Manuscript accepted on :16-12-2020
Published online on: 03-02-2021
Plagiarism Check: Yes
Reviewed by: Dr. Wioletta Wujcicka
Second Review by: Dr. Daya Shankar Gautam
Final Approval by: Dr Patorn Piromchai
Anushree Nagaraj, Sarah Andrea Wilson and Lalitha Vaidyanathan*
and Lalitha Vaidyanathan*
Department of Biomedical Sciences, Sri Ramachandra Institue of Higher Education and Research, Porur, Chennai- 600116, India.
Corresponding Author Email: lalithav@sriramchandra.edu.in
DOI : https://dx.doi.org/10.13005/bpj/2141
Abstract
Obesity, a disease involved with complex health problems, is indicated by increased BMI, triglyceride and cholesterol levels. Obese individuals are found to be highly susceptible to develop non-alcoholic fatty liver disease,cardiovascular diseases, and also type 2 diabetes mellitus. Synthetic drugs used for treating obesity have been found to be associated with side effects such as anxiety,sleeplessness,hypertension, and drug addiction. Research on natural productspossessing therapeutic biological activitieshasdiscoveredtheir potential to minimize or even completely eliminate such side effects. Medicinal properties ofCalocybe indica include antidiabetic, hypertensive, anticancer, anti-inflammatory, antibacterial, and hepatoprotective effects; however, its anti-obesity activity is obscure.In this study, the anti-obesity effects of Calocybe indicawere investigated using a diet-induced obese Zebrafish modeland compared with standard drug Atorvastatin.Results show that 200µg of C. indica was able to effectively bring down triglyceride levels (12.5± 0 mg/ml; normal control 12.7 ± 0.7 mg/ml), cholesterol (210± 15.9 mg; normal control =70.4± 0)and HMG COA Reductase levels (0.9± 0.03; normal = 1.2 ± 0.01). Excessive fat accumulation in the liver (steatohepatitis) reduced after treatment with C. indica to a greater extent than by treatment with standard drug Atorvastatin. 100 µg of C. indica was found to be optimum in decreasing the levels of the liver enzymes, AST (177.1±5.7 IU/L; normal control =177.7±43.02 IU/l), ALT (365.5±2.9 IU/L; normal control= 355.5±34.4 IU/l), and ALP (2.3±1.1μmoles of phenol liberated/mg of protein/min; normal control = 0.7±1.2 μmoles of phenol liberated/mg of protein/min).Whole-body Oil Red O staining of the zebrafishes showed that with increasing concentration of C. indica, the accumulation of triglycerides and lipids decreased.
Keywords
ALP- Alkaline Phosphatase; ALT- Alanine Transaminase; AST- Aspartate Transaminase; BMI- Body Mass Index; CVD- Cardiovascular Diseases; DIO- Diet-Induced Obesity; HFD- High Fat Diet; HMG COA- Hydroxymethyl glutaryl coenzyme A; NAFLD- Non-Alcoholic Fatty Liver Diseases; NASH- Non-Alcoholic Steatohepatitis; NFD- Normal Fat Diet; T2DM- Type 2 Diabetes Mellitus
Download this article as:| Copy the following to cite this article: Nagaraj A, Wilson S. A, Vaidyanathan L. Anti-Obesity Properties of Calocybe Indica in Zebra fishes with Short-Term High-Fat Diet Induction. Biomed Pharmacol J 2021;14(1) |
| Copy the following to cite this URL: Nagaraj A, Wilson S. A, Vaidyanathan L. Anti-Obesity Properties of Calocybe Indica in Zebra fishes with Short-Term High-Fat Diet Induction. Biomed Pharmacol J 2021;14(1). Available from: https://bit.ly/39HbMKI |
Introduction
Obesity is one of the most challenging health problems faced worldwide. Characterized by an immoderate accumulation of fat in the adipose tissue, obesity can deteriorate health by causing an increased risk of developing type 2 diabetes mellitus (T2DM) and cardiovascular diseases (CVD) such as stroke, hypertension, and coronary heart disease as well as gall bladder disease, certain cancers (endometrial, breast, prostate, colon) and non-fatal conditions including gout, respiratory conditions, gastroesophageal reflux disease, osteoarthritis, and infertility.1 It has also been associated with the pathogenesis of non-alcoholic fatty liver disease (NAFLD), observed in maximum obese individuals. NAFLD is indicated by the presence of fat in liver biopsies and has been found to reduce the life expectancy of individuals.2 The disease leads to an increased level of intrahepatic triglycerides progressinginto steatohepatitis.3
The body mass index (BMI), a parameter comparing weight and height, describes an individual as pre-obese when their BMI is above 25 kg/ 2 and obese when greater than 30 kg/m.2BMI is obtained by dividing the subject’s weight by the square of the subject’s height and is expressed in metric units (BMI = kilograms / meters2).4 Treatment regimens for obesity include physical exercise, diet modification, and anti-obesity drugs/medications, of which Hydroxymethyl glutaryl coenzyme A reductase (HMG CoA) inhibitors (commonly known as statins) are most wide-spread. Statins were shown to decrease body and liver fat accumulated in obese rats.5 In a study on patients with NAFLD, those taking statins manifested with a remarkable reduction of hepatosteatosis. It was concluded that,for NAFLD patients with elevated liver enzymes, prescription of statins could help treat the disease.6 Although there is extensive evidence suggesting the potential of statin therapy in treating obesity, many associated adverse side effects such as sleeplessness, anxiety, hypertension, and other common effects as with any other synthetic drug render it as toxic to a certain extent.7 Thus, to prevent or minimize these side effects, natural products are being studied for identifying an alternative obesity treatment regimen.
