Manuscript accepted on :24-02-2021
Published online on: 24-03-2021
Plagiarism Check: Yes
Reviewed by: Dr. Rai, Vikrant
Second Review by: Dr. Salman Ahmed
Final Approval by: Dr. em. Hans-Joachim Freisleben
Saied M. I. Al-Dalaen1 , Abdul-Wahab R. Hamad1
, Abdul-Wahab R. Hamad1 , Tayel A. AL-Hujran1
, Tayel A. AL-Hujran1 , Hayat A. Al-Btoush1
, Hayat A. Al-Btoush1 , Lidia Al-Halaseh1
, Lidia Al-Halaseh1 , Mousa K. Magharbeh1
, Mousa K. Magharbeh1 , Nariman A. Al-Jawabri1 , Islam A. Al-Kasasbeh1 and Fadhil M. Abid2
, Nariman A. Al-Jawabri1 , Islam A. Al-Kasasbeh1 and Fadhil M. Abid2
1College of Pharmacy, Mutah’ University, Al-Karak, Jordan.
2Ministry of Sciences and Technology, Baghdad, Iraq.
Corresponding Author E-mail: wahabhamad2004@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2143
Abstract
Objective: The objective of the two pharmacokinetic studies reported here was to compare the relative bio availability and bio equivalence of an ibuprofen 400 mg tablet from National Company (SDI) as a test with a reference formulation. Study Design: Evaluation of two open, randomized, cross-over studies, one single dose in healthy male volunteers.
Methods: 20 healthy volunteers were randomized in a cross-over design to single dose of Profedin 400 mg produced from National Company, ibuprofen formulation, as a test and a reference formulation produced from Pharmacia & Upjohn, Ibuprofen 400 mg. Ibuprofen and standard of ibuprofen were analyzed by utilizing HPLC, the sample extracted from 0.5ml of plasma with an organic solution of isooctane and 2-propanol. The mobile phase consisted of 44% acetonitrile and 0.1% phosphoric acid. The flow rate was 1 ml/min. The analytical column was a C-18, 5um packing size. Detection of Ibuprofen and the internal standard occurred by UV absorbance at wavelength of 220 nm.
Results: A single-dose study demonstrated that the bio availability of ibuprofen for both formulations was not significantly different. In addition, mean plasma levels of ibuprofen predictive of clinical efficacy were achieved within 1.5- 2.0 hours and the elimination of ibuprofen tablets is virtually complete in 12 hours after the single dose. The serum half-life is 1.8 to 2.0 hours. The Cmax, Tmax, Kelemin.0.5 were calculated for the test and reference. They were not significantly different.
Conclusions: Blood levels predicted that the present slow-release formulation of ibuprofen should offer reliable day and night control of pain and fever and is associated with a favorable safety profile.
Keywords
Bioavailability; Bioequivalence; Ibuprofen
Download this article as:| Copy the following to cite this article: Al-Dalaen S. M. I, Hamad A. W. R, AL-Hujran T. A, Al-Btoush H. A, Al-Halaseh L, Magharbeh M. K, Al-Jawabri N. A, Al-Kasasbeh I. A, Abid F. M. Bioavailability and Bioequivalence of Two Oral Single Dose of Ibuprofen 400 mg to Healthy Volunteers. Biomed Pharmacol J 2021;14(1). |
| Copy the following to cite this URL: Al-Dalaen S. M. I, Hamad A. W. R, AL-Hujran T. A, Al-Btoush H. A, Al-Halaseh L, Magharbeh M. K, Al-Jawabri N. A, Al-Kasasbeh I. A, Abid F. M. Bioavailability and Bioequivalence of Two Oral Single Dose of Ibuprofen 400 mg to Healthy Volunteers. Biomed Pharmacol J 2021;14(1). Available from: https://bit.ly/3fkkYb0 |
Introduction
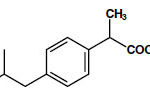
Ibuprofen Tablets, USP is (±)-2-(p-isobutylphenyl) propionic acid. It’s a white powder with a melting point of 74 – 77o C and is incredibly slightly soluble in water (<1 mg/mL) and readily soluble in organic solvents like ethanol and acetone. The molecular formula is C13H18O2, as showing below:
Ibuprofen is a non-steroidal anti-inflammatory agent. It is available in 400, 600 and 800 mg tablets for oral administration (1, 2, and 3).
