Manuscript accepted on :4-03-2021
Published online on: 30-03-2021
Plagiarism Check: Yes
Reviewed by: Dr. Aizman Roman
Second Review by: Dr. Ankur Singh Bist
Final Approval by: Dr. Josphert Ngui Kimatu
Aliaa A. Ismail1 , Dalia W. Zeidan2
, Dalia W. Zeidan2 , Amani A. Almallah3
, Amani A. Almallah3 , Aya A. Gaber4
, Aya A. Gaber4 and Heba M. A. Abdelrazek5*
and Heba M. A. Abdelrazek5*
1Department of Pathology, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt.
2Department of Home and Economics, Nutrition and Food Science Branch, Faculty of Education, Suez Canal University, Ismailia, Egypt.
3Department of Anatomy and Embryology, Faculty of Medicine, Suez Canal University, Egypt.
4Department Pharmacology Department, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt.
5Department of Physiology, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt,
Corresponding Author E-mail: hebaabdelrazekvet@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2113
Abstract
Present work aimed to study the influence of dietary ginger inclusion in female mice on gastrointestinal integrity, hepatic condition and metabolic parameters. Thirty female mice (18± 2 g) were assigned into 3 groups; control group fed standard rodents’ basal diet, ginger 2% and ginger 5% mice were basal diet supplemented with 2% and 5% ginger powder, respectively for 30 days. Weight gain, feed conversion (FCR) and efficiency (FER) ratios were recorded. Serum liver enzymes, lipid profile, total protein and albumin were measured beside estimation of hepatic reduced glutathione (GSH) and malondialdehyde (MDA). Gastric, intestinal and hepatic histopathology were performed as well as intestinal histomorphometry. Results revealed improvement in FCR, FER and most tested biochemical parameters, in 2% ginger group than control. Hepatic MDA and GSH were significantly (P<0.05) increased and decreased, respectively in 2% ginger group. However, ginger 5% group exhibited improvement in intestinal histomorphometry while adversely affected gastric mucosa and hepatic tissue histopathology. Also increased hepatic MDA and reduced GSH were prominent in 5% ginger group along with mild gastric and hepatic lesions. The administration of dietary ginger by 2% dose could be beneficial mice model however, increasing the dose to 5% could produce adverse effects on hepatic integrity and gastric mucosa.
Keywords
Dietary; Ginger; Intestinal Histomorphometry; Liver
Download this article as:| Copy the following to cite this article: Ismail A. A, Zeidan D. W, Almallah A. A, Gaber A. A, Abdelrazek H. M. A. Effect of Dietary Ginger as Feed Additive on Gastrointestinal Integrity, Hepatic Condition and Metabolic Parameters of Female Mice. Biomed Pharmacol J 2021;14(1). |
| Copy the following to cite this URL: Ismail A. A, Zeidan D. W, Almallah A. A, Gaber A. A, Abdelrazek H. M. A. Effect of Dietary Ginger as Feed Additive on Gastrointestinal Integrity, Hepatic Condition and Metabolic Parameters of Female Mice. Biomed Pharmacol J 2021;14(1). Available from: https://bit.ly/3we8XKs |
Introduction
The use of feed additives, in livestock production(Singh, 2016)as well as human feed(Wang and Shelomi, 2017) is gaining special interest owing to many reasons. Feed additives can improve digestibility and have antimicrobial, anti-inflammatory, antioxidant and immune-stimulant activities. Moreover, the ban on use of certain antibiotics due to harmful residual effects on human health and development of microbial resistance to antibiotic drugs (Sarker et al., 2010). Herbal feed additives play a significant role in health and nutrition. Several feed additives likeprebiotics,probiotics, plant extracts and organic acids had been demonstrated to have beneficial influences on animal production (Kumar et al., 2014)
Ginger(Zingiberofficinale) is one of the most well-known, safe medicinal plants with only few and insignificant adverse effects, that belongs to the Family Zingiberaceae (Mekuriya and Mekibib, 2018). Ginger is also worldwide used as cooking spice, flavoring agent and food preservation (Ajayi et al., 2013). In traditional medicine, ginger was used as a carminative or anti-flatulent. More than 60 different bio active constituents are present in ginger, which have been allocated into volatile and nonvolatile compounds (Ahmad et al., 2015). The powdered rhizome contains 3-6% fatty oil, 9% protein, 60-70% carbohydrates, 3-8% crude fiber, about 8% ash, 9-12% water and 2-3% volatile oil (Zadeh and Kor, 2014). Ginger has prominent medicinal characteristics such as its pungent and stimulant effects, where its rhizome possesses a wide range of biologically active ingredients; such as gingerol, bisabolenehogoals, salicylate, curcumin, caffeic acid, capsaicin, zingiberene and various types of lipids(Mahmood, 2019). Therefore, there are various medical applications owing to these medicinal properties have been researched before. These medicinal applications include; antipyretic, analgesic, antiemetic, antiulcer, cardio depressant and prostaglandins suppression (Semwal et al., 2015). Several studies showed that it has antioxidant, anti-hyperglycemic (Afshari et al., 2007), anticancer(Babasheikhali et al., 2019), anti-inflammatory(Tramontin et al., 2020), antiapoptotic (Li et al., 2020), and antihyperlipidemic actions (Kumar et al., 2013).
