Manuscript accepted on :29-12-2020
Published online on: 22-01-2021
Plagiarism Check: Yes
Reviewed by: Dr. Ahmed Salah
Second Review by: Dr. Ankur Singh Bist
Final Approval by: Dr. Ian James Martin
Raisa N Kazi
Department of Physiology, Al Ameen Medical College, Bijapur-586108, Karnataka, India
Corresponding Author E-mail: raisakolhar@yahoo.co.in
DOI : https://dx.doi.org/10.13005/bpj/2132
Abstract
Chronic high salt intake is well known to be linked to cause an increase in the blood pressure and one of the pathogenic effects of high salt on blood pressure is vascular functional impairment. The effect of sodium on vasculature involves an increase in the vascular resistance that could triggers a rise in the blood pressure. Sodium-induced increase in vascular resistance is primarily independent of any change in blood pressure; however, it could be an initiating factor for increase in the blood pressure. Salt induced increase in the vascular resistance involves alterations in several vasoregulatory mechanisms as evidenced in various vascular beds. A mechanism exhibiting a substantial effect on vascular function is the alpha (α1)-adrenergic system that significantly influences vascular resistance, thereby affecting peripheral vascular resistance and blood pressure. This review focused on the effects of increase dietary sodium intake on the α1-adrenergic system in renal vascular beds under normotensive and hypertensive conditions. Because the α1-adrenergic regulations of renal vascular function and renal hemodynamics affect blood pressure to a great extent, renal vascular assessment was performed. Study reports enhanced renal vascular sensitivity to α1-adrenergic agonist in high sodium normotensive and hypertensive condition, this could be due to functional alterations in the renal α1-adrenoreceptor density. This provide additional evidence on the underlying vascular pathology in salt-induced hypertension.
Keywords
Adrenergic System; Blood Pressure; Salt; Vascular Function
Download this article as:| Copy the following to cite this article: Kazi R. N. Impact of High Salt on Renal Hemodynamic In Normotensive and Hypertensive Conditions: Role of Renal Alpha 1 Adreno Receptors. Biomed Pharmacol J 2021;14(1). |
| Copy the following to cite this URL: Kazi R. N. Impact of High Salt on Renal Hemodynamic In Normotensive and Hypertensive Conditions: Role of Renal Alpha 1 Adreno Receptors. Biomed Pharmacol J 2021;14(1). Available from: https://bit.ly/2Mbst7O |
Introduction
High blood pressure is a key risk factor for mortality from cardiovascular and renal diseases. Chronic dietary sodium intake is one of the dietary influences that causean increase in blood pressure. However, the risk of high dietary sodium is not limited only to its effect on blood pressure but also its blood pressure-independent effect.1 High sodium is reported to cause an increase invascular resistance. Inability to decrease systemic vascular resistance in response to the increase in sodium intake is the primary pathological abnormality in salt-sensitive individuals.2 An abnormal vascular response to high salt intake,generally mediates the commencement of salt-induced hypertension.3 The mechanisms mediating abnormalities in vascular responses during the beginning of salt-induced hypertension may contribute to an abnormal increase in the systemic vasoconstriction that characterizedby sustained hypertension. Studies have revealed that chronic high dietary sodium intake is associated with aortic hypertrophy and decreaseddiameters of brachial and carotid arteries, suggesting vascular stiffness and distending pressure alteration.The high-salt-induced increase in vascular resistance was attenuated by a low salt period.1,4,5studies also reported that salt-induced hypertensionoccurs due to an increase in blood volume and cardiacoutput.6However,severalstudies have suggested that irregularities in the systemic vascular resistanceinitiates salt induced highblood pressure.3