Ibuprofen is a non-steroidal anti-inflammatory drug that displays potent anti-inflammatory properties and it was a strong analgesic and anti-pyretic activity. After its first registration as an anti-rheumatic drug in 1969 within the UK, it had been widely used as a prescription-only medicine (4). In 1983 it became available without prescription and currently it’s used throughout the globe as a medicine for the treatment of pain and fever. The recommended single dose of ibuprofen for the treatment of mild to moderate pain is 200 to 400mg with a maximum daily dosage of 1200 mg, compared with a daily dosage of up to 3600 mg for the treatment of rheumatic disease (5).
The primary objective of this study is to assess bio equivalence between ibuprofen formulation produced from a national company and the reference tablets produced from Pharmacia & Upjohn.
Ibuprofen has demonstrated anti-inflammatory, analgesic and antipyretic activity in animal studies designed to specifically demonstrate these effects. Its mode of action, like that of other no steroidal anti-inflammatory agents, is not completely understood, but it is associated with prostaglandin synthetase inhibition. Ibuprofen does not alter the course of the underlying disease.
As with other anti-inflammatory drugs, borderline elevations of one or more liver function tests may occur in up to 15% of patients. These abnormalities may progress, may remain essentially unchanged, or is also transient with continued therapy. The SGPT (ALT) test is a reasonably sensitive indicator of liver dysfunction. Significant (3 times the upper limit of normal) elevations of SGPT or SGOT (ALT) occurred in controlled clinical trials in but 1% of patients. A patient with symptoms and/or signs suggesting liver dysfunction, or in whom an abnormal liver test has occurred, should be evaluated for evidence of the event of more severe hepatic reactions while on therapy with ibuprofen. Severe hepatic reactions, including jaundice and cases of fatal hepatitis, are reported with ibuprofen like other non-steroidal anti-inflammatory drugs. Although such reactions are rare, if abnormal liver tests persist or worsen, if clinical signs and symptoms in step with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), Ibuprofen should be discontinued.
As with other anti-inflammatory drugs, long-term administration of ibuprofen to animals has resulted in renal papillary necrosis and other abnormal renal pathology. In humans, there are reports of acute interstitial nephritis with hematuria, proteinuria, and (10, 11).
A second type of renal toxicity has been seen in patients with prerenal conditions resulting in a decrease in renal blood flow or blood volume (12), where the renal prostaglandins have a supportive role within the maintenance of renal perfusion. In these patients, administration of a non-steroidal anti-inflammatory drug, may cause a dose dependent reduction in prostaglandin formation and will precipitate overt renal decompensating (13). Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics as well as the elderly (13). Discontinuation of anti-inflammatory drug is often followed by recovery to the pretreatment state.
However, NSAIDs are commonly utilized in the control of pain in patients with chronic inflammatory musculoskeletal conditions, (12), especially as a normal practice by general populations. (14).
Methods and Materials
Study Participants
This study included 20 male volunteers aged between 20 and 40 years (mean 29.5±5.5 years), all of them provided written consent prior to study initiation. Their body weights ranged from 58 to 84 kg (mean ± SD: 71.547±6.276kg) and their heights ranged from 165 to 178cm (169.1±4.708cm), see Table 1. All volunteers proved to be in good health as determined by a medical history, physical investigation (height, body weight, vital signs) and laboratory investigations (hematology, serum chemistry, urinalysis, HIV and hepatitis disease and test for alcohol and drugs).
Methods
This study was a randomized, two-way crossover study with a 7-day washout interval between each drug administration period. The volunteers were reserved to the clinic during each study period. Ten hours of fasting for all participants before drug administration. Water was allowed spontaneously throughout the study, apart from for 1 hour before to 4 hours after the dose. Volunteers received a one oral dose of 400 mg tablets with 200 ml of water. Blood samples were withdrawn from them at times: 0, 0.30, 1.5, 1, 2, 3, 4, 6, 10, and 12 hours of each test and reference drug.
Blood Sampling
In each period, 5ml blood samples were drawn in EDTA tubes at the subsequent times: pre-dose (0 hour) and at 0.30, 1, 1.5, 2, 3, 4, 6, 10 and 12 hours post-dose. A complete of 200 blood samples were drawn during the study for drug analysis. Plasma separated from blood by centrifugation and then frozen at -20°C, and kept frozen until assayed.