The milky white mushroom Calocybe indica was first discovered in West Bengal, India. It is edible and comprises of diverse secondary metabolites such as terpenes, phenolic compounds, and steroids, which are involved in its medicinal effects and nutritive value.8 C. indica was found to be rich in protein and fiber, with low-fat content.9 It was also found to have increased levels of calcium, phosphorus, and iron, as well as low moisture and increased fiber content.8 Several studies have identified various properties of C. indica, such as antioxidant, antidiabetic, antitumor, hepatoprotective, antimicrobial,anti-proliferative, and hypertensive effects.9-15 In one study, the hypercholesteremic effects of C. indica on human volunteers revealed a remarkable decline in the cholesterol levels of the participants. However, the subjects in this research were found to have a normal BMI of 20 and only had borderline highLDL-c levels (~230mg/dl).9 Obesity is said to be characterized by increase in LDL-c, decrease in HDL-c, and increase in triglyceride levels. Associations between the high cholesterol levels and obesity was found to be absent in most cases. Conditions that are responsible for elevating cholesterol levels include pregnancy, hypothyroidism, polycystic ovary syndrome, and other medications that increase levels of LDL-c but decrease HDL-c levels.16 Anti-obesity effects of other similar edible mushrooms like Agaricusbisporus, and Hericiumerinaceus also have reported.16 Anti-obesity properties of C. indica have not yet been ivestigated.
Zebra fishes (Danio rerio) are being progressively utilized as models of human obesity and obesity-related diseases, including visceral adiposity, hepatic steatosis, atherosclerosis, and type 2 diabetes. Oka et al., in 2010, first established the diet-induced obesity (DIO) model of Zebra fishes. The adult Zebra fishes were induced by feeding 60mg Artemia/brine shrimp (150 calories) per fish per day vs. 5mg of the same feed (20 calories) for non-induced fishes. The overfed Zebra fish exhibited increased BMI, hypertriglyceridemia, and hepatic steatosis compared to the normal fed Zebra fish. Several biochemical and histological methods have validated this model and shown that the physiological processes involved with obesity are commonly shared by obese mammals and DIO Zebra fishes.17 Besides over feeding with shrimp, several other approaches have been used to induce obesity in Zebra fishes. Meguro et al., in 2015, developed custom high-fat Zebra fish diets containing 20% corn oil or lard. They demonstrated that these high-fat diets make Zebra fish obese.18 Obesity promoted by overfeeding a normal fat diet is found to be quite different from obesity brought about by feeding a high-fat diet. Landgraf et al., in 2017, compared the metabolic phenotype of obesity-induced zebra fish established by overfeeding a normal fat diet (NFD; shrimp, 22% fat) to that of a high-fat diet (HFD; egg yolk powder/ 20% corn oil- 59% fat). Although, both the feeds prompted adiposity, fishes overfed with NFD were metabolically healthy compared with the fishes fed with HFD, which were found to have obesity-associated indications including increased glucose intolerance, fatty liver, and preferential increase of visceral fat.19
Common parameters measured that are used to confirm obesity include BMI, Cholesterol, HMG-COA Reductase, and triglyceride levels.17, 20-22 HMG-COA reductase, a rate-limiting enzyme in the cholesterol biosynthesis pathway, was found to be effectively targeted and blocked by the administration of drugs such as statins. Triglyceride and lipid accumulations in the whole body of the Zebra fishes have been visualized using the oil red o staining process.17 Furthermore, NAFLD associated with obesity can also be confirmed and the extent of liver damage is estimated by measuring levels of alkaline phosphatase (ALP), and aminotransferases including alanine aminotransferase (ALT) and aspartate aminotransferase (AST), all of which have been recognized tobe elevated in obese individuals, as released from adipose tissue into the blood circulation in excessive amounts23, 24
Natural compounds have been previously investigated for their anti-obesity effects using the DIO model. In a study by Meguro et al., in 2015, DIO Zebra fishes were supplemented with green tea extract. The extract was shown to have an anti-obesity effect, and this research also suggested that a fat-rich diet comprising of lower amounts of proteins and carbohydrates, provoke dexcessive body fat build-up in Zebra fishes in similar mechanisms to mammals.18 Another study on the anti-obesity effects of Palmariamollis (PM) using the DIO- Zebra fish and mice models demonstrated its ability to effectively decrease hepatic steatosis and visceral adiposity.25 Thus, the DIO model of Zebra fish has been proven effective for testing the effects of various supplements on body fat accumulation.
