Manuscript accepted on :3-Dec-2020
Published online on: --
Plagiarism Check: Yes
Reviewed by: Ahmed Salah
Second Review by: Belayneh Kefale
Final Approval by: Fai Poon
Theo Audi Yanto1 , Mochammad Hatta2*
, Mochammad Hatta2* , Agussalim Bukhari3
, Agussalim Bukhari3 and Rosdiana Natzir4
and Rosdiana Natzir4
1Department of Internal Medicine Faculty Medicine Universitas Pelita Harapan, Tangerang, Indonesia.
2Department of Molecular Biology and Immunology, Faculty Medicine, Hasanuddin University, Makassar, Indonesia
3Department of Nutrition, Faculty Medicine Universitas Hasanuddin, Makassar, Indonesia.
4Department of Biochemistry, Faculty Medicine Universitas Hasanuddin, Makassar, Indonesia.
Corresponding Author E-mail: hattaram@yahoo.comDOI : https://dx.doi.org/10.13005/bpj/2036
Abstract
Infectious disease is still a massive world burden of disease. It causes premature mortality and morbidity. Regardless of antibiotic therapy, the increased numbers of antibiotic resistance bring emerging problems in infectious disease. Several pathogens have unique roles to deactivate host immune response causing difficulty to treat the infection. Alack of antibiotic efficacy is crucial to modulate the immune response as a brilliant strategy to face infectious disease in years to come. Various herbal medicine has been known to have a pivotal role in enhancing immune response at the molecular level. Miana leaves extract (MLE) has a potential role in regulating the immune response to the infection. Besides its antimicrobial effect, MLE has other properties such as anti-inflammation, antioxidant. Several studies have revealedthe molecular mechanism of MLE in immune response, such as enhancing IL 37, IL 10, regulating TLR 4, and IFN-g. The objectives of this article are to review the molecular and immunological mechanism of Miana in treating various infective diseases comprehensively.
Keywords
Coleus; Infectious Disease; Immunology; Miana; Molecular
Download this article as:| Copy the following to cite this article: Yanto T. A, Hatta M, Bukhari A, Natzir R. Molecular and Immunological Mechanisms of Miana Leaf (Coleus Scutellariodes [L] Benth) in Infectious Diseases. Biomed Pharmacol J 2020;13(4). |
| Copy the following to cite this URL: Yanto T. A, Hatta M, Bukhari A, Natzir R. Molecular and Immunological Mechanisms of Miana Leaf (Coleus Scutellariodes [L] Benth) in Infectious Diseases. Biomed Pharmacol J 2020;13(4). Available from: https://bit.ly/3nojGwU |
Introduction
Infectious diseases, caused by microorganisms such as bacteria, viruses, fungi, and parasites, are associated with a significant amount of worldwide morbidity and mortality. In 2008, the World Health Organization (WHO) stated that 1 billion population suffered from one or more infectious diseases per year.1 Substantial year-to-year variation emphasizes the dynamic nature of infectious diseases.2
There was a 47% decrease in premature mortality and disability caused by infectious disease from 1990 to 2017. It correlates with better sanitation management, healthcare system, and antibiotics resistance.3However, irrational antibiotic prescribing leads to antimicrobial resistance (AMR).4The AMR bacteria cause healthcare-associated as well as community-acquired infections resulted from urinary tract infections or pneumonia.5
Furthermore, the development of novel antibiotics remains insufficient to handle the increasing AMR. Since the 1st of July 2017, eight new antibacterial agents have been approved, but their efficacy has been limited.4Therefore, adjuvant therapies are being proposed as an option in managing infectious diseases.67Herbal medicine has known that has the potential to enhance the effect of standard treatment in many diseases from degenerative diseases to infectious diseases.8910Each of them has a unique collaborative pathway in treating the ailment.Nevertheless, herbal medicine which has potential benefit as an adjuvantstill needs to evaluate its efficacy and biomolecular mechanism.111213
Furthermore, several plants and other natural resources have been used as herbal medicine such as purple miana leaves14, curcumin15, Moringa leaf extract16, Sapodilla fruit extract17, Curcuma longa extract1819, Andaliman fruit extract (Zanthoxylum acanthopodium)20, red fruit (Pandanus conoideus) extract21, and ethanol extract from Musa paradisiaca L (MPL) fruit.22Regardless of the antimicrobial effect, it has anti-inflammation effects that modulate the immune response. The other example of natural antibiotics is garlic (effective against Salmonella and E.coli).23 Honey (especially Manuka honey)have been reported to exhibit antimicrobial effect against Staphylococcus aureus and Helicobacter pylori and had been used as an ointment to accelerate wound healing, as well as Channa striata.2425Echinacea, that has been used for hundreds of years by Native American, have been studied extensively about its role in infectious disease as a model of learning pharmacognosy of the natural product serves as an example in developing the other herbal medicine.26