Table 1: Characteristic of Volunteers.
| Subject | Sex | Age\years | Height \cm | Weight\kg. |
| 1 | M | 23 | 170 | 68 |
| 2 | M | 24 | 165 | 65 |
| 3 | M | 26 | 173 | 73 |
| 4 | M | 29 | 166 | 70 |
| 5 | M | 20 | 174 | 80 |
| 6 | M | 35 | 172 | 78 |
| 7 | M | 32 | 171 | 74 |
| 8 | M | 27 | 167 | 75 |
| 9 | M | 33 | 169 | 73 |
| 10 | M | 29 | 173 | 84 |
| 11 | M | 30 | 168 | 72 |
| 12 | M | 40 | 174 | 69 |
| 13 | M | 24 | 160 | 58 |
| 14 | M | 23 | 164 | 67 |
| 15 | M | 24 | 166 | 69 |
| 16 | M | 26 | 168 | 70 |
| 17 | M | 29 | 170 | 69 |
| 18 | M | 30 | 161 | 68 |
| 19 | M | 32 | 165 | 65 |
| 20 | M | 34 | 165 | 70 |
| Mean | – | 29.458 | 169.083 | 71.547 |
| ±SD | – | 5.485 | 4.708 | 6.276 |
Analytical Methods
Extraction of Ibuprofen and ibuprofen standard by using 0.5 ml of plasma with an organic solution of isooctane and 2-propanol. The extract was evaporated to dryness, reconstituted with mobile phase, and injected into an HPLC (Shimadzu, AVP10) system. The mobile phase consisted of 44% acetonitrile in 0.1% phosphoric acid. The flow rate was 1 ml/min. The analytical column was a C-18 (Supleco, USA), 5um packing size. Ibuprofen was detected by UV absorbance (wavelength of detection 220nm). The lowest quantity detected was 0.25 mg/L by detector.
Pharmacokinetic and Statistical Methods
The pharmacokinetic and statistical analysis was based on standard deviation and ttest in particular for guidance on the investigation of bioavailability. The following pharmacokinetic parameters were calculated from the drug concentration-time data for ibuprofen:-
AUCt = area under the plasma concentration-time curve from zero to time t, where t is the time of the last measurable concentration (Ct); t1/2 = terminal elimination half-life, Cmax = highest observed plasma concentration; tmax = time to reach highest observed plasma concentration.
Statistical analysis was performed on all results obtained from participants which involved at this study. Bioequivalence standard would be met if the 90% confidence interval of the ratio of the product mean fell within the range of 80% to 125% for logarithmically transformed Cmax, and AUCt
Results and Discussion
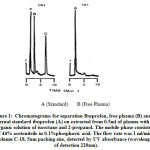
The chromatograms in Fig. 1 show that the results of standards Ibuprofen and plasma determination after oral administration of 400 mg Ibuprofen at selective results of research administration at 12 hours. The retention time of IBU was about 1.35 minutes
Linear calibration curve of Ibuprofen peak area versus plasma concentration was shown in Fig. 2. The calibration curves (obtained by rectilinear regression analysis) constructed during a period of fortnight while the assay of IBU in actual plasma samples of the bio availability trial were being dispensed. Correlation coefficients for this regression were consistently greater than 0.99 making one point calibrations feasible (13).
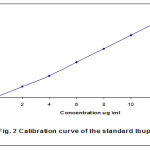 |
Figure 2: Calibration curves of the Ibuprofen. |
Ibuprofen is absorbed while administered orally. It‘s understood that ibuprofen is absolutely absorbed, making an allowance for almost 100% bio availability. However, the speed of absorption depends on its solubility within the aqueous phase of the stomach (14, 15). Peak serum ibuprofen levels are generally attained 1 to 2 hours after administration (Tables 2 and 3). With single doses up to 400 mg, a linear relationship exists between the quantity of drug administered and the built-in vicinity beneath the serum drug vs. time curve.
Table 2: Plasma concentration of Ibuprofen (National) in ug/ml with time in plasma of 20 healthy volunteers after oral dose of Profedin 400 mg tablets.