In this study, the DIO model was induced by feeding adult Zebra fishes with a high-fat calorie-rich diet. The anti-obesity properties of C. indica were evaluated using the established DIO model, and the results were compared with the treatment by a standard statin drug. The present study would open avenues for exploratory research to identify a lead phyto compound to treat obesity.
Materials and Methods
Preparation of aqueous extract of Calocybe indica
Aqueous extract of C. indica was prepared by the hot percolation method. Samples of C. indica were collected and lyophilized to a fine powder. Ten grams of the C. indica powder was dissolved in 100ml of sterile distilled water and stirred at 60°C for 1 hour using a heating mantle. It was then cooled and centrifuged, after which the residue was re-extracted twice with distilled water, and finally, the extract was concentrated by evaporation. The dried concentrate was used for phyto chemical analysis and refrigerated for further use.15
Phytochemical analysis of C. indica extract
Reagents and chemicals used: 5% W/V FeCl3 in 90% alcohol, Fehling’s solution A and B, Lead acetate, Anthrone, Concentrated H2SO4, 1.27g iodine, 2g of potassium iodide, Ether, Aqueous ammonia, Sodium hydroxide, Concentrated Nitric acid, Lead acetate, Chloroform, Acetic Anhydride-3ml, and Concentrated H2SO4– few drops. All chemicals and reagents used were commonly available in the laboratory.
Method: Phytochemical analysis was performed for the crude extract of C. indica using standard methods. Ten milligrams of the dried extract was diluted in 10 ml of distilled water and used for further qualitative analysis to detect the presence or absence of phenolic compounds, reducing sugars, flavones, glycosides, saponins, alkaloids, anthraquinones, quinones, proteins, tannins, and steroids using standard protocols.26
Establishment of diet-induced obesity model of adult Zebra fish
Materials used
Commercially available brine shrimp and corn oil.
Method
Ninety Zebra fishes were overfed with brine shrimp (450 calories/day/fish) along with a normal diet and 20% corn oil feed for five days; where 1mg of shrimp is equivalent to five calories (450 calories= 90mg of Shrimp). The normal feed was given two times a day. The Zebra fishes were fed with 30mg of artemia three times a day and with 20% corn oil at the end of each day. Excess food was removed 15 mins after feeding, and water was changed. The tank was cleaned regularly, along with water change. Zebra fishes were then divided and accommodated into different tanks, comprising of the respective groups;
Normal group
Non-obese (no induction, no treatment)
Negative control
Obesity-induced group (15 Zebra fishes)
Positive control
Treatment with standard drug Atorvastatin. (15 Zebra fishes)
Treatment with 25μg/l of C. indica (15 Zebra fishes)
Treatment with 50μg/l of C. indica (15 Zebra fishes)
Treatment with 100μg/l of C. indica (15 Zebra fishes)
Treatment with 200μg/l of C. indica (15 Zebra fishes)
Treatment with C. Indica Extract
Materials used: C. indica stock solution was prepared by weighing 10 mg in 10 ml distilled water and stored for further use.
Method: The obese Zebra fishes separated into different groups were treated with 25, 50, 100, and 200μg of C. indica (stock concentration – 1mg/ml)per liter of water. The Zebra fishes were exposed to the extract for 4 hours daily for five days. The tank water was changed after the treatment. In addition, all the fishes were provided with a normal feed at regular intervals.
Treatment with Standard Drug Atorvastatin
Materials used: Atorvastatin purchased from a local pharmacy. The stock solution of Atorvastatin was prepared by weighing 1 mg in 1 ml distilled water.
Method: The fishes in the positive standard group were treated with 100μg of Atorvastatin (1mg/ml- stock concentration) per liter of water. The Zebra fishes were exposed to the drug for 4 hours daily for five days. The tank water was changed after treatment. The fishes were given normal feed at regular intervals.
Genotoxicity Testing of C. Indica Extract
Reagents and chemicals used: Heparin, PBS, normal melting point agar (NMPA), low melting point agar (LMPA), lysis buffer, tris base, triton X, EDTA, electrophoresis buffer, neutralization buffer, ice-cold methanol, and ethidium bromide (EtBr). All chemicals and reagents used were commonly available in the laboratory.