Vascular resistance is a significant factor regulating blood pressure and tissue perfusion. High salt intake enhances vascular resistance that may increase peripheral vascular resistance and compromised tissue perfusion. This high-saltprovoked vascular effect may be an initiating factor for salt-induced hypertension.7Additionally, high-salt-induced enhanced vasoconstriction is considered a pathogenic event in salt sensitivity.8Several studies have reported the deleteriouseffect of high salt on various vascular beds, stating that high salt impairs the dilation of mesenteric and skeletal muscle resistance arteries in normotensive and hypertensive experimental rat models.5,9Moreover, high salt reduces cutaneous vasodilation, a measure of microvascular function.1
Several experimental studies have also confirmedthe adverse effect of salt onthe renal vasculature. High-salt-induced impaired renal vasodilatory mechanism involves a series of complex events that are independent of theireffect on blood pressure.However, the exact mechanism by which salt increases renal arterial constriction remains unclear. Some of these events that were extensively studied to explain the salt-induced increase in vascular resistance include endothelial dysfunction andmolecular signaling events that promote TGF-beta (β)1 production.10Studies have also reported that impaired renal vasodilatory mechanisms after salt loading in salt-sensitive Dahl ratsinclude abnormal activation of the ET-1 system, prostanoid-mediated contractions, and failure to increase nitric oxide synthase activity.11 Additionally, the role of the renin-angiotensin system (RAS) as a major determinant of salt-induced vascular dysfunction in the renal vasculature is strongly supportedby studies. The RAS is a major blood pressure regulatory mechanism; however, high-salt-induced abnormal activation of the RAS leads to enhanced renal vascular resistance, insufficient renal vasodilation, sodium retention, and hypertension development. The use of RAS blockers provides evidence of the impaired renal vascular effect of RAS in response to high sodium intake.12Thus, multiple mechanisms underlyingblood pressure-independent salt-induced renal vascular dysfunction are important pathogenic events in salt sensitivity. Salt-induced changes in renal vascular resistance can substantiallyaffect renal hemodynamics, andalterations inrenal hemodynamics play a crucial role in blood pressure regulation through its effect on sodium hemostasis and blood volumeregulation. These altered mechanisms can precede the initiation of salt-induced increase in blood pressure response. The relative significance of different mechanisms leadingto failure in normal vasodilation in response to salt intake increase remains unclear despite extensive research on the subject. However, hypersensitivity of the renal blood vessels to vasoconstrictor stimuli in salt-induced hypertensive conditions is supported by several studies. The α-adrenergic vasoconstrictor stimulation isproposed as a major cause for the increase and regulation of blood pressure in spontaneously hypertensive rat and deoxycorticosterone (DOCA) acetate salt-hypertensive rat models.13-15The vasoconstrictor effects of α-adrenergic stimulation on the renal vasculature under normotensive and hypertensive conditions remain unexplained.
This study reviewed the effect of high salt on the renal α-adrenergic mechanism (vasoconstrictor stimuli), a central regulator of renal vascular function and blood pressure. Off the various subtypes of the renal adrenoreceptors, special emphasis is being given to renal α1-adrenoreceptorsubtypes(α1-ARs), due to the keyrole of these receptors in renal vascular constriction and renal hemodynamic.16,17Recent experiments performed onAR-knockout models suggest an important role of this ARsin the overall regulation of blood pressure.
