Manuscript accepted on :25-02-2021
Published online on: 06-03-2021
Plagiarism Check: Yes
Reviewed by: Dr. Dini Damayanti
Second Review by: Dr. Ankur Singh Bist
Final Approval by: Dr Patorn Piromchai
Reena Arora1 , Mohammed Samim2
, Mohammed Samim2 and Chander Parkash1*
and Chander Parkash1*
1Department of Chemical Sciences, I.K.Gujral Punjab Technical University, Kapurthala, Punjab 144603, India.
2Department of Chemistry, School of Chemical and Life Sciences, Jamia Hamdard, New Delhi, 110062, India.
Corresponding Author E-mail: chander.ptu@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2105
Abstract
Resveratrol, a non-flavonoid phenolic phytochemical present in red grapes and berries, has been reported to have significant health benefits. Resveratrol is known for its chemopreventive and chemotherapeutic effects in multiple cancers as well as cardiovascular and inflammatory diseases. But its higher lipophilicity, poor aqueous solubility and bioavailability remains a challenge for its usage as an effective chemopreventive and chemotherapeutic agent. To overcome this,we have prepared a biocompatible calcium phosphate encapsulated resveratrol (Nanoresveratrol; NRV) and studied its antioxidant, anti-inflammatory and anti-cancer activities in the present study.Nanoresveratrol, unlike resveratrol, readily dispersed in aqueous media and showed a sustained release. Nanoresveratrol (NRV) and resveratrol (RV) showed comparable antioxidant activities. The anti-inflammatory and anticancer activities of nanoresveratrol were studied for its inhibitory effect on 7, 12-dimethylbenz[a]anthracene (DMBA)-induced/12-O-tetradecanoylphorbol-13-acetate (TPA) promoted skin inflammation and tumorigenesis mouse model. Nanoresveratrol showed a significant decrease of TPA-induced skin edema, ODC activity and thymidine incorporation when compared to resveratrol. Nanoresveratrol also inhibited chemical-induced tumorigenesis.Overall, the study results support that nanoresveratrol may represent a potential anti-cancer agent and warrants further investigations for the treatment of skin cancer.
Keywords
Antioxidant; Cancer; Chemoprevention; Inflammation; Resveratrol; Skin cancer; Tumorigenesis
Download this article as:| Copy the following to cite this article: Arora R, Samim M, Parkash C. Evaluation of Anti-inflammatory and Anti-cancer Activity of Calcium Phosphate Encapsulated Resveratrol in Mouse Skin Cancer Model. Biomed Pharmacol J 2021;14(1). |
| Copy the following to cite this URL: Arora R, Samim M, Parkash C. Evaluation of Anti-inflammatory and Anti-cancer Activity of Calcium Phosphate Encapsulated Resveratrol in Mouse Skin Cancer Model. Biomed Pharmacol J 2021;14(1). Available from: https://bit.ly/3ekzuzh |
Introduction
Cancer is the second leading cause ofmortality in developed and many developing nations across the world. Nearly eighteen million new cases and more than ten million deaths occur due to cancer annually.Melanoma and non-melanoma cancers are 19th and 5th most commonly occurring skin cancer globally [1].
Chemotherapy, radiation, targeted small molecule drugs and biologics are some of the modalities for the treatment of skin cancer [2,3]. Last decade has seen an exponential growth in the drug approvals which has increased the survival rate of patients with skin cancer. In addition, chemoprevention using natural, synthetic, or biological agents, is a promising approach to prevent or suppress carcinogenesis at different stages of skin cancer [4].
Significantly, chemoprevention of skin cancer is intensely investigated and some of the agents have also reached clinical testing [5].Agents with a variety of mechanism of action have been broadly classified according to their effects on different stages of carcinogenesis [6].Published literature including epidemiological studies has demonstrated chemoprevention as a strategy to suppress the onset and progression of carcinogenesis using natural, synthetic and biologic agents [7, 8].
Among the phytochemicals, resveratrol, a non-flavonoid phenolic compound, present in high amount in grapes, raspberries, blue berries and red wine has been reported to have proven antioxidant properties and well-known health benefits. It has been extensively reported that resveratrol has chemopreventive role in a variety of cancers [9,10].Resveratrol is also known to have a variety of biological effects, including anti-inflammatory, anti-angiogenic, pro-apoptosis and antioxidant properties, all of which are well established in preventing the onset and progression of cancer. Attempts have been made in understanding the molecular mechanisms leading to these biological pathways responsible for the anticancer effect of resveratrol [11,12].