| No. | 0.0 | 0.30 | 1.0 | 1.5 | 2.0 | 3.0 | 4.0 | 6.0 | 10.0 | 12.0 |
| 1 | 0.0 | 32.1 | 40.4 | 45.4 | 20.1 | 15.4 | 8.5 | 4.1 | 1.5 | 1.0 |
| 2 | 0.0 | 25.3 | 30.3 | 40.6 | 43.2 | 20.5 | 17.4 | 7.3 | 3.0 | 1.2 |
| 3 | 0.0 | 34.7 | 37.5 | 39.5 | 25.3 | 17.6 | 10.2 | 6.2 | 2.4 | .85 |
| 4 | 0.0 | 27.5 | 31.7 | 38.0 | 42.6 | 25.1 | 13.3 | 8.5 | 2.1 | .97 |
| 5 | 0.0 | 31.2 | 35.6 | 44.2 | 31.7 | 18.7 | 11.2 | 5.4 | 1.5 | 0.55 |
| 6 | 0.0 | 30.5 | 35.3 | 37.6 | 16.8 | 10.3 | 5.2 | 2.5 | 1.0 | 0.46 |
| 7 | 0.0 | 25.6 | 32.7 | 39.3 | 22.6 | 15.7 | 8.3 | 4.0 | 2.1 | 1.00 |
| 8 | 0.0 | 22.2 | 38.7 | 29.0 | 19.3 | 11.8 | 7.5 | 3.4 | 1.5 | 0.75 |
| 9 | 0.0 | 33.1 | 36.5 | 41.6 | 30.1 | 20.7 | 13.1 | 7.5 | 3.2 | 1.4 |
| 10 | 0.0 | 28.1 | 29.9 | 33.6 | 42.4 | 25.6 | 15.8 | 10.3 | 4.2 | 2.0 |
| 11 | 0.0 | 24.8 | 44.6 | 30.6 | 20.5 | 12.6 | 8.7 | 5.0 | 2.5 | 1.0 |
| 12 | 0.0 | 21.3 | 27.6 | 32.5 | 45.6 | 25.7 | 16.4 | 10.2 | 5.7 | 2.4 |
| 13 | 0.0 | 37.2 | 39.5 | 42.6 | 28.7 | 16.2 | 10.3 | 5.4 | 2.1 | 0.56 |
| 14 | 0.0 | 34.1 | 37.0 | 41.5 | 22.9 | 14.3 | 8.9 | 4.6 | 1.7 | 0.45 |
| 15 | 0.0 | 25.7 | 39.6 | 42.3 | 45.6 | 27.3 | 17.8 | 10.5 | 6.0 | 2.1 |
| 16 | 0.0 | 26.4 | 30.5 | 35.7 | 42.1 | 27.1 | 16.9 | 9.5 | 3.7 | 1.4 |
| 17 | 0.0 | 33.2 | 35.7 | 39.8 | 25.6 | 15.4 | 9.8 | 5.2 | 3.1 | 0.75 |
| 18 | 0.0 | 30.8 | 33.5 | 40.2 | 20.3 | 10.6 | 6.1 | 3.2 | 1.5 | 0.45 |
| 19 | 0.0 | 20.2 | 29.3 | 37.5 | 28.3 | 15.3 | 10.1 | 6.3 | 3.1 | 1.00 |
| 20 | 0.0 | 27.3 | 42.6 | 32.5 | 18.2 | 10.3 | 5.5 | 3.2 | 1.5 | 1.4 |
| Mean | 0.0 | 28.565 | 35.915 | 38.13 | 29.595 | 17.81 | 10.915 | 6.12 | 2.67 | 1.035 |
| ±SD | 0.0 | 4.734 | 5.181 | 4.455 | 10.211 | 5.777 | 3.861 | 2.562 | 1.378 | 0.576 |
Table 3: Plasma concentration of Ibuprofen (Pharmacia and Upjohn) in ug/ml with time in plasma of 20 healthy volunteers after oral dose of Ibuprofen 400 mg tablets.
| No., | 0.0 | 0.30 | 1.0 | 1.5 | 2.0 | 3.0 | 4.0 | 6.0 | 10.0 | 12.0 |
| 1 | 0.0 | 30.7 | 38.4 | 40.3 | 43.5 | 27.2 | 16.8 | 10.8 | 5.0 | 1.4 |
| 2 | 0.0 | 29.4 | 37.8 | 44.6 | 27.1 | 17.0 | 9.3 | 5.0 | 3.0 | 0.65 |
| 3 | 0.0 | 28.6 | 33.5 | 39.7 | 45.2 | 22.3 | 10.5 | 5.5 | 2.5 | 0.75 |
| 4 | 0.0 | 32.3 | 41.2 | 38.6 | 22.5 | 12.5 | 7.5 | 4.0 | 2.0 | 0.78 |
| 5 | 0.0 | 33.5 | 38.2 | 41.3 | 46.2 | 26.1 | 14.2 | 6.5 | 2.3 | 0.80 |
| 6 | 0.0 | 27.0 | 35.6 | 39.8 | 45.5 | 28.3 | 15.2 | 7.5 | 3.0 | 1.3 |
| 7 | 0.0 | 29.6 | 33.7 | 44.6 | 30.5 | 16.4 | 10.0 | 5.0 | 2.4 | 1.0 |
| 8 | 0.0 | 26.2 | 29.8 | 36.5 | 43.1 | 28.5 | 19.2 | 10.0 | 4.5 | 1.5 |
| 9 | 0.0 | 35.2 | 42.3 | 29.8 | 18.3 | 11.3 | 5.5 | 3.0 | 1.4 | 0.61 |
| 10 | 0.0 | 31.6 | 37.8 | 44.2 | 31.2 | 18.1 | 9.2 | 4.0 | 1.9 | 0.45 |
| 11 | 0.0 | 34.7 | 38.5 | 40.5 | 45.7 | 23.6 | 13.4 | 6.2 | 3.1 | 1.0 |
| 12 | 0.0 | 29.4 | 37.6 | 43.9 | 30.1 | 20.1 | 10.0 | 5.0 | 2.0 | 0.67 |