Renal alpha Adrenoreceptors
The renal sympathetic nervous system, through activation of various adrenoreceptor sub types present on the renal vasculature, mediates adrenergic regulation of the kidneys. Adrenoceptors are seven-transmembrane receptors that mediate the central and peripheral actions of noradrenaline and adrenaline. These receptors are found in nearly all the central and peripheral tissues.18In the kidneys,they are located on the renal vasculature, nephrons, and proximal tubules and contribute to renal hemodynamic and tubular functions.On the basis of pharmacological and molecular evidences, adrenergic receptorsare classified as α andβ receptors whichare further subdividedasα1, α2 andβ1, β2, and β3 receptors, respectively. Bothα1 and α2 receptors have three subtypes, all of which are G-protein-coupled receptors. The α1 receptors are Gq-coupledreceptors, whereasα2 receptors are Gi-coupledreceptors. The β receptors are also Gs-coupledreceptors.19β2 and β3 are Gi-coupled receptors. Amongst the various ARs, α-ARs are the most vital determinants of renal vascular tone.20
Signal transduction mechanism of α1-AR
During an adrenergic response,the adrenalin and noradrenalin released into the bloodstream bind to the α1 receptors (Gq protein)of the smooth muscle cells, causing activation of phospholipase C, producinginositol triphosphate (IP3). IP3diffuses into the cytosol and interacts with its receptors on the sarcoplasmic reticulummembrane, thereby causing the releaseof Ca2+into the cytosol. This results in activationof the calcium-dependent protein kinase, leading to smooth muscle contraction.21Other signaling pathways that get activated by α1 receptors include Ca2+ influx through voltage-dependent and -independent calcium channels, release of arachidonic acid, and activation of phospholipase A2 and phospholipase D, and mitogen-activated protein kinase.21
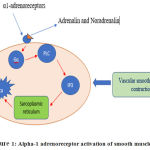 |
Figure 1: Alpha-1 adrenoreceptor activation of smooth muscle cells |
Vasoconstrictor effect of renal α1-ARs in various physiological and pathological conditions
The renal sympathetic nervous system, greatly influencesthe renal hemodynamicsby mediating catecholamine-induced effects on α1-ARs present on the renal vasculature.Based on receptor–ligand interaction and receptor-mediated signaling, α1-AR is further classified into three subtypes: α1A,α1B, and α1D.22 In the rat allsubtypes of α1-AR mediated catecholamine-induced renal vascular constriction, with α1A-AR and α1D-AR playing a significant role.20However, an alteration may occur in the functional involvement of α1-ARs under several physiological and pathological conditions.Studies have reported a role of α1A-AR and α1D-ARand a greater role of α1B-ARin mediating the renal vasoconstrictor responses in streptozotocin-induced diabetes and in a combined state of hypertension and renal failure.23In metabolic syndromes, α1B-AR is the functional subtype that mediates renal vasoconstriction inrats on high fructosediet over a long period.24In a state of hypertension and diabetes, α1A-ARs plays a vital role in enablingadrenergicallyinduced renal vascular constriction in 2K1C Goldblatt rats.The potential role of presynaptic α1-AR was reported.20The mRNA expression of all the threeα1-ARs was detected in the rat kidney cortex, andthe α1-ARgene was highlyupregulated,as confirmed by immunostaining of the smooth muscle of the arterial walls in diabetic animals.25
Renal vascular α-1 adrenergic response to high salt load in normotensive and hypertensive conditions
Renal hemodynamic adaptation plays a significant role in the regulation of blood pressure. Regulation of renal hemodynamic and renal vascular resistance is greatly influenced by α1-AR. Role of high salt on α1-ARand its subtype involvement in the regulation of renal hemodynamic in normotensive and hypertensive conditions were studied. Renal hemodynamic parameters were measured to determine renal vasoconstriction following the administration of adrenergic agonists and antagonists.26-28
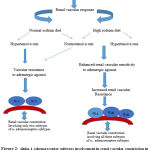 |
Figure 2: Alpha-1 adrenoreceptor subtypes involvement in renal vascular constriction in normotensive and hypertensive rats on normal and highsodium diets. |
