic mothers (Hb less than 11gm/dL) and 30 were non-anemic (Hb more than or equal to 11gm/dL). Amongst the newborns, 46.6% (n=28) were males and 53.4% (n = 32) were females. The mean gestational age of the neonates enrolled was 33.50±5.38 weeks, and the mean birth weight was 2679.25±457.9 g.
Mean ±SD of measured iron indices in maternal and cord blood and the p values obtained on comparing the data between anemic and non-anemic groups are shown in (Table 1). Hb , iron , ferritin were found to be significantly lower in anemic mothers compared to non-anemic mothers (p< 0.05 ).
The iron indices of neonates born to mothers with Hb <11 g/dL were compared with those with Hb ≥11g/dL. Lower values with significant difference (p < 0.05) was observed in iron indices (Hb, Ferritin, iron ) in cord blood of neonates born to anemic mothers (Table 1).
Table 1: student t-test, Mean ±SD and p values: group 1 values were compared with group 2. p < 0.05 was considered as significant.
| Parameters | Overall Mean ±SD | Group 1 (Anemic ) | Group 2
( Non Anemic) |
P Value |
| Hb (mother ) | 11.16±2.51 | 9.38±0.691 | 12.94±1.15 | 0.01 |
| Iron (mother ) | 41.07±13.86 | 31.26±4.26 | 50.87±14.49 | 0.02 |
| Ferritin (mother) | 59.73±19.61 | 45.87±9.29 | 73.60±9.06 | 0.02 |
| Cord Hb | 16.84±2.43 | 15.11±1.19 | 18.56±2.75 | 0.00 |
| Cord iron | 58.39±52.11 | 21.53±15.50 | 95.24±22.79 | 0.001 |
| Cord ferritin | 150.23±19.7 | 136.30±10.22 | 164.17±12.76 | 0.00 |
Multivariate regression analysis was done to correlate maternal Hb with maternal and cord iron indices . A positive linear correlation was observed between maternal Hb and maternal iron, between maternal Hb and cord Hb, between maternal Hb and cord ferritin with r =0.87, p<0.05. However, correlation between maternal Hb and maternal ferritin , between maternal Hb and cord iron was not significant. (Table 2, Fig 1 ).
Table 2: Multivariate linear Correlation between Maternal Hb with Maternal iron status, Maternal Hb with Cord blood Hb and Iron status.
| Variables | p-Value |
| Maternal Hb Vs Maternal Iron | 0.01* |
| Maternal Hb Vs Maternal Ferritin | 0.06 |
| Maternal Hb Vs Cord Hb | 0.000* |
| Maternal Hb Vs Cord Iron | 0.63 |
| Maternal Hb Vs Cord Ferritin | 0.003* |
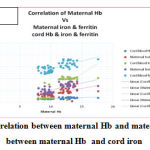 |
Figure 1: Correlation between maternal Hb and maternal ferritin, between maternal Hb and cord iron |
Discussion
One of the common nutritional deficiency seen in pregnant women is iron deficiency. Maternal iron deficiency has detrimental effects on development of the fetal and on pregnancy outcomes [18]. Results of our study suggests that cord Hb and iron is lowered in neonates born to anemic mothers, results also reveal that maternal Hb has a positive linear correlation with cord Hb and cord ferritin. However we did not find statistically significant positive correlation between maternal Hb and cord iron. Additionally, maternal Hb did not positively correlate with maternal ferritin suggesting that some of the anemic mothers may have lowered circulating iron levels with normal iron stores. Other similar studies like ours also suggest that mothers with lowered Hb levels may compromise iron homeostasis in their neonates [10].
Serum /plasma ferritin is a well-established marker for body iron stores [19]. Lower values of cord ferritin in neonates born to anemic mothers positively correlated with maternal Hb suggesting that iron stores may be reduced in neonates of anemic mothers.
It is known that a protein synthesized by fetal liver called Hepcidin serves as a regulator of iron homeostasis in early gestation as it helps the fetus to regulate placental iron uptake. Synthesis of Hepcidin is said to be regulated by maternal iron stores, inflammation, hypoxia, and RBC synthesis [20]. This suggests that several maternal factors are involved in tuning the neonates iron status pointing towards need for designing studies involving thorough search of each of the factors involved in fetal iron homeostasis.
