Richa Sharma1 , Suresh Kumar2
, Suresh Kumar2 , Prem Singh3*
, Prem Singh3* and Shikha Kapila4
and Shikha Kapila4
1Punjab Technical University, Kapurthala, Punjab, India, 144603.
2Department of Physics, MMEC, Maharishi Markandeshwar (Deemed to be University) Mullana, Ambala, Haryana, India, 133203.
3S.D. College, Ambala Cantt., Ambala, Haryana, India, 133001.
4Bahra Group of Institutions, Bhedpura, Patiala, Punjab, India, 147001.
Corresponding Author E-mail: pspundir1@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2039
Abstract
Metal oxide nanoparticles gain attention in the field of biomedical applications because of their unique physico-chemical properties and emerging out as an alternative to antibiotics. The major cause of most of the human diseases is the bacterial infection. However, antibiotics used in the cure show other complications to human health. Therefore, the purpose of the present work is to investigate the antibacterial properties of ZnO/Ag nanoparticles on the test bacterial strains, Escherichia coli (E. coli). ZnO/Ag nanoparticles are synthesized using surfactant mediated route in a single step and double step procedure. Here, CTAB and hydrazine hydrate used as a surfactant and reducing agents respectively. The synthesized nanoparticles are characterized by x-ray diffraction, scanning electron microscopy and energy dispersive x-ray spectroscopy for structure, morphology and compositional properties. The antibacterial activities of these nanoparticles are also studied using the agar-well diffusion technique. The result analysis shows that synthesized nanopaticles are spherical in shape, having particles of the size 6 nm and 13 nm in the desired elemental composition. ZnO/Ag nanoparticles possessed a strong antibacterial effect against E. coli. This study signifies that ZnO/Ag metal oxide nanoparticles exhibit stronger antimicrobial activity against pathogen bacteria E. coli which may works effectively on the antibacterial and antifungal infections.
Keywords
Antibacterial Activity; Structural Properties; Surfactant Mediated Route; ZnO/Ag Nanoparticles
Download this article as:| Copy the following to cite this article: Sharma R, Kumar S, Singh P, Kapila S. Structural, Morphological and Antimicrobial Study of ZnO/Ag Nanoparticles. Biomed Pharmacol J 2020;13(4). |
| Copy the following to cite this URL: Sharma R, Kumar S, Singh P, Kapila S. Structural, Morphological and Antimicrobial Study of ZnO/Ag Nanoparticles. Biomed Pharmacol J 2020;13(4). Available from: https://bit.ly/2U6YWNj |
Introduction
With the advancement in nanotechnology, the metal oxide nanoparticles have gained research interest in the field of biomedical applications because of their distinctive physico-chemical features, large surface to volume ratio and strong intra material interactions [1, 2]. Among different type of metal oxides, ZnO is well a known material because of its effective photocatalytic response, chemical stability, high oxidation capacity, non-toxic nature and low-cost behaviour [3, 4], which makes it an important material for biological as well as for other applications including photocatalysis, gas sensing, electronics, cosmetics, etc., [5-9]. At present different ZnO nanostructures are widely investigated because of their high excitation energy (60 meV), high optical band gap (3.3 eV) at room temperature and stable hexagonal (wurtzite) structure [10, 11]. The doping of metal ions in the pure ZnO matrix increases the surface area by reducing particle size, enhancing the light absorption and fluorescence property by changing the concentration of defects [12]. Several metals like Sb, Ag, Cu, Fe, etc., have been utilized to doped in ZnO structure in order to improve their structural, optical, electrical and catalytic properties [13-16]. It has been observed that doping of silver (Ag) and gold (Au) metals in ZnO are responsible to enhances photocatalytic activity by changing the charge separation and reducing the recombination of electron-hole pair [17, 18]. Nowadays, different bacterial infections become the main reason of the large number of diseases. Antibiotics have been frequently preferred for their treatment. However, different studies show that the extensive use of antibiotics cause many complications to public health [19]. So, as an alternative to antibiotics, metal oxide nanoparticles are establishing a promising approach to solve this problem. These nanoparticles efficiently work for the rapid neutralization of the surface electric charge of the bacterial membrane and change its penetrability which leads to bacterial death [20, 21]. Recently, Kim et. al have reported the antimicrobial effects of silver nanoparticles while Manyasree et. al have reported antibacterial activity of ZnO nanoparticles of size 35 nm against gram-negative and gram-positive organisms [20, 21]. However, the antibacterial effect of Ag doped ZnO on gram-positive bacteria are seldomly explored till date.