Oxidative stress is a common pathogenic mechanism in cancer and resveratrol has been widely investigated as an antioxidant in the prevention of a variety of cancers [13,14]. As an antioxidant, resveratrol protects critical biomolecules, including lipids, proteins and nucleic acid from free radical attack[14,15]. In doing so, resveratrol provides protection against carcinogenesis. There are several reports indicating the promising effects of resveratrol in the treatment of skin cancer, including our previous study demonstrating the in vitro effects of resveratrol and nano resveratrol [16-18].
Resveratrol has displayed potential chemopreventive effects in several in vitro and animal studies. However, several challenges compromise its bioavailability and PK properties, limiting its clinical translation [19,20]. The clinical trial data on resveratrol is limited, in contrast to the extensive literature available in laboratory and animal studies. These trials reinforce the need to use alternate drug delivery systems, including nanoparticle mediated drug delivery of resveratrol, to improve the physicochemical properties [21].
Advanced drug delivery systems can overcome the challenges associated with inherent physicochemical properties of resveratrol such as lipophilicity, poor solubility and limited bioavailability associated with resveratrol [22, 23]. For example, nanotechnology based encapsulated drugs can have significant potential in skin cancer due to their advantageous properties such as controlled drug release, a longer blood circulation time, better bioavailability, selective organ or tissue distribution and improved therapeutic window.
Nanocarriers for resveratrol have been reported in the literature that ensures the improved properties of resveratrol [24-26].The calcium phosphate-based nano delivery system has previously been successfully used as gene and drug delivery system [27-29]. Negatively charged calcium phosphate nanoparticle formulation is biocompatible and a safe agent for developing resveratrol as a chemopreventive agent [30].
In our previously reported study, we synthesized, characterized, and evaluated the calcium phosphate-encapsulated resveratrol (nanoresveratrol; NRV) for the anti-cancer activity in a murine melanoma cell line model [18]. In the present study, we evaluated calcium phosphate encapsulated resveratrol (nanoresveratrol; NRV) for the anti-inflammatory and anti-cancer activities in the animal model of skin cancer.We employed well-established 7,12-Dimethylbenz[a]anthracene (DMBA)-initiated/12-O-tetradecanoylphorbol-13-acetate (TPA)-promoted in a two-stage skin cancer model [31,32].
We have also evaluated the antioxidant activity of the nanoresveratrol. Collectively, our findings provide evidence that calcium phosphate encapsulated resveratrol (nanoresveratrol; NRV) is an effective antioxidant and exhibits anti-inflammatory and anti-cancer activities against skin cancer.
Materials and Methods
Materials
Chemicals and solvents used were ofthe highest purity available. Di-sodium hydrogen phosphate, sodium dihydrogen phosphate, sodium chloride, Tris–HCl buffer,sodium hydroxide,EDTA, sodium carbonate were obtained from Sisco Research Laboratory (SRL). Acetone and ethanol were obtained from Thomas Baker Chemicals (Pvt) Ltd. BSA, DPPH, DTT, EGTA,PMSF, 2-mercaptoethanol, Tween 80, Brij 35, citric acid, DMSO, DMBA, TPA, resveratrol were obtained from Sigma. [14C] ornithine and [3H] thymidine were purchased from Amersham Biosciences.
Methods
Entrapment efficiency and release kinetics of nano resveratrol (NRV).
Calcium phosphate encapsulated resveratrol (nano resveratrol; NRV) particles were prepared in the aqueous core of an AOT (Aerosol-OT)/hexane/water reverse micellar system. The synthesis and characterization of the nano resveratrol has already been reported in our earlier study [18]. Entrapment efficiency of resveratrol in the resveratrol loaded calcium phosphate nano particles was measured and calculated as:
Entrapment efficiency (%) =
Resveratrol present in nano particles/ Initial amount of resveratrol taken to prepare nano resveratrol × 100
The release behaviour of resveratrol from the nano particles was evaluated using thedialysis method reported by Lu et al[33].
The time dependent resveratrol released was determined as:
Release percentage = [Resveratrol]release/[Resveratrol] total × 100.
Antioxidant activity using DPPH assay
Antioxidant activity of resveratrol (RV) and nanoresveratrol (NRV) was measured using DPPH (1,1-Diphenyl-2-Picrylhydrazyl) free radical scavenging assay using a previously reported method[34]. Ascorbic acid was used as a positive control in the assay.
Animals
Swiss albino mice (6-8 weeks), weighing 20-25 g,were obtained from the Jamia Hamdard University animal house facility, New Delhi, India. The mice were housed in a well-ventilated room at 25±2 °C under a 12 h day and night cycle. Mice were provided with standard laboratory feed (Hindustan Lever Ltd. Bombay, India) and drinking water ad libitum. Studies were conducted using protocols approved by the Institutional Animal Ethical Committee (Reg. No. 173/GO/Re/2000/CPCSEA). Appropriate care of the mice was taken according to the guidelines established by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Govt. of India. After shaving the dorsal skin of mice with an electric clipper, hair removal cream (Anne French, Bombay, India) was applied. This was done two days prior to treatment.