Method: Comet assay was performed to test if any potential genotoxic effects of C. indica treatment are observed in adult Zebra fishes,using a method proposed by Rocco et al.,in 2011. The blood samples were collected from each group and were run by gel electrophoresis on precoated slides. Following this, the slides were stained and viewed under a fluorescent microscope.27
Assessment of biochemical parameters in the established models to confirm obesity and to compare the anti-obesity effect of C. indica extract with Atorvastatin in Zebra fish obese model.
Measurement of BMI and blood glucose levels
Method: Weekly measurements of length and whole body weight of the Zebra fishes were noted. By dividing the body weight (g) of the fished by the square of the body length (cm), the BMI of the fishes was obtained. The tail of the Zebra fish was cut below the caudal fin and using a glucometer, and the blood glucose was measured for all the groups.17
Preparation of Tissue Homogenate
Materials used
O.25M sucrose, commonly available in the laboratory.
Method
The livers were homogenized with 500µl of 0.25M sucrose, and 200µl of 0.25M sucrose was used for washing the mortar and pestle.The homogenate was centrifuged at 700xg for 10 mins.The supernatant obtained was and centrifuged once again at 10,000rpm for 20 mins, and the acquired supernatant was stored in the freezer until further use.
Biochemical analysis
Chemicals and reagents used: 1. Cholesterol estimation: Ferric chloride acetic acid reagent, Concentrated H2SO4, and Cholesterol working standard- 40 µg/ml in ferric chloride acetic acid reagent. 2. Triglyceride estimation: Chloroform-Methanol mixture – 2:1 (v/v), Saturated sodium chloride solution, Activated silicic acid (LOBA), 0.2 N Sulphuric acid, 0.4% Potassium hydroxide in alcohol (prepared fresh), 0.1 M Sodium meta periodate (LOBA) (prepared fresh), 0.5M Sodium meta arsenite(LOBA), Chromotropic acid reagent (LOBA), 7.0% Thiourea solution and Standard tripalmitin. 3. HMG-COA reductase assay: Saline arsenate (LOBA),Dilute perchloric acid, Hydroxylamine hydrochloride reagent- Stock, for mevalonate mixed with an equal volume of water and for HMG COA mixed with 4.5 N NaOH, and Ferric chloride reagent. 4. AST and ALT: Buffered substrate (0.1M Phosphate buffer, pH – 7.4, 1.0M aspartic acid/0.2M L- alanine, 2 mM 2 – oxoglutarate), DNPH, 0.4 N NaOH, Standard pyruvate, and 0.33 M Sucrose. 5. ALP: 0.1M disodium phenyl phosphate, 0.1M MgCl2, Carbonate-bicarbonate buffer, Folin’s reagent, 15% sodium carbonate, Stock, and working standard phenol solutions, stock and standard protein working standard solutions.
All chemicals used were either purchased from the Precision Scientific Co., India, or available in the laboratory and were of analytical grade.
Methods
Levels of tissue cholesterol, tissue triglyceride, and HMG-COA reductase activities were analyzed using standard methods proposed by Zlatkis et al. in 1953, Rice in 1970, and Rao and Ramakrshnan in 1975, respectively.28-30
NAFLD associated with obesity was tested by measuring the levels of liver enzymes Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST) by standard protocols presented by Mohun and Cook in 1957, and Alkaline phosphatase by protocol from Kind and King in 1954.31, 32
Oil Red O Staining
The zebra fishes were fixed with 4% para formaldehyde, embedded in paraffin, cross-sectioned, stained with oil red o stain, and viewed under the microscope.17
Results
Phytochemical analysis of crude extract of Calocybe indica
Phytochemical analysis of the crude extract of C. indica was performed. It revealed the presence of various phytoconstituents, as seen in table 1.
Table 1: Phytochemical analysis of aqueous extract of C. indica
| PHYTOCHEMICAL TEST | INFERENCE(‘+’ indicates presence, ‘-’ indicates absence) |
| Phenolic compounds | + |
| Reducing sugars | – |
| Flavones | + |
| Glycosides | + |
| Saponins | – |
| Alkaloids | + |
| Anthraquinones | – |
| Quinones | + |
| Proteins | + |
| Tannins | + |
| Steroids | – |
Comet Assay
Comet assay was performed to determine the genotoxicity of various concentrations of C. indica in adult Zebrafishes, and the results were recorded and compared with the normal group. No significant genotoxicity was observed with all the concentrations of C. indica, as identified by the absence of comet-like appearance in the fluorescent images shown in figure 1.
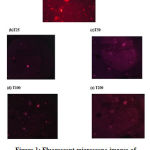 |
Figure 1: Fluorescent microscope images of DNA of Zebra fish treated with aqueous extract of C. indica. (a) Normal group; (b) T 25; (c) T 50; (d) T 100 and (e) T 200. |
DIO Model of Obesity
Obesity was induced in the Zebra fishes by prompting them with a high-fat calorie-rich diet. The BMI was recorded before and after treatment with different concentrations of C. indica and was compared with the group treated with standard drug Atorvastatin and normal control. Whole-body weight comparisons made between the fishes of different groups can be seen in figure 2. Negative control groups show increased weight gain, which reduced upon treatment with all concentrations of C. indica, and standard drug Atorvastatin.