Hence, in the present work, we are trying Ag metal as a dopant in ZnO material using single step and double step processes. The high solubility, minimum orbital energy and large ionic radius [18] of Ag metal may help to improve the functionality of ZnO material. The antibacterial activity of ZnO/Ag nanoparticles is investigated against gram-negative pathogenic bacteria, Escherichia coli (E. coli).
Materials and Methods
Chemicals
To pursue the work, analytical grade chemicals, purchased from S.D. Fine Chem. Limited and HiMedia Laboratories Pvt. Ltd. India, were used without any further purification. Zinc sulphate heptahydrate (ZnSO4.7H2O) and silver nitrate (AgNO3) were used as zinc and silver ions sources respectively whereas CTAB used as a surfactant and hydrazine hydrate as a reducing agent. All the solutions for experimentation were prepared using the deionized water.
Synthesis of ZnO/Ag nanoparticles
ZnO/Ag nanoparticles were synthesized by the surfactant mediated route using single step and double step procedure.
Single step procedure for the synthesis of ZnO/Ag nanoparticles
ZnO/Ag nanoparticles were prepared by single step procedure as: initially 0.576 gm of ZnSO4.7H2O, 20 mg of AgNO3 and 0.74 gm of CTAB were dissolved in 100 ml of deionized water individually to prepare 0.01 M solution of each reagent. All the solutions were then mixed, sonicated for 1 h and kept at continuous stirring for 1 h using a magnetic stirrer. Finally, ammonia solution (~78 ml) was added which maintain the solution pH at ~10. The whole solution was kept under continuous stirring and left to complete the precipitation process at room temperature. The residue of this chemical reaction was filtered and washed using deionized water three times. The precipitates in the powder form are collected and dried for 2 h at temperature 75 °C using a microwave oven. This powder is then re-dispersed in 20 ml deionized water containing 0.2 ml of 1 M hydrazine hydrate under vigorous stirring. During this reaction process, Ag nanoparticles were deposited on the surface of ZnO nanoparticles, which leads to the formation of ZnO/Ag precipitates (AZO-1). Finally, this powder was collected by filtration, washed with deionized water successively and dried well again in the microwave oven at 75 °C for 2 h.
Double step procedure for the synthesis of ZnO/Ag nanoparticles
In this procedure, ZnO nanoparticles were prepared in the step-1 and then Ag mixing was done in step-2 to obtain ZnO/Ag nanoparticles.
Step-1: Preparation of ZnO Nanoparticles
For the preparation of ZnO nanoparticles, initially, 4 ml of ZnSO4 (1 M) and 4 ml of CTAB (1 M) were dissolved in 400 ml of deionized water so that the final concentration of both become 0.01 M. The whole solution is then sonicated for 30 minutes at temperature of 42 °C. Finally, 6 ml of ammonia solution is added to this solution to maintain pH = 10. The solution became cloudy slowly due to the formation of Zn(OH)2 and then the precipitation occurs. The white precipitates were filtered and washed with deionized water consecutively. The obtained precipitates were dried at 75 °C for 2 h in a microwave oven.
Step-2: Preparation of ZnO/Ag Nanoparticles
The process of mixing Ag with ZnO nanoparticles to form ZnO/Ag nanoparticles consists of dissolution of 0.1 g of ZnO in 10 ml of deionized water. The sonication of the solution was done for ~30 min. Then, a solution of 0.17 g of AgNO3 in 10 ml of water (0.01 M) was prepared. The two solutions were mixed together under continuous stirring to make precipitates, which were collected and washed. After that, the precipitates were re-dispersed in 20 ml of water and 0.2 ml of 1 M hydrazine hydrate and vigorously stirred. Ag nanoparticles were deposited on the surface of ZnO nanoparticles, which results in the formation of ZnO/Ag precipitates (AZO-2). Finally, this powder was collected, washed with deionized water repeatedly and dried well again in the microwave oven at 75 °C for 2 h.
Finally, both the synthesized samples (AZO-1 and AGO-2) were annealed in a muffle furnace at 500 °C for 1 hour before further characterizations.