Moreover, Ginger has gastrointestinal protective effect as it contains several active ingredients which are known to promote digestion, absorption and counteracting constipation as well as flatulence via accelerating activity of gut muscles (Priyashantha and Mahendranathan, 2020). Ginger roots contains ingredients like Aryl alkanes that give ginger pungent taste that enhancing the appetite of animal and improve the nutrients palatability which finally caused increased feed intake(Roufogalis, 2014).
Although, the previously reviewed protective effects of ginger it had been found to produce some adverse effects. These effects include gastro intestinal upset and troubles (Zick et al., 2009). The safety and optimum dose for dietary ginger is not fully elucidated. Therefore, the target of the hereby study was to research the influence of two levels of dietary ginger on body weight, biochemical parameters, hepatic oxidative stress, guthistomrphometry and hepatic histopathology.
Material and Methods
Animals and Treatment
Thirty female mice weighing (18± 2 g) obtained from the Animal House of Theodor Bilharz Research Institute, Cairo, Egypt, were used for the present study. Animals were kept in standard plastic cages at the Laboratory Animal House, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt. All experimental protocols were executed according to the “Guide for the Care and Use of Laboratory Animals”. After acclimating for 14 days, mice were randomly divided into three equal groups, each containing 10 mice and treated as follows:
Group A (control): served as negative control mice, fed standard rodents’ basal diet
Group B (Ginger 2%): mice were fed standard rodents’ basal diet supplemented with ginger powder (Captain Co., Egypt) at rate 2%
Group C (Ginger 5%): mice were fed standard rodents’ basal diet supplemented with ginger powder (Captain Co., Egypt) at rate 5%
The experimental diets were offered for 30 days.
Body and liver weights, Feed conversion ratio (FCR) and Feed Efficiency Ratio (FER).
Experimental mice were weighed weekly during the experimental period. The final body weight was subtracted from the initial one to obtain the weight gain. Food intake was recorded all over the thirty days of the experimental time. FCR was obtained from the formula described by Abdelrazek et al. (2018) as follows: FCR = (Feed consumption (g) /mice/30 days)/ (body weight gain (g) /mice/30 days). Feed efficiency ratio (FER) was obtained from the formula described by Helmy et al. (2018) as follow:
FER = body weight gain (g) after 30days/food intake (g) for 30 days. The liver weight was recorded then relative hepatic eight in relation to body weight was calculated.
Sampling
After thirty days from the start of treatment, serum samples tissue specimens were collected from diestrus females in all groups. Mice were exposed to light tetrahydrofurane anesthesia, and blood was obtained from retro-orbital plexus. The blood was let to coagulate for 20 minutes then subjected to centrifugation at 3000 rpm for 15 minutes to get the serum. The sera were kept at -20°C. After blood collection, mice were dissected and the postmortem findings were recorded; then samples from the internal organs (liver and intestine) were taken. The liver was excised, washed with ice-cold phosphate buffer saline (PBS) to remove any extraneous matter blotted to dry, and weighed, and small sections were cut. The first pieceof the liver was subjected to homogenization to be applied for the antioxidant level determination. The other liver pieceas well as different small intestine segments were put in 10% neutral buffered formalin for the histopathologicalinspection.
Serum biochemical parameters
Serum alanine aminotransferase(ALT) and alkaline phosphatase (ALP) were measured using calorimetric Diamond Diagnostic Co., (Egypt) kits. The total protein (TP) and albumin levels were estimated in sera using calorimetric Diamond Diagnostic Co., (Egypt) kits. The level of globulin was obtained by subtracting albumin from total protein value. Albumin/ globulin (A/G) ratio was calculated.
Serum total cholesterol (TC)level, triglycerides (TGs) and high density lipoprotein cholesterol (HDLC) were determined by Spectra diagnostic, Egypt kits. The procedures were done according the enclosed kits’ pamphlet.
Liver of each experimental mice was subjected to homogenization in potassium phosphate buffer PH= 7.4. One-gram liver was added to 5 mL of the later buffer then homogenized with Teflon Homogenizer, (Spain). The samples were centrifuged at 3000 rpm at 4oC to obtain supernates. The hepatic reduced glutathione (GSH) content and malondialdehyde (MDA) as lipid peroxidation marker were estimated in supernatant using Biodiagnostic Co., (Egypt) kits.