| 13 | 0.0 | 30.7 | 35.8 | 45.2 | 31.0 | 17.4 | 9.2 | 4.5 | 1.9 | 0.55 |
| 14 | 0.0 | 28.2 | 33.6 | 36.8 | 43.8 | 29.2 | 13.5 | 6.7 | 3.1 | 0.90 |
| 15 | 0.0 | 27.6 | 39.8 | 30.2 | 20.0 | 13.4 | 6.5 | 3.4 | 1.5 | 0.62 |
| 16 | 0.0 | 33.5 | 39.4 | 28.3 | 16.2 | 10.2 | 5.1 | 2.6 | 1.2 | 0.45 |
| 17 | 0.0 | 31.7 | 36.2 | 43.0 | 31.5 | 14.2 | 3.7 | 3.6 | 1.5 | 0.52 |
| 18 | 0.0 | 27.2 | 33.4 | 42.5 | 46.3 | 28.6 | 18.0 | 7.6 | 3.0 | 1.0 |
| 19 | 0.0 | 26.8 | 29.8 | 35.7 | 42.5 | 29.1 | 17.3 | 8.2 | 3.4 | 1.4 |
| 20 | 0.0 | 30.1 | 38.4 | 42.5 | 28.7 | 19.0 | 10.0 | 5.1 | 2.2 | 0.95 |
| Mean | 0.0 | 30.175 | 36.54 | 39.4 | 34.445 | 20.02 | 11.385 | 5.81 | 2.695 | 0.89 |
| ±SD | 0.0 | 2.692 | 4.776 | 5.123 | 10.387 | 6.362 | 4.294 | 2.319 | 1.106 | 0.340 |
However, the place underneath the curve will increase much less than proportional with increase in dose above 400 mg. There’s no evidence of drug accumulation due to action of enzymes. Ibuprofen is metabolized, eliminated and secreted in the urine. The excretion of ibuprofen is virtually complete 12 hours after the last dose. The serum half-life is 1.8 hours for both formulations (Tables 4 and 5).
Table 4: Pharmacokinetic of Profedin (National) 400 mg tablet after oral administration to 20 healthy volunteers.
| No., | Ka | Ka0.5t | Kelem | Kelem0.5 | Cmax | Tmax | AUC |
| 1 | 0.460 | 1.506 | 0.308 | 2.25 | 45.4 | 1.5 | 120.00 |
| 2 | 0.293 | 2.364 | 0.319 | 2.17 | 43.2 | 2.0 | 136.42 |
| 3 | 0.318 | 2.364 | 0.343 | 2.02 | 39.5 | 1.5 | 132.580 |
| 4 | 0.212 | 3.269 | 0.367 | 1.89 | 42.6 | 2.0 | 110.613 |
| 5 | 0.264 | 2.626 | 0.454 | 1.525 | 44.2 | 1.5 | 120.218 |
| 6 | 0.292 | 2.370 | 0.379 | 1.826 | 45.3 | 1.0 | 93.36 |
| 7 | 0.236 | 2.936 | 0.425 | 1.632 | 39.3 | 1.5 | 96.417 |
| 8 | 0.386 | 1.795 | 0.449 | 1.541 | 38.7 | 1.0 | 87.517 |
| 9 | 0.218 | 3.179 | 0.452 | 1.530 | 41.6 | 1.5 | 130.622 |
| 10 | 0.178 | 3.876 | 0.282 | 2.407 | 42.4 | 2.0 | 98.116 |
| 11 | 0.358 | 5.390 | 0.376 | 1.845 | 44.6 | 1.0 | 114.625 |
| 12 | 0.362 | 1.914 | 0.393 | 1.762 | 45.6 | 2.0 | 120.226 |
| 13 | 0.378 | 1.833 | 0.345 | 2.01 | 42.6 | 1.5 | 110.318 |
| 14 | 0.657 | 1.053 | 0.373 | 1.856 | 41.5 | 1.5 | 116.812 |
| 15 | 0.865 | 1.801 | 0.328 | 2.116 | 45.6 | 2.0 | 124.580 |
| 16 | 0.302 | 2.295 | 0385 | 1.799 | 42.1 | 2.0 | 171.325 |
| 17 | 0.384 | 1.805 | 0.423 | 1.639 | 39.8 | 1.5 | 168.516 |
| 18 | 0.412 | 1.682 | 0.449 | 1.543 | 40.2 | 1.5 | 156.312 |
| 19 | 0.158 | 4.386 | 0.429 | 1.612 | 37.5 | 1.5 | 110.636 |
| 20 | 0.348 | 1.991 | 0.454 | 1.528 | 42.6 | 1.0 | 98.116 |
|
Mean |
0.354 | 2.472 | 0.387 | 1.826 | 42.215 | 1.55 | 120.866 |
|
±SD |
0.164 | 1.119 | 0.0533 | 0.266 | 2.459 | 0.359 |
23.442 |
Table 5: Pharmacokinetic of Ibuprofen (Pharmacia and Upjohn) 400 mg tablet after oral administration for 20 healthy volunteers.