TPA-induced skin edema, Ornithine decarboxylase (ODC) activity and Thymidine incorporation assay
Treatment effect of both resveratrol (RV) and nanoresveratrol (NRV) on skin edema, ODC activity and thymidine incorporation, induced by TPA, was studied using swiss albino mouse model of skin cancer. Mice were randomly assigned into five groups, each group having six mice. Group 1 mice served as vehicle control and received topical application of 200 μl of the vehicle (acetone alone). Group 2 mice treated with TPA served as positive control. Group 3 mice received topical applications of 10μg resveratrol/200 μl acetone (RV), Group 4 mice received topical applications of 0.5μg nanoresveratrol/200μl acetone (NRV1) and Group 5 mice received 1μg nanoresveratrol/200μl acetone (NRV2). Thirty minutes post-treatment, mice in each of the group 2, 3, 4, 5 received topical applications of TPA (2μg/200μl acetone). Group 1 which represented the vehicle control group did not receive these treatments. All the treatments were administered consecutively for three days. After 12 h of the last TPA treatment the mice were sacrificed by cervical dislocation. To evaluate skin edema,skin of each mouse was excised and processed.
To assay ODC activity in cutaneous cytosol, the distribution and treatment schedule of mice was same as described for skin edema. After 6 h of the last TPA treatment, the mice from all the groups were sacrificed and further processed for cytosolic preparation for ODC activity assay.
For cutaneous [3H] thymidine in corporation studies, the treatment schedule followed was same as described above. The only variation was that intraperitoneal injection of [3H]thymidine (20 μCi/animal/0.2ml saline) was given to the mice of all groups after 18 h of TPA/acetone treatment. Following this the mice were sacrificed by cervical dislocation after 2 h of [3H]thymidine treatment.
Evaluation of skin edema
The effect of resveratrol (RV) and nano resveratrol (NRV) on skin edemainduced by TPA was assessed using the method of Afaqet al[35].
Ornithine decarboxylase (ODC) activity
To assay ODC activity, skin tissue from the mice in each group was excised and washed with ice cold normal saline. The extraction of epidermal extract and estimation of ODC activity was performedusing the method of Verma et al[36].
[3H] Thymidine incorporation
The isolation of skin DNA and assessment of incorporation of [3H] thymidine was carried out using the method of Smart et al[37].
In vivo skin tumorigenesis studies
Chemically induced two-stage skin tumorigenesis model was employed to study the cancer prevention activity of the nano resveratrol (NRV) as elaborated by Chaudhary et al [38] . To induce tumor formation, a single dose of DMBA (50µg/200 µl acetone) was applied topically to the shaved dorsal mouse skin. After one week of DMBA treatment, topical treatment of TPA (2µg/200µ lacetone; thrice weekly for 18 weeks) was given to promote tumor growth . Group 1 mice was used as vehicle control. Group 2 mice, treated with TPA alone, was used as positive control. Thirty minutes prior to TPA treatment,Group 3 received 0.5µg nano resveratrol/200μlacetone (NRV1) and Group 4 received 1.0µg nano resveratrol/200μl acetone (NRV2) thrice weekly till 18 weeks. The experiment was followed up for 18 weeks and mice were observed for the appearance of tumors and their enumeration. The data collected at 6, 12 and 18 weeks is expressed as tumor incidence (percentage of mice with tumors) and tumor burden (number of tumors/mouse).
Statistical Analysis
The level of significance between different groups is based on the analysis of variance test followed by Dunnett’s test. P-values less than 0.05 is considered to be significant.
Results and Discussion
Entrapment efficiency and release kinetics of nano resveratrol (NRV)
Here we have determined the release kinetics of the synthesized nano resveratrol. We have reported detailed synthesis and characterization of nano resveratrol in our previous report [18].The entrapment efficiency of resveratrol in the nano particles was greater than 85%.
As shown in Figure 1 resveratrol was released from nano resveratrol in a sustained manner at physiological buffer (pH 7.4). Sustained release of drug from the nano particles helps in the availability of the drug for a longer period at the treatment site.
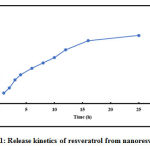 |
Figure 1: Release kinetics of resveratrol from nano resveratrol. |
Antioxidant activity
DPPH free radical scavenging assay is a well-established method for determining the antioxidant activity. As shown in Figure 2, resveratrol and nanoresveratrol both displayed a significant DPPH radical activity. Ascorbic acid (positive control) displayed free radical scavenging activity. The IC50 value of ascorbic acid (AA), resveratrol (RV) and nanoresveratrol (NRV) was found to be 52.72 μg/ml, 57.81μg/ml, and 50.3μg/mlrespectively.