Histopathological examination and histochemistry
Formalin- fixed liver and intestine were washed under running tap water, dehydrated in ascending concentration gradesof ethyl alcohol and stained with H&E stain according to the standard method then examined microscopically (Slaoui and Fiette, 2011).
Other 5 μm thickness liver sections were imperiled to periodic acid Schiff (PAS) stain for glycogen according to Pearse (1968)
Image Analysis
Morphometric analysis for crypt depth (CD, μm), intestinal villus width (VW, μm) and villus height (VH, μm) was performed by Image J software. CD was dogged as the deepness of the invagination between two adjacent villi whereas the VH was assessed from the villus top to the lamina propria. The PAS stained liver slides were subjected to color quantification using Image J program. 5 random fields / slide/ animal were subjected to this analysis.
Statistical Analysis
Statistical analyses were doneby the aid of SPSS software version 20.0. One-way analysis of variance (ANOVA) was used to distinguish the significant differences between the groups followed by Duncan’s multiple comparison test to find if there was any significant difference between groups. Statistical significance was deliberated when P ≤ 0:05. All the values were conveyed as mean ± SE (standard error of the mean).
Results
Body and liver weight, FCR and FER
The final body weight and body weight gain were significantly reduced (P<0.05) in mice fed with ginger 5% when matched to that of control. There were non-significant variances observed in the latter parameters in group B (Ginger 2% supplemented group) compared to control and ginger 5% fed groups.
In addition, feed intake and FER showed a significant (P < 0.05) decrease in ginger 5% supplemented group compared to control. However, FCR was significantly (P<0.05) increased in ginger 5% supplemented group than control and 2% ginger supplemented group. Ginger 2% demonstrated significant improvement in FCR and FER than control.
Relative liver weights in group B (Ginger 2% fed mice) was significantly decreased (P < 0.05) in comparison tothose in the control group and 5% ginger supplemented group.
Table 1: Effect of dietary ginger supplementation on body weight, weight gain, feed intake, feed conversion ratio (FCR), feed efficiency ratio (FER) and liver weight in female albino mice.
| Control (group A) | Ginger 2% (group B) | Ginger 5% (group C) | |
| Initial body weight (g) | 19.02±0.61 | 19.05 ±0.50 | 18.91±0.41 |
| Final body weight (g) | 27.02a±1.00 | 25.05ab ±1.00 | 22. 40 b ±0.61 |
| Weight gain (g) | 8.00 a ±0.41 | 5.10a±0.92 | 3.51 b ±0.81 |
| Feed intake (g) | 37.81a±1.10 | 30.50 a ±2.40 | 25.6 b ±0.7 |
| FCR | 5.62b ±0.90 | 4.01 c±0.31 | 9.82 a ±1.90 |
| Feed efficiency | 0.21 b ±0.03 | 0.26a±0.01 | 0.10c±0.03 |
|
Liver weight (g) |
1.50 a ±0.20 | 0.52b ±0.30 |
1.81 a ±0.20 |
Different letters (a & b) indicate significant differences between interacting groups at P < 0:05.
Serum biochemical parameters
There were non-significant alterations in ALT levels between animal so fall experimental groups. Meanwhile, the serum ALP activity was markedly decreased (P<0.05) in group B (Ginger 2%)supplemented mice in comparison to the control group and ginger 5% fed mice.
Interestingly, Group B (Ginger 2%) fed mice revealed a significant (P<0.05) elevation in total protein, albumin and globulin levels in comparison to other experimental groups.
Ginger supplementation significantly (P<0.05) decreased the serum total cholesterol levels in both ginger fed groups (2% and 5%) when matched to the control one. A significant (P<0.05) promotion in serum HDLC concentrations were noted in mice fed ginger 2% supplemented diet compared with other groups. Furthermore, TGs levels demonstrated a significant (P<0.05) decrease in both ginger treated groups in comparison to control ones.
Compared with control group, Ginger 2% supplementation caused significant increases in the contents of hepatic reduced glutathione (GSH). Moreover, MDA level was markedly (P<0.05) suppressed by ginger 2% treatment while it was significantly (P<0.05) increased than control in ginger 5% supplemented group.
Table 2: Effect of dietary ginger supplementation on serum biochemical parameters, lipid profile, reduced glutathione (GHS) and lipid peroxidation (MDA) in female albino mice.