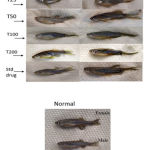 |
Figure 2: Comparison between untreated, standard drug-treated, and extract-treated models. |
Measurement of BMI
BMI increased by 2 and 2.2 folds in obese male and female fishes, respectively. A dose-dependent reduction in BMI was seen for the groups treated with C. indica. Figure 3. shows the comparison of BMI of both male and female fishes of different groups on a graph. Treatment with Atorvastatin was found to be as equally effective as 50 µg of the extract. 200 µg of the extract brought about a decrease in the BMI levels, closer to levels seen in the normal group.
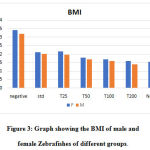 |
Figure 3: Graph showing the BMI of male and female Zebrafishes of different groups. |
Measurement of blood glucose
Blood glucose measured has been graphically represented in figure 4. An increase in the blood glucose levels was observed in fishes belonging to the obese group (negative control). A dose-dependent reduction in the levels was seen for the groups treated with C. indica. The blood glucose level for fishes treated with 200µg of C. indica was comparable with the normal group. No reduction in the blood glucose levels was observed for the groups treated with the standard drug Atorvastatin.
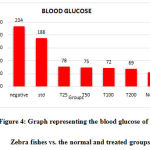 |
Figure 4: Graph representing the blood glucose of DIO Zebra fishes vs. the normal and treated groups. |
Estimation of tissue cholesterol and triglycerides
Cholesterol and TG levels increased in the Zebrafishes belonging to the negative control group. Cholesterol levels of fishes treated with standard drug Atorvastatin decreased to a greater extent compared with the fishes treated with lower concentrations ofC. indica. Treatment with 200μg of C. indica was able to effectively bring down the triglyceride levels as close to the normal control. A dose-dependent reduction in the cholesterol and triglyceride levels were seen between the C. indica treatedgroups as represented in Table 2.& Figure 5.
Table 2. Comparison of the level of cholesterol between the groups
| GROUPS | Cholesterol conc. (mg in 100ml) |
| Normal | 70.4 (±0) |
| Negative | 544 (±17.17) |
| Standard | 91.7 (±11.9) |
| T25 | 480 (±24.9) |
| T50 | 448 (±19.8) |
| T100 | 403.2 (±7.01) |
| T200 | 210 (±15.9) |
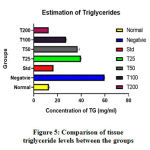 |
Figure 5: Comparison of tissue triglyceride levels between the groups. |
Estimation of HMG-COA Reductase
An increase in the ratio of HMG-COA and mevalonate is said to be due to the decrease in the activity of enzyme HMG-COA Reductase. An increase in the enzyme activity was seen in the negative control. Group treated with Atorvastatin had the lowest enzyme activity. C. indica was able to decrease the enzyme activity in a dose-dependent fashion, as shown in figure 6. Fishes treated with 200 μg of C. indicawas able to bring about a decrease in HMG COA Reductase activity when compared with standard drug Atorvastatin which was not very effective in decreasing the activity.
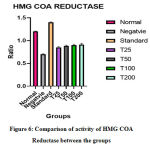 |
Figure 6: Comparison of activity of HMG COA Reductase between the groups. |
Assay of AST, ALT and ALP
AST, ALT, and ALP levels increased in the negative control (obese fishes) group. In C. indica treated groups, the levels of all the three enzymes decreased in a dose-dependent manner. The AST and ALT level of fishes treated with 100µg of C. indica was comparable with the levels of non-obese fishes. AST level of fishes treated with 50µg and ALT levels of fishes treated with 25µg of C. indicawere comparable with the fishes treated with standard drug Atorvastatin. ALP level for fishes treated with 100µg of C. indica was found to be optimum.However, at 200 µg concentration, C. indica increased the level of these enzymes; suggesting maximum efficacy at 100 µg. The levels of ALT, AST, and ALP enzymes in each group have been represented in Table 3, figure 7, and Figure 8, respectively.
Table 3: Comparison of the level of ALT between the groups.
| GROUPS | ACTIVITY OF ENZYME (IU/L) |
| Normal | 355 (±34.4) |
| Negative | 522 (±54.4) |
| Standard | 461.1 (±49.06) |
| T25 | 405.5 (±8.57) |
| T50 | 383.2 (±25.8) |
| T100 | 365.2 (±2.69) |
| T200 | 299.9 (±29.8) |
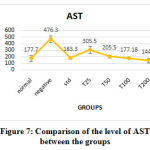 |
Figure 7: Comparison of the level of AST between the groups |
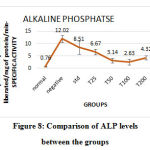 |
Figure 8: Comparison of ALP levels between the groups. |
Oil Red O Staining
Accumulation of triglycerides and lipids were seen under the microscope in obese fishes (negative control group). Treatment with C. indica showed a decrease in both lipid accumulation and lipid droplet size.Figure 9. Shows the accumulation of triglycerides and lipids after staining with oil red o stain for all the groups.