The antioxidant activity of nanoresveratrol indicates that resveratrol released from the nanoformulation preparation is functionally active. The antioxidant activities of both nanoresveratrol and resveratrol was comparable, as shown in Figure 2.
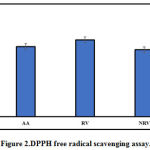 |
Figure 2: DPPH free radical scavenging assay. |
Ascorbic acid (AA), resveratrol (RV) and nanoresveratrol (NRV). Data is represented as mean IC50 ± S.D (n=6)
In Vivo Anti-Cancer Studies
In the present study we evaluated the chemopreventive effect of resveratrol and nano resveratrol on TPA-induced edema and DMBA/TPA tumorigenesis using swiss albino mouse skin cancer model.
Previous reports have shown that the treatment of mouse skin with TPA or treatment of epidermal cells with chemical carcinogens such as DMBA represent excellent models to study a variety of biological responses upon drug treatment [35-38]. These include examining the impact of drugs on inflammation, oxidative responses, ODC levels, thymidine incorporation and cell growth resulting in tumor promotion in DMBA-initiated mouse model of skin cancer. Topical treatment of skin with TPA induces inflammatory hallmarks, such as edema and COX2 induction. TPA has been reported to induce hyperplasia, several biochemical changes by inducing ROS and the malignant transformation of epidermal cells to tumors [35-38]. Of note, DMBA-mediated effects in animal models are similar to those noted in human cancers indicating the relevance of these pre-clinical models and the translatability of the findings from these models. Hence DMBA-induced/TPA-promoted murine skin tumorigenesis has been used as a suitable model to study the chemopreventive potential of compounds.
Skin Edema
Inflammation is known to have a potential role in tumor initiation and progression [39,40]. TPA has been reported to induce inflammatory response like skin edema and hyperplasia in mouse skin [35-38]. Inflammation is an important pathway known to drive the malignant transformation and promotes tumor angiogenesis and metastasis in skin cancer. It is, therefore, relevant to examine the impact of anti-cancer drugs on inflammation [39, 40].
We examined skin edema as an indicator of inflammation using a TPA-treated mouse model. Table 1 shows that significant edema was induced in the mice from Group 2 (TPAalone) in comparison to mice from Group 1 (vehicle control). Edema was observed to decrease significantly in mice from Group 3 (10 μg resveratrol; RV), Group 4 (0.5 μg nano resveratrol; NRV1) and Group 5 (1 μg; NRV2) as compared to Group 2 mice (TPA alone). Further, topical treatment of RV, 30 min before TPA application, demonstrated 59.4% inhibition against skin edema. Treatment with NRV1 showed 78.3% and NRV2 showed 94% inhibition against skin edema, indicating that a greater effect was observed at a higher dose.
Table 1: Topical pre-treatment of RV and NRV on TPA-induced skin edema in swiss albino mice.
| Groups | Skin punch
(Weight in mga) |
% inhibition (edema)b |
| Group 1 (Vehicle control) | 23.20 ± 0.23 | — |
|
Group 2 (TPA) |
33.80 ± 0.62⁎⁎ |
— |
| Group 3 (RV+TPA) | 27.50 ± 1.20⁎⁎ | 59.4 |
|
Group 4 (NRV1+TPA) |
25.50 ± 1.30⁎⁎ |
78.3 |
| Group 5 (NRV2+TPA) | 23.80 ± 1.20⁎⁎⁎ | 94.34 |
Values in the table are shown as mean ± SE of three mice for each group (⁎⁎ p<0.01, ⁎⁎⁎ p<0.001).
bGroup 1 (vehicle control) was compared to the Group 2 (TPA alone) to observe the change in skin edema. Group 3 (10 µg RV), Group 4 (0. 5µg NRV1) and Group 5 (1 µg NRV2) are compared to Group 2 (TPA alone) for calculating percentage inhibition of edema.
The study demonstrates RV and NRV, when applied topically before TPA treatment, showed a significant reduction in skin edema induced by edema. It is noteworthy that nanoresveratrol, used at 10-20 fold lower dose relative to resveratrol, is more effective in skin edema reduction.
Our data indicates that the nano resveratrol at lower doses shows significant anti-inflammatory response and may play a pivotal role in the inhibition of tumor promotion.
Ornithine decarboxylase and Thymidine assay
Ornithine decarboxylase (ODC) is a critically essential enzyme in the polyamine biosynthetic pathway. These polyamines bind to polyanionic DNA, RNA and phospholipids, affecting DNA replication, transcription, translation, thereby playing a role in cell proliferation[41-44]. ODC expression is tightly controlled by various cellular mechanisms in normal cells. However, ODC expression is upregulated, yielding constitutively elevated levels of ODC activity in neoplastic cells. ODC is known to be induced by tumor promoters such as TPA in skin cancer and marks an important event during skin tumor development. Several reports suggest that targeted inhibition of ODC in non-melanoma skin cancer is anattractive therapeutic strategy for developing inhibitors of skin tumorigenesis [43, 44].