| Control (group A) | Ginger 2%
(group B) |
Ginger 5
(group C) |
|
| ALT (IU/l) | 24.40a ±0.21 | 24.91a ±0.22 | 24.30a ±0.14 |
| ALP (IU/l) | 64.41a ±0.32 | 60.72b ±1.52 | 66.52 a ±0.84 |
| Total protein | 5.91b ±0.23 | 8.71a ±0.20 | 5.70b±0.22 |
| Albumin | 4.22b ±0.01 | 5.62a ±0.21 | 4.10b ±0.20 |
| Globulin | 1.71b ±0.20 | 3.10a±0.20 | 1.60 b ±0.30 |
| A/G ratio | 2.81ab ±0.32 | 1.91b ±0.22 | 3.41a±0.60 |
| Total cholesterol | 83.30a ±1.70 | 68.70b ±2.50 | 73.11b ±3.21 |
| HDLC | 44.71b ±1.51 | 54.70a±1.40 | 41.71b ±0.91 |
| TGs | 83.11a ±0.81 | 63.42c±1.71 | 71.41b ±4.01 |
| GSH (mmol/g tissue) | 25.60b ±1.00 | 31.10a±0.50 | 22.80c±1.00 |
| MDA (nmol/g tissue) | 0.60b ±0.02 | 0.50c±0.01 | 0.70a±0.03 |
Different letters (a, b and c) indicate significant differences between interacting groups at P < 0:05.
Histopathological examination and histochemistry
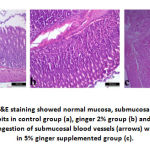
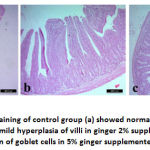
Stomach of control group (group A) and group B showed normal histological structure of mucosa, submucosa and serosa. Normal gastric glands, gastric pits were observed. Group C showed normal structure of all layers in addition to congestion of submucosal blood vessels. On the same hand, duodenum showed normal intact tall villi with normal enterocytes, submucosal glands and normal crypts in all examined groups. Jejunum of control group had normal long columnar villi with columnar enterocytes, while group B showed mild hyperplasia of enterocytes lining the villi and proliferation of goblet cells in group C. Examination of ileum revealed normal histological picture in all groups.
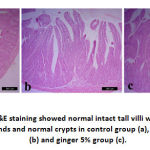 |
Figure 2: Duodenum H&E staining showed normal intact tall villi with normal enterocytes, submucosal glands and normal crypts in control group (a), ginger 2% group (b) and ginger 5% group (c). |
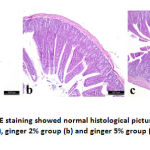 |
Figure 4: Ileum H&E staining showed normal histological picture in control group (a), ginger 2% group (b) and ginger 5% group (c). |
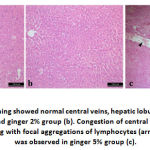
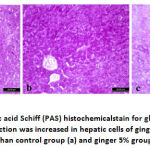
Liver of group A and B showed normal hepatic lobules, central veins and intact radiating hepatic cords around central veins. Group C had congestion of central veins along with focal aggregations of lymphocytes around the congested blood vessels. Histochemical reaction of the liver cells to PAS showed increased the positive reaction of hepatic cells of group B to PAS than other two groups.
Image Analysis
The morphometric analysis for duodenalvilli height revealed significant (P<0.05) increase in VH and CD in both ginger treated groups (2% and 5%) than control however duodenal VW showed no significant differences among groups. Jejunal VH and VW revealed significant increase in ginger 2% group than control while ginger 5% treated group was non-significantly differed than control. Jejunal CD showed significant increase in both ginger 2% and ginger 5% groups than control. Ilial VH was significantly (P<0.05) increased in both ginger 2% and ginger 5% groups than control group. However, VW and CD were significantly (P<0.05) promoted in ginger 2% group than the control. Ginger 5% group showed non-significant alteration in VW and CD than control.
Table 3: Effect of dietary ginger supplementation on intestinal morphometric analysis and liver color quantification in female albino mice.
| Control (group A) | Ginger 2%
(group B) |
Ginger 5
(group C) |
||
| Duodenum | Villus height (μm) | 435.10±28.51b | 1280.11 a ±52.31 | 1613.21±60.82a |
| Villus width (μm) | 154.30 ± 19.20 | 166.12 ± 25.60 | 141.42 ± 75.61 | |
| Crypt depth (μm) | 219.7 b± 9.71 | 265.45a ± 12.71 | 290.21a ± 53.72 | |
| Jejunum | Villus height (μm) | 488.01b±81.28 | 721.25a± 7.61 | 407.31b±24.26 |
| Villus width (μm) | 117.11b±12.55 | 278.41a±14.30 | 138.13b±21.51 | |
| Crypt depth (μm) | 220.59b±38.31 | 317.88a±40.33 | 291.29a±24.27 | |
| Ilium | Villus height (μm) | 372.75b±20.96 | 656.49a± 19.07 | 455.33a±15.02 |
| Villus width (μm) | 187.66b±23.75 | 251.98a±24.9 | 132.85b±8.71 | |
| Crypt depth (μm) | 218.48ab±33.51 | 249.96a±37.1 | 138.43b±8.1 | |
| Liver | Staining intensity | 55.21b±3.98 | 79.32a ±7.89 | 45.23c±5.88 |
| Staining area % | 66.32b±6.23 | 85.98 a±5.87 | 51.35c±6.99 |
Different letters (a, b and c) indicate significant differences between interacting groups at P < 0:05.