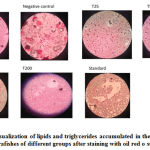 |
Figure 9: Visualization of lipids and triglycerides accumulated in the body of the Zebrafishes of different groups after staining with oil red o stain. |
Discussion
Natural medicine has recently attracted curiosity for its potential health perks in curbing metabolic diseases like obesity and diabetes.33
indica has been reported to have the following effects: anti-cancer effect, anti-oxidant effect, anti-lipid peroxidative effect, anti-microbial effect, anti-hyperglycemic effect, anti-hypertensive effect, and Hepatoprotective effect.9-15 The main aim of our study was to evaluate the anti-obesity effects of C. indica in DIO Zebrafish.
Dyslipidemia, commonly seen in obesity, is distinguished by an increased flux of free fatty acids, elevated TG levels, low high-density lipoprotein cholesterol levels, and increased low-density lipoprotein level.17 Zebrafishes fed with HF diet (shrimp and 20% corn oil) showed an increase in BMI, blood glucose, tissue cholesterol, and triglyceride levels. DIO fishes also showedan increase in the activity of the enzymes AST, ALT, ALP, and HMG COA Reductase.
DIO Zebrafishes had greater bodyfat volumes than the control group, which was indicated by the increase in BMI (2.2 folds for females and 2 folds for males). Treatment with C. indica was found to suppress body weight and body fat distribution in male and female Zebrafishes.
Elevation of tissue cholesterol and triglyceride levels was seen in the liver homogenate of DIO Zebrafishes. Treatment with Atorvastatin and C. indica resulted in decreased cholesterol and triglyceride levels. It has been previously reported of the hypercholesterolemic effectsof C. indica,9,which is consistent with our results.
HMG COA reductase is a rate-limiting enzyme of the mevalonate pathway, which is a metabolic pathway responsible for the production of cholesterol and other isoprenoids. It catalyzes the reaction converting HMG COA to mevalonate. A class of drugs called statins can inhibit this enzyme.34 The ratio between the absorbance of the HMG COA and mevalonate was measured as HMG COA reductase activity;the lower the ratio higher the activity. Activity significantly increased in the DIO obese group. Groups treated with Atorvastatin showed maximum reduction in enzyme activity, indicated by a higher ratio. Treatment with C. indica found to reduce the enzyme activity in a dose-dependent manner.
Visceral fat is a key mediator of NASH and is strongly associated with the level of aminotransferases in the obese population. The importance of visceral fat in NAFLD caused by obesity has also been shown in many animal models, including fa/fa obese rats. In the study done on obese rats, surgical resection of intra-abdominal fat depots reversed hepatic insulin resistance and steatosis.35Obesity generally presents with adecrease in the level of adiponectin, a hormone found in the adipose tissue usually responsible for β oxidation of fatty acids in the fat tissues. Such a decrease in the level of adiponectin leads to the accumulation of free fatty acids and triglycerides in the liver. This results in Non-alcoholic fatty liver disease, which, when left untreated, can lead to liver cirrhosis.36 AST, ALT and ALP levels are said to be elevated in liver diseases.23, 24Hepaticsteatosis associated with obesity could be the cause for the increased levels of these enzymes in the DIO Zebrafishes. Anti-obesity effects of C. indicawas indicated by a decrease in AST, ALT, and ALP levels, which correlates with its already known hepatoprotective effects. Treatment with Atorvastatin also brought about a decrease in the enzyme levels. Many recent reports showthat specific statins can ameliorate NAFLD/NASH. Statin treatment may also protect from hepatocellular carcinoma (HCC) related to NAFLD/NASH.37
100 µgof C. indicawas identified to exert maximum efficacy by reducing liver enzymes demonstrated hepatoprotective properties at this concentration. However, 200µg was found to be required to bring about a reduction in cholesterol, triglyceride, and HMG-COA reductase levels. Differences in hepatic uptake and plasma concentration levels of drugs could have brought about these varying activities at different concentrations.38
This suggests that the therapeutic effect of C. indica could be beneficial towards systemic lipid metabolism of visceral adiposity and hepatic steatosis. As the crude extract of C. indica has been used for the study, the anti-obesity effect could have been due to the synergistic activity of the phytocompounds present. Further purification, extraction, and testing of the individual phytocomponents on all the parameters could provide with the opportunity to designa drug that can be used for the treatment of obesity.