We conducted experiments to examine the effect of resveratrol (RV) and nano resveratrol (NRV) on ODC activity and thymidine incorporation assay. As shown in Figure 3, our study shows that both RV and NRV treatment resulted in significant inhibition of TPA-mediated induction of epidermal ODC activity. However, nano resveratrol was more efficient in inhibiting ODC activity at lower doses as compared to resveratrol. A single topical application of TPA on mouse skin induced a robust 6.1-fold increase in ODC activity relative to the vehicle control.RV, NRV1 and NRV2 pre-treatment showed 23%, 42.7% and 68% reduction of ODC activity, respectively as compared to the ODC activity in the TPA-induced group. These data indicate that nano resveratrol, even at lower dose (0.5 μg) had significant inhibition on ODC activity as compared to that of resveratrol. Since elevated levels of ODC activity is related with cell proliferation and neoplastic transformation leading to tumorigenesis, inhibition of ODC activity by nano resveratrol in our study indicates its anti cancer potential in skin cancer [42]. Therefore, in a separate experiment, we measured tumor burden and tumor incidence in skin carcinogenesis mouse model.
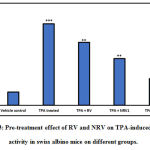 |
Figure 3: Pre-treatment effect of RV and NRV on TPA-induced ODC activity in swiss albino mice on different groups. |
Data represents mean ± S.E. of six mice per group.
Group 1 (vehicle control) is compared to the Group 2 (TPA treated). Group 3 (10μg RV), Group 4 (NRV1, 0.5μg) and Group 5 (NRV2, 1μg) are compared to Group 2 (TPA-treated)
(⁎ p<0.05, ⁎⁎ p<0.01, ⁎⁎⁎ p<0.001).
The rate of DNA synthesis was measured using thymidine incorporation assay, as shown in Figure 4. Treatment of mice with TPA led to a 2.7-fold increase in DNA synthesis in the mouse skin as revealed by the thymidine incorporation. RV, NRV1 and NRV2 pre-treatment significantly decreased the unscheduled DNA synthesis caused by TPA. The amount of reduction observed in the DNA synthesis in RV, NRV1 and NRV2 pre-treated groups was 21%, 55% and 61%, respectively as compared to the TPA-induced group. Taken together, these observations showed that nanoresveratrol at both the doses inhibited the DNA synthesis more efficiently as compared to resveratrol.
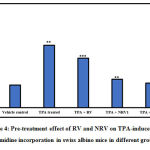 |
Figure 4: Pre-treatment effect of RV and NRV on TPA-induced [3H] thymidine incorporation in swiss albino mice in different groups. |
Results represent mean ±S.E. of six mice per group. Group 1 (vehicle control ) is compared to Group 2 (TPA treated).
Group 3 (RV,10μg ), Group 4 (NR1, 0.5μg ) and Group 5 (NR2, 1μg ) were compared to Group 2 (TPA treated) (⁎ p<0.05, ⁎⁎ p<0.01, ⁎⁎⁎ p<0.001)
Therefore,ODC activity and thymidine incorporation assays confirm that both resveratrol and nano resveratrol were effective at reducing the ODC activity and decreasing the DNA synthesis. Collectively, these studies indicate that nano resveratrol at low concentrations significantly inhibited DNA synthesis, implying a negative effect on cell proliferation which may point towards the chemopreventive action of resveratrol during tumor promotion and progression stage. To directly test the chemopreventive action of nanoresveratrol, we conducted experiments to evaluate nano resveratrol’s effect on tumor incidence and tumor burden.
Skin Tumorigenesis
TPA-induced skin inflammation, increased ODC activity and enhanced DNA synthesis have been reported to contribute to the malignant transformation of epidermal cells leading to tumorigenesis [45,46]. Since nanoresveratrol was effective in reducing inflammation, decreasing the ODC activity, significantly abrogating the DNA synthesis, we examined its effect on tumor formation.
We evaluated the effect of nano resveratrol using DMBA-initiated/TPA-promoted two-stage skin tumorigenesis in a mouse model. As shown in Table 2, the topical treatment of NRV on skin tumorigenesis in mice resulted in a significant decrease in tumor incidence and tumor burden in comparison to the mice treated with DMBA/TPA alone. The data collected at 6, 12 and 18 weeks is expressed as tumor incidence (percentage of mice with tumors) and tumor burden (number of tumors/mouse).