Discussion
In the hereby study, different levels of ginger powder (2% and 5%) were added to female mice dietand their influences on final body weight, weight gain, liver weight, serum biochemical parameters, lipid profile, oxidative stress, lipid peroxidation and organ histopathology were evaluated.
The inclusion of ginger powder in diet of female mice (Ginger 2%) showed no significant variations in final weight gain as compared to control group. However, ginger 5% group (C) treated mice showed announced reduction in body weight when compared to control group (A) and ginger 2% group (B). This result was similar toSayed et al. (2020)who reported significant decrease in body weight and weight gain after ginger administration. Moreover, food intake was observed to be significantly decreased in ginger 5% group (C). This effect may be explained by the evidences demonstrated by several publications which stated that; many of medical herbal plants in high dose may cause reduction in food intake due to its strong bitter taste (Mansoub, 2011; Ficker et al., 2003). In our study, increasing ginger % in diet has pungent smell and tastethat may attribute to the observedreduction of food intake. Also ginger at 5% level has been confirmed to reduce blood leptin levelas well as fat mass (Misawa et al., 2015). Leptinhas acentralpart in regulation of appetite via influencing orexigenic neuropeptide Y (Kelesidis et al., 2010). Consequent to food intake reduction the FER and FCR were significantly decreased and increased, respectively in group (C). our results concerning this point were in harmony with those obtained by Kim et al. (2018).
Ginger has a gastro protective activity and the ability to protect gastric mucosa against any necrotizing agent suggesting increased digestion (Eltazi, 2014). It also stimulates digestion beneficially, enhancing the digestive activities like the intestinal lipase, the disaccharides, sucrase and maltase(Platel and Srinivasan, 1996)therefore it increased FER and reduced FCR in 2% ginger supplemented group.
Concerning the serum biochemistry results, we noticed a significant decline in serum ALT and ALP values in group B (ginger 2%). Comparatively, ginger 2% supplementation appear to exhibit higher protection of liver compared with ginger 5%. These results may be due to the existence of hepatoprotective natural bioactive ingredients in the ginger powder which are capableofdiminishing free radical-produced liverinjury (Butt and Sultan, 2011). This indicates that ginger aided in the regeneration of hepatocyte, enhanced the liver histology and function, inhibitedhepatic damage, arrested further hepatic parenchymal destruction and preserved cell membrane integrity, accordingly, inhibited the enzyme release. These results came in agreement with (Patrick-Iwuanyanwu et al. (2007); Mahmoud and Elnour, 2013; Shanmugam et al., 2011).
Concerning lipid profile results, a significant reduction was observed in serum TC, TGs levels in both ginger fed groups (2% and 5%). Ginger has been confirmed to lower serum TGs and TCand raise HDLC(Misawa et al., 2015; Thomson et al., 2002; Fuhrman et al., 2000; Verma et al., 2004). It was established that ginger active ingredients could reduce cholesterol biosynthesis as well as cholesterol conversion to bile acids(Fuhrman et al., 2000). Ginger and its active contents could stimulate the activity of hepatic cholesterol-7-alpha hydroxylase, which in turn stimulates the conversion of cholesterol to bile acids, an important pathway of elimination of cholesterol from the body(Mahmoud and Elnour, 2013). Also ginger promotes fecal cholesterol excretion (Verma et al., 2004).Also, ginger can inhibit the absorption of lipids in diet by prohibiting their hydrolysis, Furthermore, (Ramakrishna Rao et al., 2003) cleared that ginger enhanced the activity of pancreatic lipase and amylase when they were directly in contact with the enzymes. Collectively, comparison between group B and group C, showed that the influence of ginger 2% treatment on reducing levels of TGs was more apparent than that of ginger 5%. The prohibiting effect of ginger on TC and TGs seen in group B and group C was consistent with previous reports that demonstrating the hypocholesterolemic and anti-atherosclerotic effects of ginger(Bhandari and Pillai, 2005; Ahmed et al., 2000; Sakr et al., 2009).
Addition of dietary ginger 2% produced significant rise in serum HDLC concentrations in comparison to the control group. HDLC is considered as protective against atherosclerosis because it moves cholesterol from marginal tissues to the liver thus serves as good transporterfor plasma cholesterol (Blaha et al., 2008).
The active constituents of ginger such as zingerone, gingeriol, zingibrene, gengerols and shogoals have antioxidant potential(Zancan et al., 2002). Besides, other researchers showed that ginger oils have dominative protective effect on DNA damage induced by H2O2 and might act as a scavenger of oxygen radicals and might be used as an antioxidant (Grzanna et al., 2005).