A slight elevation in dietary sodium intake increased the sensitivity of the renal vasculature to α1-ARagonists in high-sodiumfed normotensive and hypertensive rats. Notably, a slight increase in dietary sodium increased the renal vascular response to vasoconstrictor stimuli even in normotensive rats. Moreover, the adrenergically induced renal vasoconstrictions were reduced by specific antagonists of α1A-AR and α1D-AR in both normotensive and hypertensive rats on high and normal sodium diets. However, unexpectedly, a decrease in the renal vasoconstrictor effect for α1B-AR antagonistswas observed in rats on highsodium diet. Irrespective of alterations in dietary sodium intake, α1A-ARand α1D-AR are the functional subtypes mediating the adrenergically induced renal vascular constriction in both normotensive and hypertensive rats. Additionally, α1B functionally involved in mediating the renal cortical vasoconstriction in rats fed with a high salt diet. The enhanced sensitivity could be explained on the basis of maximum presser response to the α1-AR agonist and additional involvement of the ARs in rats feda highsodium diet. A moderate salt load caused functional alterations in the renal vascular α1-AR density that was indicated asenhanced sensitivity of the renal vasculature to α1-AR agonists. Despite the fact that these changes were independent of any additionalrise in arterial blood pressure. This mechanism provides critical insights into how high salt load can enhance the vascular response to vasoconstrictor stimuli that can increase the vascular resistance which could be an initiating factor for the salt-induced increase in the arterial blood pressure.26-28
This result strengthen the earlier view that harmful effects of salt loading are not limited toincrease in blood pressure.1Moreover, the obtained data suggests that even the lowest amount of salt intake (0.9% NaCl), nearly equivalent to the average salt intake currently observed in industrialized and urbanized countries, may promote and increase the adrenergic responsiveness of the renal vasculature to adrenergic vasoconstrictor stimuli, leading to alterations in the vascular resistance. This greater vascular smooth muscle responsiveness may lead increased vascular resistance for perfusionof blood causingan increase in pressure, which then predisposes the individual to increased arterial wall thickness and remodeling mechanisms. In theseconditions, hypertension is mediated by enhanced vascular resistance, leading to vasoconstriction and additionalincrease of total peripheral vascular resistance. Studies have revealed that increase in vascular reactivity occurring after sodium loading might be due to the sodium-dependent impairment of noradrenaline uptake. Augmented vascular responsiveness provide greater resistance to blood flow and predisposes an individual to salt-induced blood pressure response.29Studies using other salt-related hypertensive rat models, the DOCA-salt-hypertensive rats, have stated that the enhanced responsiveness of the mesenteric vascular bed to α1-AR agonists could be due to a local alteration in the α1-AR density. Suzuki et al. found an increase in both density and affinity of α1-AR in the mesenteric vasculature of DOCA-salt hypertensive rats.30 An increased affinity of the small mesenteric artery α1-ARwas demonstrated in spontaneously hypertensive rats compared with normotensive Wistar-Kyoto rats.31 Higher renal densities of α1-AR and α2-ARwere demonstrated in both spontaneously hypertensive rats and Dahl salt-sensitive rats.32 Additional studies in other salt-related hypertensive animal models have revealed that the enhanced responsiveness of the vasculature to catecholamine might be due to a local alteration in the α1-AR density.33 These differences in sensitivity of different vascular beds due to high salt load could cause a change in neurovascular transduction processes.33 Evidences also suggest disturbance in nitric oxide and intrarenal RAS activitiescausingabnormal vasodilatory response to salt,which usually precede and initiate salt-induced hypertension. Hence,the underlying mechanisms that promote vascular salt sensitivity are complex involving genetic and environmental influences on the vasculature that are independent of blood pressure.The relative significance of different mechanisms leading to failure in normal vasodilation in response to increases in salt intake remainsunclear. Present study reports that enhanced renal vascular response to adrenergic agonist is due to α1-AR functional alterations and that this may be one of the causes for salt-induced impaired renal vasodilatory response.Abnormal relationship between high salt intake and renal vascular α1-AR can have an implication on the renal vascular resistance and renal hemodynamics. Altered renal hemodynamic parameters can have greater effect on blood pressure response through altered sodium tubular handling. Additionally, we suggest that the relation between salt and α-adrenergic system in other vascular beds need to be further considered.
Conclusion
High salt intake enhances the renal vascular responsiveness to vasoconstrictor stimuli. The enhanced sensitivity was not only observed in hypertensive conditions but unexpectedlyalso in normotensive conditions. Increased renal vascular sensitivity is because of functional alterations in the renal α1-ARdensity. These findings provide additional evidence on the underlying vascular pathology in salt-induced hypertension.