After 18weeks, tumor incidence in the DMBA/TPA induced mice treated with NRV2 was significantly reduced to 44.3% as compared to 96.5% with DMBA/TPA induced group. Similarly, after 18 weeks, thetumor burden in the DMBA/TPA induced mice treated with NRV2 were significantly reduced to 5.1 ± 0.92 as compared to 13.7 ±1.2 with DMBA/TPA group. Further, pre-treatment with NRV1 showed a significant inhibition of tumor incidence and tumor burden, as shown in the Table 2. In addition, these striking effects of nanoresveratrol were associated with a prolonged latency period of tumor induction and incidence.
Table 2: Pre-treatment effect of nanoresveratrol on DMBA-initiated /TPA-promoted tumor formation in swiss albino mice.
|
Groups
|
(Tumorincidence)
Percentage of mice with tumors |
(Tumor burden)
Tumors/mouse b |
|||||
| 6 weeks | 12 weeks | 18 weeks | 6weeks | 12 weeks | 18 weeks | ||
| Group 1 (Vehicle Control) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Group 2 (DMBA/TPA) | 47.8 | 87.7 | 96.5 | 4.3 ± 1.2⁎⁎ | 7.9± 1.0⁎⁎ | 13.7±1.2⁎⁎ | |
| Group 3 (RV1+DMBA/TPA) | 22.9 | 33.4 | 54.2 | 2.1 ± 0.32⁎ | 3.9± 0.10⁎⁎ | 6.2 ± 1.10⁎ | |
| Group 4(NRV2+DMBA/TPA) | 18.5 | 25.9 | 44.3 | 0.81± 0.21⁎ | 2.1 ± 0.09⁎ | 5.1 ± 0.92⁎ | |
Mouse was treated with single topical application of DMBA (50μg/200μl acetone) and promoted with TPA (2μg/200μl acetone) by thrice weekly application for 18 weeks. Group 1(vehicle control) was compared to Group 2 (DMBA/TPA), Group 3 (NRV1, 0.5μg) and group 4 (NRV2, 1μg) were compared to Group 2 (DMBA/TPA-treated).
aTumor incidence is expressed as percentage.
bTumor burden is expressed as the mean ± SE from 15 mice. (⁎ p<0.05, ⁎⁎ p<0.01).
In two step skin tumorigenesis, initiated by a single topical application of DMBA and promoted by repeated application of TPA, we observed that the NRV treatment resulted in a significant decrease in tumor incidence and tumor burden when compared to mice treated with DMBA/TPA. It has been previously reported that the topical application of resveratrol on the skin significantly reduced tumorigenesis in the mice [16,17]. However, this study demonstrates that the nano resveratrol tightly suppresses the tumorigenesis. In addition, we previously reported that nano resveratrol induces apoptosis in a murine skin cancer cell line [18].
Taken together, the topical application of NRV to swiss albino mice led to reduction in the process of DMBA/TPA-promoted skin tumorigenesis. Suppression of the inflammatory responses, ODC activity inhibition, inhibition of DNA synthesis may contribute towards resveratrol’s effect on controlling tumorigenesis. In addition, it is well established that the oxidative stress facilitates tumorigenesis through the free radical attack of biomolecules, which ultimately aids in the onset of neoplastic transformation. Oxidative stress also promotes inflammation, which in turn aids in tumorigenesis. The antioxidant property exhibited by nano resveratrol may provide protection against harmful radical attack due to the oxidative stress.
Conclusion
The studydemonstrates that the calcium phosphate nano resveratrol formulation has a potent in vivo anticancer activity against chemical-induced skin cancer. Enhanced inflammation, ODC activity, DNA synthesis, oxidative stress are known to promote carcinogenesis. We have observed that calcium phosphate encapsulated nano resveratrol is able to prevent these processes, suggesting nano resveratrol’s potential as a chemopreventive agent. Nano resveratrol also displayed a remarkable inhibitory effect on tumor incidence and tumor burden. The biocompatible calcium phosphate nano resveratrol formulation holds potential for overcoming challenges such as poor aqueous solubility and bioavailability. Nano resveratrol shows a sustained release over a period of time and displays a higher potency at lower doses as compared to free resveratrol. Taken together, this study displaysthe in vivo anti-cancer property of nanoresveratrol and suggests further investigation of its therapeutic potential against skin cancer.
Acknowledgments
We are thankful to the Department of Chemical Sciences, I.K. Gujral Punjab Technical University, Kapurthala (India) and Department of Chemistry, Jamia Hamdard University, New Delhi(India) for providing necessary laboratory facilities.
Conflict of Interest
Authors do not have any financial or non-financial conflict of interest.