In this investigation, hepatic GSH levels were dramatically promoted in group B (ginger 2%). These results harmonized with Mohamed et al. (2015). The possible explanation may be due to prevention of ROS generation consistent with free radical scavenging potential, omitting lipid peroxidation and protein oxidation and declined ROS-DNA interaction demonstrating the restoration of the antioxidant system of the liver (Al-Suhaimi et al., 2011).
Moreover, MDA was significantly reduced in 2% ginger supplemented group that could be seemingly linked to ginger ability to hunt reactive oxygen species particularly the most active peroxyland hydroxyl radicals that initiated lipid peroxidation and counteract the oxidative steps(Mekuriya and Mekibib, 2018). These results were parallel to increased hepatic synthetic power of TP, albumin and globulin as well as glycogen storage in group (B). On the other side, liver content of GSH was significantly declined while MDA increased in 5% ginger supplemented group that denoted adverse effect of such dose in hepatic tissue.some researches demonstrated that increasing the dosage of herbal medicinal plants may encounter some sort of toxicity (Mansoub, 2011; Ficker et al., 2003).
Previous results were in harmony with the hepatic histopathological examination. Whereas mild histological lesions were observed in ginger 5% fed mice; including congestion in central vein as well as focal aggregation of lymphocytes. The lipid peroxidation and oxidative stress were known to be potent stimulant of hepatic proinflamatory cytokines production (Li et al., 2016; Elgawish et al., 2019) that manifested by lymphocytes aggregation. The hepatic lesion in such group was reflected on hepatic glycogen storage capacity that manifested by reduced staining area % and intensity. Consequently, the hepatic capability to synthesize TP, albumin and globulin were reduced in such group.
Gastric and intestinal histomorphometry results revealed that usage of 2% dietary ginger had more pronounced protective effect on gastric mucosa and intestinal VH, CD and CW. The later histomorphic parameters promotion were attributed to increased digestive as well as absorption surface (Ribeiro et al., 2004) that increased the feed efficiency in current study. Contrary, administration of 5% dietary ginger produced congestion in submucosal gastric vessels while increasing intestinal VH and CD with no alteration in VD. Other authors confirmed that higher dose of ginger could cause gastric irritation (Desai et al., 1990).
Conclusion
From the present study,it could be concluded that the incorporation of ginger in mice diet as feed additive at 2% level significantly has a great positive effect on feed effeciency, promoting blood biochemical parameters, increasing hepatic antioxidants and reduced lipid peroxidation. Along with positive change in the histological and histochemical appearance of digestive organs were observed. However, adverse effects were observed in ginger 5% dose especially concerning feed efficiency, hepatic antioxidants and hepatic histopathology as well as lipid peroxidation. Therefore, ginger should not be increased otherwise it would produce retrogressive changes or adverse effects. Further investigations using several doses of ginger must be done to elucidate an endpoint for its safe use in diet.
Conflict of Interest
The authors have no conflict to disclose.
Funding Source
This work received no external funding.
References
- Singh P. (2016). Use of nano feed additives in livestock feeding. International Journal of Livestock Research 6, 1-14.
CrossRef - Wang Y.-S., and Shelomi M. (2017). Review of black soldier fly (Hermetia illucens) as animal feed and human food. Foods 6, 91.
CrossRef - Sarker M., Ko S., Lee S., Kim G., Choi J., and Yang C. (2010). Effect of different feed additives on growth performance and blood profiles of Korean Hanwoo calves. Asian-Australasian Journal of Animal Sciences 23, 52-60.
CrossRef - Kumar M., Kumar V., Roy D., Kushwaha R., and Vaiswani S. (2014). Application of herbal feed additives in animal nutrition-a review. Int J Lives Res 4, 1-8.
CrossRef - Mekuriya W., and Mekibib B. (2018). Review on the medicinal values of ginger for human and animal ailments. J Vet Sci Technol 9.
CrossRef - Ajayi O.B., Akomolafe S.F., and Akinyemi F.T. (2013). Food value of two varieties of ginger (Zingiber officinale) commonly consumed in Nigeria. International Scholarly Research Notices 2013.
CrossRef - Ahmad B., Rehman M.U., Amin I., Arif A., Rasool S., Bhat S.A., Afzal I., Hussain I., and Bilal S. (2015). A review on pharmacological properties of zingerone (4-(4-Hydroxy-3-methoxyphenyl)-2-butanone). The Scientific World Journal 2015.
CrossRef - Zadeh J.B., and Kor N.M. (2014). Physiological and pharmaceutical effects of Ginger (Zingiber officinale Roscoe) as a valuable medicinal plant. European Journal of Experimental Biology 4, 87-90.
- Mahmood S. (2019). A critical review on pharmaceutical and medicinal importance of ginger. Acta Scientific Nutritional Health 3, 78-82.
CrossRef - Semwal R.B., Semwal D.K., Combrinck S., and Viljoen A.M. (2015). Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 117, 554-568.