Conclusion
Treatment with Calocybe indica was able to bring down the cholesterol, triglyceride, and HMG COA Reductase levels in a dose-dependent manner. It was able to reduce the accumulation of fat in the liver (steatohepatitis), which resulted due to obesity, indicated by the reduction in the levels of the liver enzymes AST, ALT, and ALP. From the experimental evidence of these biochemical tests, we conclude that C. indica has a beneficial effect on obesity. Further studies are essential to determine the effects of individual, and/or synergistic effects of the phytocompounds in the milky white mushroom.
Conflict of Interest
There is no conflict of interest.
Ethics
Experiments were performed after approval from the Ethics Committee for students projects, Sri Ramachandra Institute of Higher Education and Research, Porur, Chennai- 600 116
References
- Ofei F. Obesity – a preventable disease. Ghana medical journal, 2005;39(3):98-101.
- KailaB, and Raman M. Obesity: a review of pathogenesis and management strategies. Can. J. Gastroenterol., 2008;22(1):61-68.
CrossRef - Fabbrini E, Sullivan S, and Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology, 2010;51(2):679-689.
CrossRef - Nuttall F. Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutrition today, 2015;50(3):117-128.
CrossRef - Aguirre L, Hijona E, Macarulla M. T, Gracia A, Larrechi I, Bujanda L, Hijona L, and Portillo M. P. Several statins increase body and liver fat accumulation in a model of metabolic syndrome. J. Physiol. Pharmacol., 2013;64:281-8.
CrossRef - Sigler M.A, Congdon L, and Edwards K. L. An Evidence-Based Review of Statin Use in Patients With Nonalcoholic Fatty Liver Disease. Med. Insights. Gastroenterol., 2018;11: 1179552218787502-1179552218787502.
CrossRef - Ramkumar S, Raghunath A, and Raghunath S. Statin Therapy: Review of Safety and Potential Side Effects. Acta Cardiol. Sin., 2016;32(6):631-639.
- Subbiah K.A, and Balan V. A. Comprehensive Review of Tropical Milky White Mushroom (Calocybe indica P&C). Mycobiology, 2015;43(3): 184-194.
CrossRef - Anju R. P, and Ukkuru M. Health Impact and Medicinal Properties of Nutritionally Edible Milky Mushroom (Calocybe Indica). Int. J. Adv. Res. Sci. Eng. Technol., 2016;3(11): 235-237.
CrossRef - R, and Rao G. N. Antioxidant properties and electrochemical behavior of cultivated commercial Indian edible mushrooms. J. Food Sci. Technol., 2013;50(2): 301-308.
CrossRef - PrabuD, and Kalavalli K. In vitro and in vivo antidiabetic activity of Calocybe indica. Proceedings of 8th International Conference on Mushroom Biology and Mushroom Products (ICMBMP8), 2014: 19-22.
- Ganapathy J, and Renitta E. Evaluation of Anti-Cancer Activity and Anti-Oxidant Status of Calocybe Indica (Milky Mushroom) On Dalton’s Lymphoma Ascites Induced Mice. 2014;8: 466-475.
- Chatterjee S, Dey A, Dutta R, Dey S, Acharya K. Hepatoprotective effect of the ethanolic extract of Calocybe indica on mice with CCl4 hepatic intoxication. Int. J. PharmTech. Res., 2011;3(4):2162-8.
- Krishnaveni M, and Manikandan M. Antimicrobial Activity of Mushrooms. Research J. Pharm. and Tech., 2014; 7(4): 399-400.
- Sumathy R, Kumuthakalavalli R, Krishnamoorthy A. S, Balan V. Effect of phytochemicals and antioxidant compounds enriched extract from Calocybe Indica var. APK2 on proliferation of human MCF-7 breast carcinoma cells. Der Pharmacia Sinica, 2015;6: 6-11.
- Ganesan K, and Xu B. Anti-Obesity Effects of Medicinal and Edible Mushrooms. Molecules, 2018; 23(11).
CrossRef - Oka T, Nishimura Y, Zang L, Hirano M, Shimada Y, Wang Z, Umemoto N, Kuroyanagi J, Nishimura N, Tanaka T. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC physiology, 2010;10: 21-21.
CrossRef - Meguro S, Hasumura T, and Hase T. Body fat accumulation in zebrafish is induced by a diet rich in fat and reduced by supplementation with green tea extract. PloS one, 2015;10(3): e0120142-e0120142.
CrossRef - LandgrafK, Schuster S, Meusel A, Garten A, Riemer T, Schleinitz D, Kiess W, Körner A. Short-term overfeeding of zebrafish with normal or high-fat diet as a model for the development of metabolically healthy versus unhealthy obesity. BMC physiology, 2017;17(1): 4-4.
CrossRef - Stępień A, Stępień M, Wlazeł R. N, Paradowski M, Banach M, Rysz J. Assessment of the relationship between lipid parameters and obesity indices in non-diabetic obese patients: a preliminary report. Med Sci Monit., 2014;20: 2683-2688.