References
- Worldwide Cancer Data. World Cancer Research Fund, 6 Aug. 2018, https://www.wcrf.org/dietandcancer/cancer-trends/worldwide-cancer-data
- Domingues B., Lopes J.M., Soares P., Pópulo H. Melanoma treatment in review. Immunotargets. Ther., 2018; 7: 35‐49.
CrossRef - Lee A.Y., Berman R.S. The Landmark Series: Non-melanoma skin cancers. Ann.Surg.Oncol., 2020; 27(1): 22‐27.
CrossRef - P., Brown K. Cancer chemoprevention: a rapidly evolving field. Br. J. Cancer., 2013; 109(1): 1‐7.
CrossRef - Jeter J.M. et al. Chemoprevention agents for melanoma: A path forward into phase 3 clinical trials. Cancer., 2019; 125(1): 18‐44.
- De Flora S., Ferguson L.R. Overview of mechanisms of cancer chemopreventive agents. Mutat. Res., 2005; 591(1-2): 8‐15.
CrossRef - Fontana F. et al. Unravelling the molecular mechanisms and the potential chemopreventive/therapeutic properties of natural compounds in melanoma. Semin. Cancer. Biol., 2019; 59: 266‐282.
CrossRef - De Melo F.H.M., Oliveira J.S., Sartorelli V.O.B., Montor W.R. Cancer chemoprevention: classic and epigenetic mechanisms inhibiting tumorigenesis. what have we learned so far?. Front. Oncol., 2018; 8: 644.
CrossRef - Katta R., Brown D.N. Diet and skin cancer: The potential role of dietary antioxidants in nonmelanoma skin cancer prevention. J. Skin.Cancer., 2015; 2015: 1-10.
CrossRef - Jang M., Cai L., Udeani G.O. et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science., 1997; 275(5297): 218‐220.
CrossRef - Kim S.M., Kim S.Z. Biological activities of resveratrol against cancer. Phys. Chem. Biophys.,2018; 8: 1-16.
CrossRef - Shankar S., Singh G., Srivastava R.K. Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front. Biosci., 2007; 12: 4839‐4854.
CrossRef - Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: how are they linked?. Free. Radic. Biol. Med., 2010; 49(11): 1603‐1616.
CrossRef - Pandey K.B., Rizvi S.I. Anti-oxidative action of resveratrol: Implications for human health. Arabian J. of Chem., 2011; 4( 3): 293–98.
CrossRef - Ndiaye M., Philippe C., Mukhtar H., Ahmad N. The grape antioxidant resveratrol for skin disorders: promise, prospects, and challenges. Arch. Biochem. Biophys., 2011; 508(2): 164‐170.
CrossRef - Roy P., Kalra N., Prasad S., George J., Shukla Y. Chemopreventive potential of resveratrol in mouse skin tumors through regulation of mitochondrial and PI3K/AKT signaling pathways. Pharm. Res., 2009; 26(1): 211‐217.
CrossRef - Afaq F., Adhami V.M., Ahmad N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol. Appl. Pharmacol., 2003; 186(1): 28‐37.
CrossRef - Arora R., Parkash C. Synthesis, characterization, and evaluation of anticancer activity of nanoresveratrol in B16 melanoma cell line. J. Drug Del.Therap., 2019; 9(4A): 625-631.
- Singh C.K., Ndiaye M.A., Ahmad N. Resveratrol, and cancer: Challenges for clinical translation. Biochim. Biophys. Acta., 2015; 1852(6): 1178‐1185.
CrossRef - Berman A.Y., Motechin R.A., WiesenfeldM.Y., Holz M.K. The therapeutic potential of resveratrol: a review of clinical trials. NPJ. Precis. Oncol., 2017; 1:35.
CrossRef - Siddiqui I.A., Sanna V., Ahmad N., Sechi M., Mukhtar H. Resveratrol nanoformulation for cancer prevention and therapy. Ann. N Y Acad. Sci., 2015; 1348(1): 20‐31.
CrossRef - Rigon R.B. et al. Nanotechnology-based drug delivery systems for melanoma antitumoral therapy: A review. BioMed Res Int., 2015; 2015: 1–22.
CrossRef - ummerlin N., Soo E., Thakur S., Qu Z., Jambhrunkar S., Popat A. Resveratrol nano formulations: challenges and opportunities. Int. J. Pharm., 2015; 479(2): 282‐290.
CrossRef - Wan S., Zhang L., Quan Y., Wei K. Resveratrol-loaded PLGA nanoparticles: enhanced stability, solubility, and bioactivity of resveratrol for non-alcoholic fatty liver disease therapy. R. Soc. Open Sci., 2018; 5(11): 181457.
CrossRef - Chaudhary Z. et al. Encapsulation and controlled release of resveratrol within functionalized mesoporous silica nanoparticles for prostate cancer therapy. Front. Bioeng. Biotechnol., 2019; 7:
CrossRef - Bano S., Ahmed F., Khan F., Chaudhary S.C., Samim, M. Enhancement of the cancer inhibitory effect of the bioactive food component resveratrol by nanoparticle-based delivery. Food & Function., 2020 ; 11(4): 3213–26.