| No., | Ka | Ka0.5t | Kelem | Kelem0.5 | Cmax | Tmax | AUC |
| 1 | 0.289 | 2.406 | 0.313 | 2.215 | 43.5 | 2.0 | 188.275 |
| 2 | 0.503 | 1.378 | 0.423 | 1.638 | 44.6 | 1.5 | 156.321 |
| 3 | 0.357 | 1.941 | 0.395 | 1.756 | 45.2 | 2.0 | 178.275 |
| 4 | 0.248 | 2.794 | 0.484 | 1.432 | 41.2 | 1.0 | 144.632 |
| 5 | 0.209 | 3.310 | 0.402 | 1.723 | 46.2 | 2.0 | 182.412 |
| 6 | 0.388 | 1.785 | 0.376 | 1.841 | 45.5 | 2.0 | 169.225 |
| 7 | 0.257 | 2.696 | 0.380 | 1.826 | 44.6 | 1.5 | 172.315 |
| 8 | 0.372 | 1.863 | 0.395 | 1.754 | 43.1 | 2.0 | 146.328 |
| 9 | 0.258 | 2.686 | 0.438 | 1.582 | 42.2 | 1.0 | 171.422 |
| 10 | 0.358 | 1.934 | 0.415 | 1.664 | 44.2 | 1.5 | 150.616 |
| 11 | 0.155 | 4.483 | 0.388 | 1.787 | 45.7 | 2.0 | 163.500 |
| 12 | 0.228 | 3.039 | 0.378 | 1.832 | 43.9 | 1.5 | 168.533 |
| 13 | 0.362 | 1.914 | 0.327 | 2.122 | 45.2 | 1.5 | 142.718 |
| 14 | 0.522 | 1.328 | 0.309 | 2.236 | 43.8 | 2.0 | 110.312 |
| 15 | 0.732 | 0.946 | 0.388 | 1.785 | 39.8 | 1.0 | 125.466 |
| 16 | 0.324 | 2.136 | 0.443 | 1.564 | 39.4 | 1.0 | 92.475 |
| 17 | 0.256 | 2.707 | 0.414 | 1.672 | 43.0 | 1.5 | 148.754 |
| 18 | 0.342 | 2.026 | 0.387 | 1.792 | 46.3 | 2.0 | 131.138 |
| 19 | 0.473 | 1.465 | 0.344 | 2.016 | 42.5 | 2.0 | 137.816 |
| 20 | 0.487 | 1.423 | 0.404 | 1.714 | 42.5 | 1.5 | 133.870 |
| Mean | 0.356 | 2.213 | 0.390 | 1.798 | 43.625 | 1.625 | 150.740 |
| ±SD | 0.135 | 0.826 | 0.043 | 0.209 | 1.950 | 0.393 | 24.650 |
AUCt = area under the plasma concentration-time curve from zero to the time of the last measurable concentration; AUCinfinity = area under the plasma concentration-time curve from zero to infinity; Cmax = maximum observed plasma concentration; tmax = time to reach Cmax; t1/2 = terminal elimination half-life.