Results of our study shows that, though maternal Hb correlates to cord Hb and cord ferritin it does not correlate well with cord iron. This may be because several factors and complex mechanisms may be involved in iron homeostasis in the fetus. However, our results certainly points that iron status indicators are reduced in neonates born to anemic mothers which is indicative of insufficient iron reserves in the neonates at birth. Similar finding have been reported earlier as well [21, 22]. However some studies have reported that maternal iron status is not reflected in the neonates and iron status of fetus is independent of iron status in the mothers [23,24]. Few other studies suggest that the fetus receives iron from mothers as per its requirements irrespective of maternal stores [6,25] thus contradicting the results of our study.
Transport of maternal iron to maternal side of syncytiotrophoblast is aided by serum transferrin binding to transferrin receptor and expression of transferrin receptors is reported to be upregulated in maternal iron deficiency [25], while other studies show that upregulation of receptors in iron deficiency fail to provide enough iron to fetus [21].
Our results make it evident that neonates delivered from anemic mothers show low Hb levels. And that it is important to understand that Hb is primarily required for oxygen and carbon-dioxide transport and as a body buffer and many other functions [26]. Decreased Hb in the neonates will place the neonates at a risk for upper respiratory tract infections and sepsis , apart from negatively affecting the normal functions attributed by Hb [27].
Further studies are required for definitive conclusion to be drawn as to whether iron available in the mother has any influence on the iron transferred across the placenta. Inherent challenges in the interpretation of Hb, Iron and Fe status in umbilical cord blood, exposes a requirement for larger cohort studies so as to obtain conclusive data on the relationship and the effect of maternal iron status on fetal iron homeostasis. Need for sufficient number studies to deduce reference values for iron indices in cord blood are highlighted in our study.
Conclusions
Maternal anemia can effect neonatal iron stores. Hemoglobin, Iron and ferritin was significantly lower in cord blood of neonates born to anemic mothers compared to those born to non-anemic mothers . Additionally neonate’s hemoglobin and ferritin concentration had a significant correlation with hemoglobin concentration of the mother. Based on our results we conclude that maternal anemia may have an effect on the neonates Hb and iron stores. Our findings, combined with a review of the published literature, indicate a need for further analysis of the relations between multiple iron indices to assess iron and hematologic status at birth. Our study also indicates the need for further studies that will explore mechanisms involved in iron transfer between the mother and fetus.
Acknowledgement
None.
Conflict of Interest
No conflict of interest between any of the authors.
Funding Source
This project was funded by Medicity Diagnostics and Health Care Center , Karnataka , India.
References
- Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Longlasting neural and behavioral effects of iron deficiency in infancy”. Nutrition Reviews, 2006;64:S34-43.
CrossRef - Cunningham-Rundles, S., Lin, H., Ho-Lin, D., Dnistrian, A., Cassileth, B. R., & Perlman, J. M. “Role of nutrients in the development of neonatal immune response”. Nutrition Reviews, 2009; 67;1753-4887.
CrossRef - Lozoff B, Klein NK, Nelson EC, McClish DK, Manuel M, Chacon ME. “Behavior of infants with iron-deficiency anemia”. Child Development, 1998;69(1):24-36.
CrossRef - Sun D, Mcleod A, Gandhi S, Malinowski AK, Shehata N. “Anemia in Pregnancy”.. Obstetrical & Gynecological Survey. 2017;72(12):730–737.
CrossRef - Lee, Sunmin, et al. “.Prevalence of Anemia and Associations between Neonatal Iron Status, Hepcidin, and Maternal Iron Status among Neonates Born to Pregnant Adolescents”.. Pediatric Research, 2015; 79(1):42–48
CrossRef - Moreno-Fernandez, Jorge, et al. “Iron Deficiency and Iron Homeostasis in Low Birth Weight Preterm Infants: A Systematic Review”..Nutrients,2019;11(5):1090.
CrossRef - Noble, K. G., et al. “Academic Achievement Varies With Gestational Age Among Children Born at Term”.. Pediatrics, 2012;2:2011-2157.
CrossRef - Lozoff, Betsy. “Iron Deficiency and Child Development”. Food and Nutrition Bulletin,2007; 28,suppl4: s560–571.