References
- Greaney J.L, DuPont J.J, Lennon‐Edwards S.L, Sanders P.W, Edwards D.G. and Farquhar W.B. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. Journal of physiology, 2012; 590: 5519-5528.
CrossRef - Edwards D. G, and Farquhar W. B. Vascular effects of dietary salt. Current opinion in nephrology and hypertension, 2015; 24: 1-8.
CrossRef - Morris R. C, Jr, Schmidlin O, Sebastian A, Tanaka M, and Kurtz T. W. Vasodysfunction That Involves Renal Vasodysfunction, Not Abnormally Increased Renal Retention of Sodium, Accounts for the Initiation of Salt-Induced Hypertension. Circulation, 2016; 133; 881–893.
CrossRef - Safar M. E, Temmar M, Kakou A, Lacolley P, and Thornton S. N. Sodium intake and vascular stiffness in hypertension. Hypertension, 2019: 54; 203-209
CrossRef - Zhu J, Huang T, Lombard J. H. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. Journal of vascular research. 2007; 44:382–90.
CrossRef - Hall J.E, Textbook of Medical Physiology. 13th. Philadelphia: Elsevier; 2015
- Delong C, Sharma S. Physiology and Peripheral Vascular Resistance. StatPearls Publishing.2020: 245; 1-6.
- Schmidlin O, Forman A, Tanaka M, Sebastian A, and Morris Jr R. C. NaCl-induced renal vasoconstriction in salt-sensitive African Americans: antipressor and hemodynamic effects of potassium bicarbonate. Hypertension, 1999: 33; 633-639.
CrossRef - Lenda D.M, Boegehold M.A. Effect of a high-salt diet on oxidant enzyme activity in skeletal muscle microcirculation. American journal of physiology Heart and circulatory physiology. 2002; 282:395–402.
CrossRef - Ying W. Z, Aaron K, and Sanders P. W. Mechanism of dietary salt-mediated increase in intravascular production of TGF-β1. American Journal of Physiology-Renal Physiology, 2008: 295; 406-414.
CrossRef - Barton M, Vos I, Shaw S, Boer P, D’uscio L.V, andGröne H. J. Dysfunctional renal nitric oxide synthase as a determinant of salt-sensitive hypertension: mechanisms of renal artery endothelial dysfunction and role of endothelin for vascular hypertrophy and glomerulosclerosis. Journal of the American Society of Nephrology, 2000: 11; 835-845.
- Van P. P, Zeeuw D, Navis G, Jong P. E. Does the renin-angiotensin system determine the renal and systemic hemodynamic response to sodium in patients with essential hypertension? Hypertension. 1999: 27; 202-208.
CrossRef - Suzuki S, Takata Y, Kubota S, Ozaki S, Kato H. Characterization of the α1adrenoceptors in the mesenteric vasculature from deoxycorticosterone-salt hypertensive rats: studies on vasoconstriction, radioligand binding and postreceptor events. J Pharmacol Exp Ther. 1994; 268: 576–583.
- Ibarra M, Lopez-Guerrero J. J, Villalobos-Molina R. Further evidence for the predominance of α1D-adrenoceptors in arteries of normotensive and spontaneously hypertensive rats. Pharmacol Rev Commun. 1998:10; 135-139.
- Takata Y, Kato H, Adrenoceptors in SHR. alterations in binding characteristics and intracellular signal transduction pathways. Life Sci. 1996;58: 91–106.
CrossRef - Ahmad A, Sattar M. A, Azam M, Khan S. A, Bhatt O, Johns E.J. Interaction between nitric oxide and renal α1-adrenoreceptors mediated vasoconstriction in rats with left ventricular hypertrophyin Wistar Kyoto rats. PloS one. 2018: 15;13-19.