Following oral administration of a drug, extent and rate of absorption is influenced by number of factors. With relation to the formulation of the drug, these factors include: the release of the active ingredient, the dissolution within the stomach and/or intestine, the transport of the solid or the dissolved active ingredient from the stomach into the intestine, and the absorption of the substance through the mucosa (12). The pharmacokinetic profiles of ibuprofen tablets were observed differences in the result of one or more differences in the course of these processes. The techniques grow to be become quite obvious that the maximum reasons for the determined variations are the dissolution properties of the opportunity formulations.
Ibuprofen is a relatively weak acid and its solubility in water or at acid pH is incredibly low. This leads to a comparatively long continuance within the acid environment of the stomach, which slows down absorption of the substance (16, 17).
An acceleration of this method can be achieved by the use of ibuprofen salts, which show faster solubility than the free acid, or by the use of buffered effervescent formulations.
The impractical considerations have been confirmed several times in different pharmacokinetic research and were also confirmed in this particular study. The fast-dissolving ibuprofen proved to have a significantly faster absorption, resulting in a greater Cmax and a shorter tmax (17, 18). The results of plasma ibuprofen concentration in this study are found in tables (4, 5) and figures (3, 4, 5).
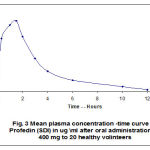 |
Figure 3: Mean plasma concentration-time curve of Profdin (SDI) in ug/ml after oral administration of 400 mg to 20 healthy volunteers. |
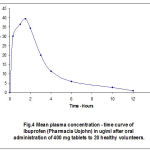 |
Figure 4: Mean plasma concentration-time curve of Ibuprofen (Pharmacia and Upjohn) in ug/ml after oral administration of 400 mg to 20 healthy volunteers. |
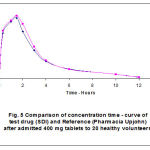 |
Figure 5: comparison of concentration-time curve of test durg (SDI) and Reference (Pharmacia and Upjohn) After admitted 400 mg tablets to 20 healthy volunteers. |
The perfect mechanisms of action of disintegration, dissolution and absorption that led to the results seen in this study are not fully known and are the topic of upcoming research. Most absorption is probably occur in the small intestine, and one potential theory is that there is greater retention in the stomach of the particles from the disintegrating ibuprofen tablets of both test and reference. Ibuprofen tablets have a better solubility than ibuprofen free acid in the stomach, which again may reduce its retention and it passes rapidly into the small intestine (18, 20).
Single doses of 400mg ibuprofen are most frequently used for the treatment of mild and moderate pain. In the treatment of pain, a rapid onset of action is desirable from a medical point of view and patient expectations.
Increase ibuprofen plasma concentration will increase analgesia in relation to dosage demonstrated for the first time by Laska et al. 1986. They matched the analgesic activity of ibuprofen aluminium salt with low bioavailability to a regular-release ibuprofen acid formulation. The advantage of therapeutic is fast-dissolving or soluble forms of ibuprofen has been confirmed in several independent studies.
In a recent study (22), the analgesic effects of two oral formulations of ibuprofen, effervescent formulation and ibuprofen acid tablets were compared. The bubbling method proved to have a maximum plasma concentration of 15 to forty minutes, while most concentrations had been reached inside 60 to ninety minutes after tablet intake.
Twenty healthy volunteers completed the study. The overall mean pharmacokinetic parameters and statistical comparisons are summarized in tables 4 and 5. The confidence intervals (90%) for logarithmically transformed AUCt and AUCinfinity were 96.3% to 102.2% and 96.5% to 102.3%, respectively. The 90% confidence interval for logarithmically transformed Cmax was 146.4% to 168.6%, which was outside the specified bioequivalence range.
Conclusions
In accordance with previous results, this study confirmed that the pharmacokinetic differences between a reference ibuprofen tablet formulation and test formulations are not significant.
Acknowledgements
The authors are expressing their appreciations to all those who have assisted in the research, namely the staff of Pharmacy College, the Mutah’ University, and researchers at the Medical Research Centre at College of Pharmacy, Mutah’ University, Jordan.
Conflict of Interest
There are no conflict of interest .
References
- The American Society of Health-System Pharmacists. Archived from the original on 9 September 2017. Retrieved 12 October 2016.
- The American Society of Health-System Pharmacists. Archived from the original on 9 September 2017. Retrieved 12 October 2016.
- British National Formulary, March 2014–September 2014 (2014 ed.). London: British Medical Association. 2014. pp. 686–688.
- Busson M. Update on ibuprofen: review article. J Int Med Res 1986; 14: 53-62.
CrossRef - Davies NM. Clinical pharmacokinetics of ibuprofen. The first 30 years. Clin Pharmacokinet. 1998;34:101-154
CrossRef - Albert KS, Gernaat CM. Pharmacokinetics of ibuprofen. Am J Med 1984; 77: 40-46.