CrossRef - Bisht S., Bhakta G., Mitra S., Maitra A. pDNA loaded calcium phosphate nanoparticles: highly efficient non-viral vector for gene delivery. Int. J. Pharm., 2005; 288(1): 157‐168.
CrossRef - Huang D., He B., Mi P. Calcium phosphate nanocarriers for drug delivery to tumors: imaging, therapy and theranostics. Biomater. Sci., 2019; 7(10): 3942‐3960.
CrossRef - Mingzhen Z., Kataoka K. Nano-structured composites based on calcium phosphate for cellular delivery of therapeutic and diagnostic agents. Nano Today., 2009; 4 (6), 508–17.
CrossRef - Wu J., Lin L.,Yurong C., Juming Y. Recent advances of calcium phosphate nanoparticles for controlled drug delivery. Mini-Reviews in Medi. Chem., 2013; 13: 1501-1507.
CrossRef - Filler R. B., Roberts S.J., GirardiM. Cutaneous two-stage chemical carcinogenesis. CSH Protoc., 2007; 2007.
CrossRef - Katiyar S.K., Mukhtar H. Inhibition of phorbol ester tumor promoter 12-O-tetradecanoylphorbol-13-acetate-caused inflammatory responses in SENCAR mouse skin by black tea polyphenols. Carcinogenesis.,1997; 18(10): 1911‐1916.
CrossRef - Lu X. et al. Resveratrol-loaded polymeric micelles protect cells from Abeta-induced oxidative stress. Int. J. Pharm., 2009; 375(1-2): 89‐96.
CrossRef - Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. Lebenson Wiss. Technol., 1995; 28: 25-30.
CrossRef - Afaq F., Saleem M., Krueger C.G., Reed J.D., Mukhtar H. Anthocyanin‐ and hydrolyzable tannin‐rich pomegranate fruit extract modulates MAPK and NF‐κB pathways and inhibits skin tumorigenesis in CD‐1 mice. Int. J. Cancer., 2005; 113: 423-433.
CrossRef - Verma A.K., Shapas B.G., Rice H.M., Boutwell R.K. Correlation of the inhibition by retinoids of tumor promoter-induced mouse epidermal ornithine decarboxylase activity and of skin tumor promotion. Cancer Res., 1979; 39 (2): 419‐425.
- Smart R.C., Huang M., M., Conney A.H. sn-l, 2-Diacylglycerols mimic the effects of 12- 0-tetradecanoylphorbol-13-acetate in vivo by inducing biochemical changes associated with tumor promotion in mouse epidermis. , 1986; 7(11): 1865–1870.
CrossRef - Chaudhary S.C., Alam M.S., Siddiqui M.S., Athar M. Perillyl alcohol attenuates Ras-ERK signalling to inhibit murine skin inflammation and tumorigenesis. Chemico-bio. Inter., 2009 ; 179(2-3): 145-153.
CrossRef - Maru G.B., Gandhi K., Ramchandani A., Kumar G. The role of inflammation in skin cancer. Adv. Exp. Med. Biol. 2014; 816: 437‐469.
CrossRef - Neagu M. et al. Inflammation: A key process in skin tumorigenesis. Oncol. Lett., 2019; 17: 4068-4084.
CrossRef - Auvinen M., Paasinen A., Andersson L.C., Holtta E. Ornithine decarboxylase activityis critical for cell transformation. Nature., 1992; 360(6402): 355‐358.
CrossRef - O’Brien T.G., Megosh L.C., Gilliard G., Soler A.P. Ornithine decarboxylase overexpression is a sufficient condition for tumor promotion in mouse skin. Cancer Res.,1997; 57(13): 2630‐2637.
- ElmetsA.,Athar M.Targeting ornithine decarboxylase for the prevention of nonmelanoma skin cancer in humans. Cancer Prev. Res (Phila)., 2010; 3(1): 8‐11.
CrossRef - Tang X. et al. Ornithine decarboxylase is a target for chemoprevention of basal and squamous cell carcinomas in Ptch1+/- mice. J. Clin. Invest. 2004; 113(6): 867‐875.
CrossRef - Rundhaug J.E., Fischer S.M. Molecular mechanisms of mouse skin tumor promotion. Cancers(Basel)., 2010; 2(2): 436‐482.
CrossRef - Nowotarski S.L, Feith D.J, Shantz L.M. Skin Carcinogenesis Studies Using Mouse Models with Altered Polyamines. Cancer Growth Metastasis. 2015; 8(1): 17‐27.
CrossRef