Characterization Techniques
The structural and phase identification of ZnO/Ag nanoparticles were analyzed from the XRD spectra obtained by Panalytical x-ray diffractometer in the 2θ range of 10° to 80°. The surface morphology was observed by field effect scanning electron microscope (FE-SEM; Jeol JSM-6100). The elemental composition of nanoparticles identified using Bruker Quantax energy dispersive x-ray analyzer (EDX).
Antibacterial activity by agar well diffusion method
The antibacterial activity of the ZnO/Ag nanoparticles was done on E. coli bacterial test strains using agar-well diffusion technique. This method involves the inoculation of E. coli in agar plate with the synthesized ZnO/Ag nanoparticles. The plate was prepared with nutrient broth (medium used to grow various types of bacteria and contains many nutrients needed for the bacterial growth) and nutrient agar (also a medium used to grow various types of bacteria and contains many nutrients needed for the bacterial growth and is obtained from algae). The plates were then incubated for 24 h at 37 °C and the growth was observed.
Results and Discussion
XRD analysis of ZnO/Ag nanoparticles
The crystallinity and phase of the synthesized nanoparticles AZO-1 and AZO-2 were analyzed from XRD spectra (Fig. 1a and Fig. 1b) respectively.
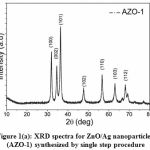 |
Figure 1(a): XRD spectra for ZnO/Ag nanoparticles (AZO-1) synthesized by single step procedure |
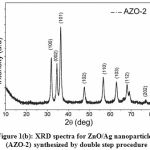 |
Figure 1(b): XRD spectra for ZnO/Ag nanoparticles (AZO-2) synthesized by double step procedure |
Fig. 1(a) and 1(b) clearly displayed well defined XRD peaks positioned at 2θ values of 31.9°, 34.5°, 36.3°, 47.6°, 56.6°, 63.0°, 68.1°, 69.2° and 77.4° which belongs to (100), (002), (101), (102), (110), (103), (112), (201) and (202) crystal planes of wurtzite (hexagonal) phase of ZnO respectively. These XRD observations were matched with the standard jcpds card # 36-1451 and reported values for the ZnO synthesized by facile hydrothermal process [22]. A little variation in 2θ values for AZO-1 and AZO-2 may be due to the difference in the synthesis procedure which results in particle size variation. The average particle size (D) of the ZnO/Ag nanoparticles has been determined using full-width at half maximum (β) of the XRD peaks by using Debye-Scherer equation [23]
where K (= 0.89) is the shape parameter, λ is the x-rays wavelength and θ is the Bragg angle. The average size of particle is estimated to be 13.4 nm and 6.9 nm for AZO-1 and AZO-2 respectively. However no distinguish peak for Ag detected in the XRD spectra which may be probably due to the small quantity of Ag nanoparticles in ZnO structure.
FE-SEM analysis of ZnO/Ag nanoparticles
The surface morphology of the synthesized nanoparticles was carried out using FE-SEM and shown in Fig. 2(a) and 2(b). From surface morphology analysis, it has been observed that the size of particles is clearly of the order of few nanometers. The synthesized particles exhibit spherical symmetry, agglomerated in cluster form which are randomly scattered and have a uniform size distribution. The size of nanoparticles estimated by inception method for both the samples is found in the range of 40-50 nm.
 |
Figure 2(a): FE-SEM images for ZnO/Ag nanoparticles (AZO-1) synthesized by single step procedure at the scale of 1 um and 500 nm. |
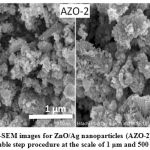 |
Figure 2(b): FE-SEM images for ZnO/Ag nanoparticles (AZO-2) synthesized by double step procedure at the scale of 1 μm and 500 nm |
EDX analysis of ZnO/Ag nanoparticles
EDX technique utilizes the interaction of the excitation source with the sample for the elemental analysis. Fig. 3(a) and 3(b) show the EDX spectra for Ag doped ZnO nanoparticles. It is clear from the spectra that different peaks belong to zinc (Zn), oxygen (O) and silver (Ag) are identified in both the samples.
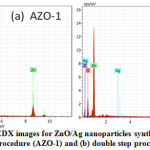 |
Figure 3: EDX images for ZnO/Ag nanoparticles synthesized by (a) single step procedure (AZO-1) and (b) double step procedure (AZO-2) |
Antibacterial activity of ZnO/Ag nanoparticles
To investigate the antibacterial activity of the synthesized ZnO/Ag nanoparticles against gram-negative bacteria (E. coli), three petri-dics/plates with different compositions have been prepared (Table 1). Inoculation of E. coli has been done with 1.5 ml.