CrossRef - Delaney, Katherine M, et al. “Umbilical Cord Serum Ferritin Concentration Is Inversely Associated with Umbilical Cord Hemoglobin in Neonates Born to Adolescents Carrying Singletons and Women Carrying Multiples”. The Journal of Nutrition, 2019;149(3):406–415.
CrossRef - Collard, K.J.”Iron homeostasis in the neonate”. Pediatrics, 2009; 123:1208–1216.
CrossRef - Sullivan, Jerome L. “Iron Metabolism and Oxygen Radical Injury in Premature Infants.” Iron and Human Disease, 2018:447–457.
CrossRef - Rao, Raghavendra, and Michael K. Georgieff. “Iron Therapy for Preterm Infants.” Clinics in Perinatology, 2009;36(1): 27–42.
CrossRef - O’Brien KO, Zavaleta N, Abrams SA, Caulfield LE. “Maternal iron status influences iron transfer to the fetus during the third trimester of pregnancy”. American Journal of Clinical Nutrition, 2003;77(4):924-927
CrossRef - Rehu, Mari, Kari Punnonen, Vaughn Ostland, Seppo Heinonen, Mark Westerman, Kari Pulkki, and Ulla Sankilampi. “Maternal Serum Hepcidin Is Low at Term and Independent of Cord Blood Iron Status.” European Journal of Haematology ,2010;85(4): 345–52
CrossRef - Obrien, Kimberly O, et al. “Maternal Iron Status Influences Iron Transfer to the Fetus during the Third Trimester of Pregnancy.” The American Journal of Clinical Nutrition, 2003;77(4):924–930.
CrossRef - K Swetha, P Tarakeswararao, M Saisunilkishore ‘’Relationship between maternal iron and cord blood iron status: A prospective study’’Indian Journal of Child Health,2019(4);595-598.
CrossRef - World Health Organization. Children’s Fund and UN University. Iron Deficiency Anemia. Assessment, Prevention and Control. A Guide for Programme Managers. Geneva: World Health organisation 2001
- Scholl, Theresa O. “Iron Status during Pregnancy: Setting the Stage for Mother and Infant.” The American Journal of Clinical Nutrition, 2005; 81(5):1218S-1222.
CrossRef - Puolakka, J., et al. “Evaluation by Serum Ferritin Assay of the Influence of Maternal Iron Stores on the Iron Status of Newborns and Infants.” Acta Obstetricia Et Gynecologica Scandinavica, 1980;55(95) : 53–56.
CrossRef - Nemeth E, Ganz T. “Regulation of iron metabolism by hepcidin”. Annual Review of Nutrition, 2006;26:323–42.
CrossRef - Siddique H, Ayyub M, Nadeem M. “Correlation of iron status between pregnant women and their corresponding new borns”. Journal of Rawal Medical College, 2013;17(1):84-87.
CrossRef - Terefe, Betelihem, et al. “Effect of Maternal Iron Deficiency Anemia on the Iron Store of Newborns in Ethiopia.” Anemia,2015:1–6.
CrossRef - R. Hadipour, A. K. Norimah, B. K. Poh, F. Firoozehchian, R. Hadipour, and A. Akaberi, “Haemoglobin and serum ferritin levels in newborn babies born to anaemic Iranian women: a cross-sectional study in an Iranian Hospital,” Pakistan Journal of Nutrition,2010;9(6):562–566.
CrossRef - A. de Azevedo Paiva, P. H. C. Rond´o, R. A. Pagliusi, M. D. R. D. O. Latorre, M. A. A. Cardoso, and S. S. R. Gondim, “Relationship between the iron status of pregnant women and their new borns,”Revistade Sa´ude P´ublica,2007;.41()3:321– 327.
CrossRef - Liao QK, Kong PA, Gao J, Li FY, Qian ZM. “Expression of ferritin receptor in placental microvilli membrane in pregnant women with different iron status at mid-term gestation”. European Journal of Clinical Nutrition, 2001;55(8):651-656.
CrossRef - Ganong WF. Review of Medical Physiology. 22nd ed. New York: Mc Graw-Hill; 2005. Gas transport between the lungs and the tissues; pp. 666–669.
- Hussain, Sheikh Quyoom. “Low Hemoglobin Level a Risk Factor for Acute Lower Respiratory Tract Infections (ALRTI) in Children.” Journal Of Clinical And Diagnostic Research, 2014;8(4):1-4.
CrossRef