CrossRef - Christian A.H, Mochari H, and Mosca L. J. Waist circumference, body mass index, and their association with cardiometabolic and global risk. Cardiometab. Syndr., 2009;4(1): 12-19.
CrossRef - Pose E, Trebicka J, Mookerjee R. P, Angeli P, Ginès P.Statins: Old drugs as new therapy for liver diseases? of Hepatol., 2019;70(1): 194-202.
CrossRef - Khan A. R, Awan F. R, Najam S. S, Islam M, Siddique T, Zain M., Elevated serum level of human alkaline phosphatase in obesity. J. Pak. Med. Assoc., 2015;65: 1182-1185.
- Das A. K, Chandra P, Gupta A, Ahmad N.Obesity and the levels of liver enzymes (ALT, AST & GGT) in East Medinipur, India. Asian Journal of Medical Sciences, 2014;6.
CrossRef - NakayamaH,Shimada Y, Zang L, Terasawa M, Nishiura K, Matsuda K, Toombs C, Langdon C, and Nishimura N. Novel Anti-Obesity Properties of Palmaria mollis in Zebrafish and Mouse Models. Nutrients, 2018;10(10): 1401.
CrossRef - Wadood A. Phytochemical Analysis of Medicinal Plants Occurring in Local Area of Mardan. Biochemistry & Analytical Biochemistry, 2013;02.
CrossRef - Rocco L,Frenzilli G, Zito G, Archimandritis A, Peluso C, and Stingo V. Genotoxic effects in fish induced by pharmacological agents present in the sewage of some Italian water-treatment plants. Environmental Toxicology, 2012;27(1): 18-25.
CrossRef - Singh K, Kaur J, Ahluwalia P, and Sharma J. Effect of monosodium glutamate on various lipid fractions and certain antioxidant enzymes in arterial tissue of chronic alcoholic adult male mice. Toxicology international, 2012;19(1): 9-14.
CrossRef - Yogeeta S.K, Hanumantra R. B, Gnanapragasam A, Subramanian S, Rajakannu S, and Devaki T. Attenuation of Abnormalities in the Lipid Metabolism during Experimental Myocardial Infarction Induced by Isoproterenol in Rats: Beneficial Effect of Ferulic Acid and Ascorbic Acid. Basic & Clinical Pharmacology & Toxicology, 2006;98(5): 467-472.
CrossRef - Shaik A.H, Shaik N. R, Mohammed A. K, Al Omar S. Y, Mohammad A, Mohaya T. A,and Kodidhela L. D. Terminalia pallida fruit ethanolic extract ameliorates lipids, lipoproteins, lipid metabolism marker enzymes and paraoxonase in isoproterenol-induced myocardial infarcted rats. Saudi Journal of Biological Sciences, 2018;25(3): 431-436.
CrossRef - Mohun A.F, and Cook I. J. Simple methods for measuring serum levels of the glutamic-oxalacetic and glutamic-pyruvic transaminases in routine laboratories. Journal of clinical pathology, 1957; 10(4): 394-399.
CrossRef - R, and King E. J. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. Journal of clinical pathology, 1954;7(4):322-326.
CrossRef - Tabatabaei-Malazy O, Larijani B, and Abdollahi M. Targeting metabolic disorders by natural products. Journal of diabetes and metabolic disorders, 2015; 14: 57-57.
CrossRef - Jiang S.Y,Li H, Tang J. J, Wang J, Luo J, Liu B, Wang J. K, Shi X. J, Cui H. W, Tang J, and Yang F. Discovery of a potent HMG-CoA reductase degrader that eliminates statin-induced reductase accumulation and lowers cholesterol. Nature Communications, 2018;9(1): 5138.
CrossRef - Finelli C, and Tarantino G. What is the role of adiponectin in obesity related non-alcoholic fatty liver disease? World journal of gastroenterology, 2013;19(6): 802-812.
CrossRef - BuechlerC, Wanninger J, and Neumeier M. Adiponectin, a key adipokine in obesity related liver diseases. World journal of gastroenterology, 2011;17(23): 2801-2811.
- Karavia E.A, and Kypreos K. E. Pharmacological management of nonalcoholic fatty liver disease: Limitations, challenges and new therapeutic. Journal of Atherosclerosis Prevention and Treatment, 2019;10(1):28–37.
- Chu X, Korzekwa K, Elsby R, Fenner K, Galetin A, Lai Y, Matsson P, Moss A, Nagar S, Rosania G. R, Bai J. P, Polli J. W, Sugiyama Y, and Brouwer K. L. Intracellular drug concentrations and transporters: measurement, modeling, and implications for the liver. Clin Pharmacol Ther., 2013;94(1):126-41.
CrossRef