Table 1: The Composition of the plates for the testing of antibacterial activity
| Plates with AGO nanoparticles | Nutrient broth
(gm) |
Nutrient agar (gm) | Water (ml) | Dose of nanoparticles (ml) |
| Plate 1 (no AGO) | 0.65 | 1.65 | 50 | – |
| Plate 2 (AZO-1) | 0.65 | 1.65 | 50 | 0.5 |
| Plate 3 (AZO-2) | 0.65 | 1.65 | 50 | 0.5 |
These prepared plates have been kept for the incubation at 37 oC for 24 h. The growth of E. coli strain has been observed in plate 1 as shown in fig. 4(a), whereas no growth of E. coli strain is obtained in plates 2 and 3 as shown in Fig. 4(b) and 4(c) respectively. It means that the ZnO/Ag samples have been prevented the growth of E. coli. Hence, it can be concluded that the synthesized nanoparticles show excellent antibacterial response against gram-negative bacteria E. coli in the present study.
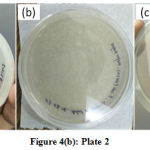 |
Figure 4: (a): Plate 1, (b): Plate 2 and (c): Plate 3 |
A strong antimicrobial activity of ZnO/Ag nanoparticles has been found against the E. coli bacterial strain. It may be due to the interaction between nanoparticles and CTAB surfactant which leads to nano-size particle formation. The small size of the ZnO/Ag nanoparticles and CTAB surfactant may promote the antibacterial activity. The small size nanoparticles can easily penetrate the small thickness of the bacterial cell wall. These ZnO/Ag nanoparticles discharge ions that interact with the protein cell membrane and able to disrupt their functions which finally lead to the death of microorganism [21, 24].
Conclusion
ZnO/Ag nanoparticles have been synthesized by the surfactant mediated route through the single step and double step procedure. The study of the structure, morphology and composition of the synthesized nanoparticles has been performed by XRD, FE-SEM and EDX. The average particle size is 13.4 nm and 6.9 nm for both ZnO/Ag nanoparticles synthesized via single step and double step procedure respectively. EDX analysis confirms the presence of Zn, O and Ag elements in the nanoparticles. The antibacterial activity of spherical shaped ZnO/Ag nanoparticles has been tested against oral pathogen E. coli and is found to have a good antimicrobial response. The results of the present work clearly indicate that ZnO/Ag nanoparticles synthesized by above described procedures can be utilized as an antimicrobial agent against the antibacterial and antifungal infections and also in other industrial and agricultural practices.
Acknowledgments
The authors want to thanks microelectronics lab of Ambala College of Engineering and Applied Research, Devsthali, Ambala for the preparation of the samples and their antibacterial activity. They are grateful to Panjab University, Chandigarh for providing characterization facilities of XRD, FE-SEM and EDX. They are also thankful to Punjab Technical University, Kapurthala for their timely guidance.
Conflicts of Interests
There is no Conflict of Interest.
Funding Source
There is no Funding Source.
Authors Contributions
All authors have contributed equally in this work.
References
- Moser WR, editor, Advanced catalysts and nanostructured materials: modern synthetic methods, 1st ed San Diego: Academic Press; 1996.
- Valden M, Lai X, Goodman DW. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science; 281(5383): 1647-1650 (1998).
CrossRef - Hoffmann MR, Martin ST, Choi W, Bahnemann DW. Environmental applications of semiconductor photocatalysis. Chemical Review; 95(1): 69-96 (1995).
CrossRef - Periyat P, Pillai SC, McCormack DE, Colreavy J, Hinder SJ. Improved high-temperature stability and sun-light-driven photocatalytic activity of sulfur-doped anatase TiO2. The Journal of Physical Chemistry C; 112(35): 7644-7652 (2008).
CrossRef - Huang WJ, Fang GC, Wang CC. A nanometer – ZnO catalyst to enhance the ozonation of 2,4,6-trichlorophenol in water, Colloids and Surfaces A; 260(1-3): 45-51 (2005).