CrossRef - Afshari A.T., Shirpoor A., Farshid A., Saadatian R., Rasmi Y., Saboory E., Ilkhanizadeh B., and Allameh A. (2007). The effect of ginger on diabetic nephropathy, plasma antioxidant capacity and lipid peroxidation in rats. Food chemistry 101, 148-153.
CrossRef - Babasheikhali S.R., Rahgozar S., and Mohammadi M. (2019). Ginger extract has anti-leukemia and anti-drug resistant effects on malignant cells. Journal of Cancer Research and Clinical Oncology 145, 1987-1998.
CrossRef - Tramontin N.d.S., Luciano T.F., Marques S.d.O., de Souza C.T., and Muller A.P. (2020). Ginger and avocado as nutraceuticals for obesity and its comorbidities. Phytotherapy Research 34, 1282-1290.
CrossRef - Li J., Thangaiyan R., Govindasamy K., and Wei J. (2020). Anti-inflammatory and anti-apoptotic effect of zingiberene on isoproterenol-induced myocardial infarction in experimental animals. Human & Experimental Toxicology, 0960327120975131.
CrossRef - Kumar S., Saxena K., Singh U.N., and Saxena R. (2013). Anti-inflammatory action of ginger: A critical review in anemia of inflammation and its future aspects. Int J Herb Med 1, 16-20.
- Priyashantha A., and Mahendranathan C. (2020). Traditional uses of medicinal plants in Sri lanka with special reference to herbal drinks: A review. Journal of Biology and Nature, 1-13.
- Roufogalis B.D. (2014). Zingiber officinale (Ginger): a future outlook on its potential in prevention and treatment of diabetes and prediabetic states. New Journal of Science 2014.
CrossRef - Zick S.M., Ruffin M.T., Lee J., Normolle D.P., Siden R., Alrawi S., and Brenner D.E. (2009). Phase II trial of encapsulated ginger as a treatment for chemotherapy-induced nausea and vomiting. Support Care Cancer 17, 563-572.
CrossRef - Abdelrazek H., Zeidan D.W., Soliman M.T., and Khodry A.K.A. (2018). Effect of Dietary Curcumin on Heptorenal Alfla-Toxicosis in Mice. Egyptian Academic Journal of Biological Sciences, F Toxicology & Pest Control 10, 99-110.
CrossRef - Helmy S.A., Eltamany D.A., Abdelrazek H., and Alashrey R.H. (2018). Immunomodulatory Effect of Dietary Turmeric against Aflatoxins in Mice: Histological and Immunohistochemical Study. Egyptian Academic Journal of Biological Sciences, D Histology & Histochemistry 10, 79-90.
CrossRef - Slaoui M., and Fiette L. (2011). Histopathology procedures: from tissue sampling to histopathological evaluation. Methods Mol Biol 691, 69-82.
CrossRef - Pearse A.G. (1968). Histochemistry, theoretical and applied. Sayed S., Ahmed M., El-Shehawi A., Alkafafy M., Al-Otaibi S., El-Sawy H., Farouk S., and El-Shazly S. (2020). Ginger Water Reduces Body Weight Gain and Improves Energy Expenditure in Rats. Foods (Basel, Switzerland) 9, 38.
- CrossRef
- Mansoub N.H. (2011). Comparison of using different level of black pepper with probiotic on performance and serum composition of broiler chickens. J Basic Appl Sci Res 1, 2425-2428.
- Ficker C.E., Smith M.L., Susiarti S., Leaman D.J., Irawati C., and Arnason J.T. (2003). Inhibition of human pathogenic fungi by members of Zingiberaceae used by the Kenyah (Indonesian Borneo). Journal of ethnopharmacology 85, 289-293.
CrossRef - Misawa K., Hashizume K., Yamamoto M., Minegishi Y., Hase T., and Shimotoyodome A. (2015). Ginger extract prevents high-fat diet-induced obesity in mice via activation of the peroxisome proliferator-activated receptor δ pathway. J Nutr Biochem 26, 1058-1067.
CrossRef - Kelesidis T., Kelesidis I., Chou S., and Mantzoros C.S. (2010). Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med 152, 93-100.
CrossRef - Kim S., Lee M.-S., Jung S., Son H.-Y., Park S., Kang B., Kim S.-Y., Kim I.-H., Kim C.-T., and Kim Y. (2018). Ginger Extract Ameliorates Obesity and Inflammation via Regulating MicroRNA-21/132 Expression and AMPK Activation in White Adipose Tissue. Nutrients 10, 1567.
CrossRef - Eltazi S.M. (2014). Effect of using ginger powder as natural feed additive on performance and carcass quality of broiler chicks. Assiut Veterinary Medical Journal 60, 87-94.
- Platel K., and Srinivasan K. (1996). Influence of dietary spices or their active principles on digestive enzymes of small intestinal mucosa in rats. Int J Food Sci Nutr 47, 55-59.