CrossRef - Raisa N. K,Munavvar A S, Nor A. A, Hassaan A. R, Anand S. K, Nurjannah M. H, Mohammed H. A, Ibrahim M. S, Abdul H. K, Edward J. J.Influence of high dietary sodium intake on functional contribution of renal α1a‐adrenoceptor of SHR Adv Clin Exp Med. 2011:20; 47–55.
- Guimaraes S, Moura D. Vascular adrenoceptors: an update. Pharmacol Rev.2001: 53; 319-356.
- Farzam, Khashayar, and Anand D. Lakhkar. “Adrenergic Drugs.” (2018).
- Armenia A, Sattar M, Abdullah N. Functional subtypes of renal α1-adrenoceptor in diabetic and non-diabetic 2K1C Goldblatt renovascular hypertension. Acta Pharmacol Sin. 2008: 29; 564–572.
CrossRef - Bylund, D. B. “Norepinephrine: Adrenergic Receptors.” (2009): 1231-1236.
CrossRef - KhalidM, Giudicelli Y,DausseJ.P. An up-regulation of renal alpha (2)A-adrenoceptors is associated with resistance to salt-induced hypertension in Sabra rats. J. Pharmacol. Exp. Ther. 2001: 299; 928–933.
- Hye Khan M. A, Sattar M. A, Abdullah N. A, Johns E. J. Influence of combined hypertension and renal failure on functional α1‐adrenoceptor subtypes in the rat kidney. British journal of pharmacology. 2008: 153; 1232-41.
CrossRef - Abdulla M. H, Sattar M. A, Abdullah N. A, Khan M. A. H, Swarup K. R. A, and Johns, E. J. The contribution of α 1B-adrenoceptor subtype in the renal vasculature of fructose-fed Sprague–Dawley rats. European journal of nutrition. 2011: 50; 251-260.
CrossRef - Zhao X, Zhang Y, Leander M, Li L, Wang G, and Emmett N. Altered Expression Profile of Renal-Adrenergic Receptor in Diabetes and Its Modulation by PPAR Agonists. Journal of diabetes research 2014: 25; 1-11.
CrossRef - Kazi R. N, Munavvar A. S, Abdullah N. A, Khan A. H, and Johns, E. J. Influence of high dietary sodium intake on the functional subtypes of α1‐adrenoceptors in the renal cortical vasculature of Wistar–Kyoto rats. Autonomic and Autacoid Pharmacology. 2009: 29; 25-31.
CrossRef - Kazi R. N, Sattar M. A, Abdullah N. A, Rathore H. A, Kolla A. S, Hussain N. M, Johns E. J. Influence of high dietary sodium intake on functional contribution of renal α1a-adrenoceptor of SHR.Advances in Clinical and Experimental Medicine. 2011: 20; 47-55.
- Kazi, R. N. A. “Renal Denervation and Salt Induced Hypertension.” Adv kidney Dis Treat1 (2017): 2.
CrossRef - K, Oberleithner H. An emerging concept of vascular salt sensitivity. F1000 biology reports. 2012: 4; 1-7.
CrossRef - Suzuki S, Takata Y, Kubota S, Ozaki S, Kato H. Characterization of the alpha-1 adrenoceptors in the mesenteric vasculature from deoxycorticosterone-salt hypertensive rats: Studies on vasoconstriction, radioligand binding and postreceptor events. J Pharmacol Exp Ther. 1994: 268; 576–583
- Nyborg N. B, Bevan J. A. Increased α-adrenergic receptor affinity in resistance vessels from hypertensive rats. Hypertension. 1988: 11; 635–638.
CrossRef - Brodde O. E, Michel M. C. Adrenergic receptors and their signal transduction mechanisms in hypertension. J Hypertens. 1992: 10;133–145.
CrossRef - Caveney S. W, Taylor D. A, FlemingW. W. Examination by radioligand binding of the α1-adrenoceptors in the mesenteric arterial vasculature during the development of salt-sensitive hypertension. Naunyn-Schmiedeberg’s archives of pharmacology. 1997: 356; 374-382
CrossRef