CrossRef - Annapoorani R, Dhananjeyan MR, Renganathan R. An investigation on ZnO photocatalysed oxidation of uracil. Journal of Photochemistry and Photobiology A; 111(1-3): 215-221 (1997).
CrossRef - Kumar S, Arora D, Dhupar A, Sharma V, Sharma JK, Sharma SK, Gaur A. Structural and optical properties of polycrystalline ZnO nano powder synthesized by direct precipitation technique, Journal of Nano- and Electronic Physics; 12(4): 04027-5 (2020).
CrossRef - Hariharan C. Photocatalytic degradation of organic contaminants in water by ZnO nanoparticles: revisited, Applied Catalysis A-General; 304(1): 55-61 (2006).
CrossRef - Yang LY, Dong SY, Sun JH, Feng JL, Wu QH, Sun SP. Microwave-assisted preparation, characterization and photocatalytic properties of a dumbbell-shaped ZnO photocatalyst. Journal of Hazardous Materials; 179(1-3): 438-443 (2010).
CrossRef - Ozgur U, Alivov YI, Liu C, Teke A, Reshchikov MA, Dogan S, Avrutin V, Cho SJ, Morkoc H. A comprehensive review of ZnO materials and devices, Journal of Applied Physics; 98(4): 041103-041301 (2005).
CrossRef - Dey A. Semiconductor metal oxide gas sensors: a review, Materials Science and Engineering B; 229: 206-217 (2018).
CrossRef - Pal B, Sharon M. Enhanced photocatalytic activity of highly porous ZnO thin films prepared by sol–gel process, Materials Chemistry and Physics; 76(1): 82-87 (2002).
CrossRef - Xiu FX, Yang Z, Mandalapu LJ, Zhao DT, Liu JL. Photoluminescence study of Sb-doped p-Type ZnO films by molecular-beam epitaxy, Applied Physics Letters; 87(25): 252102-3 (2005).
CrossRef - Chauhan R, Kumar A, Chaudhary RP. synthesis and characterization of silver doped ZnO nanoparticles, Archives of Applied Science Research; 2(5): 378-385 (2010).
- Shabannia R. Synthesis and characterization of Cu-doped ZnO nanorods chemically grown on flexible substrate. Journal of Molecular Structure; 1118: 157-160 (2016).
CrossRef - Kanchana S, Chithra MJ, Ernest S, Pushpanathan K, Violet emission from Fe-doped ZnO nanoparticles synthesized by precipitation method, Journal of Luminescence; 176: 6-14 (2016).
CrossRef - Merga G, Cass LC, Chipman DM, Meisel D. Probing silver nanoparticles during catalytic H2 evolution, Journal of the American Chemical Society; 130(22): 7067-7076 (2008).
CrossRef - Gouvea CAK, Wypych F, Moraes SG, Duran N, Nagata N, Zamora PP. Semiconductor-assisted photocatalytic degradation of reactive dyes in aqueous solution, Chemosphere; 40(4): 433-440 (2000).
CrossRef - Malarkodi C, Kumar SR, Kumar P, Vanaja MK, Gnanajobitha G, Annadurai G. Biosynthesis and antimicrobial activity of semiconductor nanoparticles against oral pathogens. Journal of Biological Inorganic Chemistry; 347167: 1-10 (2014).
CrossRef - Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH. Antimicrobial effects of silver nanoparticles, Nanomedicine; 3(1): 95-101 (2007).
CrossRef - Manyasree D, Kiranmayi P, Kolli VR. Characterization and antibacterial activity of ZnO nanoparticles synthesized by co-precipitation method. International Journal of Applied Pharmaceutics;10(6): 224-228 (2018).
CrossRef - Umar A, Khan MA, Kumar R, Algarni H. Doped ZnO nanoparticles for enhanced ethanol gas sensing application, Journal of Nanoscience and Nanotechnology; 18(5): 3557-3562 (2018).
CrossRef - Kumar S, Sharma P, Sharma V. CdS nanofilms: Synthesis and the role of annealing on structural and optical properties, Journal of Applied Physics; 111: 043519-6 (2012).
CrossRef - Vanaja M, Kumar SR, Kumar PK, Gnanajobitha G, Malarkodi C, Annadurai G. Photosynthesis and characterization of silver nanoparticles using stem extract of coleus aromaticus, International Journal of Materials Science and Applications; 3(1): 1-4 (2013).
CrossRef