CrossRef - Butt M.S., and Sultan M.T. (2011). Ginger and its health claims: molecular aspects. Critical reviews in food science and nutrition 51, 383-393.
CrossRef - Patrick-Iwuanyanwu K., Wegwu M., and Ayalogu E. (2007). Prevention of CCI4-induced liver damage by ginger, garlic and vitamin E. Pak J Biol Sci [Internet] 10, 617-621.
CrossRef - Mahmoud R., and Elnour W. (2013). Comparative evaluation of the efficacy of ginger and orlistat on obesity management, pancreatic lipase and liver peroxisomal catalase enzyme in male albino rats. Eur Rev Med Pharmacol Sci 17, 75-83.
- Shanmugam K.R., Mallikarjuna K., Nishanth K., Kuo C.H., and Reddy K.S. (2011). Protective effect of dietary ginger on antioxidant enzymes and oxidative damage in experimental diabetic rat tissues. Food Chemistry 124, 1436-1442.
CrossRef - Thomson M., Al-Qattan K., Al-Sawan S., Alnaqeeb M., Khan I., and Ali M. (2002). The use of ginger (Zingiber officinale Rosc.) as a potential anti-inflammatory and antithrombotic agent. Prostaglandins, leukotrienes and essential fatty acids 67, 475-478.
CrossRef - Fuhrman B., Rosenblat M., Hayek T., Coleman R., and Aviram M. (2000). Ginger extract consumption reduces plasma cholesterol, inhibits LDL oxidation and attenuates development of atherosclerosis in atherosclerotic, apolipoprotein E-deficient mice. The Journal of nutrition 130, 1124-1131.
CrossRef - Verma S., Singh M., Jain P., and Bordia A. (2004). Protective effect of ginger, Zingiber officinale Rosc on experimental atherosclerosis in rabbits.
- Ramakrishna Rao R., Platel K., and Srinivasan K. (2003). In vitro influence of spices and spice‐active principles on digestive enzymes of rat pancreas and small intestine. Food/Nahrung 47, 408-412.
CrossRef - Bhandari U., and Pillai K. (2005). Effect of ethanolic extract of Zingiber officinale on dyslipidaemia in diabetic rats. Journal of ethnopharmacology 97, 227-230.
CrossRef - Ahmed R.S., Seth V., Pasha S., and Banerjee B. (2000). Influence of dietary ginger (Zingiber officinales Rosc) on oxidative stress induced by malathion in rats. Food and Chemical Toxicology 38, 443-450.
CrossRef - Sakr S., Okdah Y., and El-Adly E. (2009). Effect of ginger (Zingiber officinale) on Mancozeb fungicide induced testicular damage in albino rats. Aust J Basic Appl Sci 3, 1328-1333.
- Blaha M.J., Blumenthal R.S., Brinton E.A., Jacobson T.A., and Cholesterol N.L.A.T.o.N.-H. (2008). The importance of non–HDL cholesterol reporting in lipid management. Journal of clinical lipidology 2, 267-273.
CrossRef - Zancan K.C., Marques M.O., Petenate A.J., and Meireles M.A.A. (2002). Extraction of ginger (Zingiber officinale Roscoe) oleoresin with CO2 and co-solvents: a study of the antioxidant action of the extracts. The Journal of supercritical fluids 24, 57-76.
CrossRef - Grzanna R., Lindmark L., and Frondoza C.G. (2005). Ginger—an herbal medicinal product with broad anti-inflammatory actions. Journal of medicinal food 8, 125-132.
CrossRef - Mohamed O., el-nahas A., El-Sayed Y., and Ashry K. (2015). Ginger extract modulates Pb-induced hepatic oxidative stress and expression of antioxidant gene transcripts in rat liver. Pharmaceutical Biology.
CrossRef - Li S., Hong M., and Tan H.Y. (2016). Insights into the Role and Interdependence of Oxidative Stress and Inflammation in Liver Diseases. 2016, 4234061.
CrossRef - Elgawish R.A., Abdelrazek H.M.A., Ismail S.A.A., Loutfy N.M., and Soliman M.T.A. (2019). Hepatoprotective activity of Uncaria tomentosa extract against sub-chronic exposure to fipronil in male rats. Environmental Science and Pollution Research 26, 199-207.
CrossRef - Ribeiro S.R., Pinto Júnior P.E., Miranda A.C.d., Bromberg S.H., Lopasso F.P., and Irya K. (2004). Weight loss and morphometric study of intestinal mucosa in rats after massive intestinal resection: influence of a glutamine-enriched diet. Revista do Hospital das Clínicas 59, 349-356.
CrossRef - Desai H., Kalro R., and Choksi A. (1990). Effect of ginger and garlic on DNA content of gastric aspirate. Indian journal of medical research 92, 139-141.