CrossRef - Marija Tubic-Grozdanis, Michael B. Bolger, and Peter Langguth. Application of Gastrointestinal Simulation for Extensions for Biowaivers of Highly Permeable Compounds. AAPS J. 2008 Mar; 10(1): 213–226.
CrossRef - R.A. Matyas, S.L. Mumford, K.C. Schliep,1 K.A. Ahrens, L.A. Sjaarda, N.J. Perkins, A.C. Filiberto, D. Mattison, S.M. Zarek, J. Wactawski-Wende, and E.F. Schisterman. Effects of over-the-counter analgesic use on reproductive hormones and ovulation in healthy, premenopausal women. Hum Reprod. 2015 Jul; 30(7): 1714–1723.
CrossRef - Whitehall-Much GmbH, Münster, Germany. Comparative Pharmacokinetics of Two Fast-Dissolving Oral Ibuprofen Formulations and a Regular-Release Ibuprofen Tablet in Healthy Volunteers. International, Havant, Hants, England. Clin Drug Invest. 2001; 21(1)
CrossRef - Paul Nderitu*, Lucy Doos, Peter W Jones, Simon J Davies and Umesh T Kadam. Non-steroidal anti-inflammatory drugs and chronic kidney disease progression: a systematic review. Family Practice 2013; 30:247–255.
CrossRef - Andrea Arfè, biostatistician, Lorenza Scotti, Cristina Varas-Lorenzo, Federica Nicotra, Antonella Zambon, etal. 2016. Non-steroidal anti-inflammatory drugs and risk of heart failure in four European countries: nested case-control study. BMJ 2016; 354.
CrossRef - Bhopal S, Chan J, Ellis O et al. Non-steroidal anti-inflammatory drugs prescribing in chronic kidney disease: an observational study. Prim Health Care Res Dev 2010; 11(03): 280–84.
CrossRef - Bill H. McCarberg. NSAIDs in the Older Patient: Balancing Benefits and Harms. Pain Medicine, Volume 14, Issue suppl_1, December 2013, Pages S43–S44,
CrossRef - AntonioMatji, , AdriannaGagol, EnriqueMorale, LuisCarvajalaDolores R.Serrano, Zelalem A.Worku, Anne MarieHealy, Juan Jose Torrado.Predicting the critical quality attributes of ibuprofen tablets via modelling of process parameters for roller compaction and tableting. International Journal of Pharmaceutics. Volume 565, 30: 2019, 209-218
CrossRef
- Brooks P. Use and benefits of nonsteroidal anti-inflammatory drugs. Am J Med 1998; 104(3A): 9– 13.
CrossRef - Maxwell CJ, Dalby DM, Slater M et al. The prevalence and management of current daily pain among older home care clients. Pain 2008; 138: 208–16.
CrossRef - Whitehall-Much GmbH, Münster, Germany; , , , Whitehall International, Havant, Hants. Comparative Pharmacokinetics of Two Fast-Dissolving Oral Ibuprofen Formulations and a Regular-Release Ibuprofen Tablet in Healthy Volunteers. Clin Drug Invest. 2001; 21(1); 14-21.
CrossRef - Davies NM. Clinical pharmacokinetics of ibuprofen. The first 30 years. Clin Pharmacokinet 1998; 34: 101-54
CrossRef - Geisslinger G, Dietzel K, Bezler H, et al. Therapeutically relevant differences in the pharmacokinetical and pharmaceutical behavior of ibuprofen lysinate as compared to ibuprofen acid. Int J Clin Pharmacol Ther Toxicol 1989; 27: 324-8
- Chiarini A, Tartarini A, Fini A. pH-solubility relationship and partition coefficients for some anti-inflammatory arylaliphatic acids. Archiv Pharmazie 1984; 317: 268-73
CrossRef - Ceppi MN, Gazzaniga A, Gianesello V, et al. Activity and pharmacokinetics of a new oral dosage form of soluble ibuprofen. Arzneimittelforschung 1992; 42: 556-9
- Laska EM, Sunshine A, Marrero I, et al. The correlation between blood levels of ibuprofen and clinical analgesic response. Clin Pharmacol Ther 1986; 40: 1-7
CrossRef - Hummel T, Cramer O, Mohammadian P, et al. Comparison of the antinociception produced by two oral formulations of ibuprofen: ibuprofen effervescent vs ibuprofen tablets. Eur J Clin Pharmacol 1997; 52: 107-14
CrossRef