In Indonesia, there are abundant potential herbal resources such as moringa leaf16,Ajwa dates27, Channa striata28. In the last two decades, the discovery of novel therapeutics to combat multi-drug resistance (MDR) has begun, exclusively with ground plants and deep-sea flora.29 Biological theories behind these natural products are related to molecular biology, genetics, physiology, and pathology. Food, diseases, poisons, and antidotes have complex interactions that create a wide range of possibilitiesof their application for secondary metabolites as well as their synthetic or semisynthetic derivatives.3031
Phytochemicals have become a source of new molecules that leads to the development of novel drugs with potential immunoregulator and antimicrobial agent used as adjuvant or alternative therapy.2432One of the herbal medicines that havepromising possibleimmune regulator effects is Miana. Torajapeople in South Sulawesi, Indonesia,uses Miana leaves to treat infectious diseases or boost their immunity.33 A 2013 survey conducted in the Toraja community of South Sulawesi showed that 85.71% of patients chose to use traditional medicine and applyMiana leaves as the complement of tuberculosis treatment.34 However, there are no comprehensive reviews on the molecular and immunologic mechanism of Miana in infectious disease. This review will serve as the first extensive study on the molecular mechanism and immune response of Miana in infectious disease as a potential agent for combating infection in years to come.
Search Strategies
A comprehensive search of the literature was conducted in PubMed (NIH), Scopus, EMBASE, Google Scholar, and Portal Garuda database using keyword combinations of the medical subject headings (MeSH) of ‘miana’, ‘iler’, ‘coleus scutellarioides’, ‘infection’,‘infectious disease’, ‘immune response’, ‘cytokine’, ‘anti-inflammation’,’pro-inflammation’, and ‘transcription factor’.A relevant reference list was also manually searched.
Miana Leaves Extract
Miana is one of the flowering plant species that belongs to the family Lamiaceae. It is native to South East Asia and its neighbouring regions. It is also known as Coleus blumei or Plectranthusscutellarioides, or Ocimumscutellarioides. orSolenostemonscutellarioides.The Coleus genus contains more than 500 species. Plants need moist-drained soil to grow and typically grow 0.5-1 m, though some may grow as tall as 2 meters. Plants are pretty bushy, woody-based evergreen perennial and mostly grown due to its decorative variegated leaves.35 In Indonesia, Miana has a rectangular-shaped stem appearance. Their leaves have a triangular-shaped or ovoid shape whichcolour varies from green to purplish-red. Its flower has a form of stacked strands on its buds with red or white, purple or yellow variations.
Mine are tropical plants that are generally grown as annuals. The various shaped leaves have several colours like chartreuse, rust red, cream, and purple-black. Some cultivars sport almost all of these colours combined. The darker the red spots in the leaves, the more they can tolerate the sunlight. Miana blooms in the summer. The blue to white nettle like flowers are unremarkable. Thus it tends to detract visually from the impact of the foliage. Miana grows in warm soils with adequate drainage. Miana grown in too much sunlight may wilt. On the side, those grown in too much shade may become leggy.36
Miana is one of the plants included in the list of 66 biopharmaceutical crop commodities based on the Decree of the Minister of Agriculture No. 511/Kpts/PD.310/9/2006. The leaves are used by people in the health field such as herbs to treat ophthalmia and dyspepsia, concoctions to reduce swelling in the wound (inflammation), headaches, asthma, coughing, smoothing the menstrual cycle, enhancing appetite, accelerating the maturation of boils, diarrhoea, and worm medicine.3738 In Toraja ethnic communities (in the province of South Sulawesi, Indonesia), it is one of the medicinal plants which is commonly used.34
Mianaleaves needs to be extracted. The simplest extraction method is mixing all the ingredients with a solvent, and material will show different solubility in different solvents depending on the polarity of the compound to be extractedso that a pure combination from the plant has medicinal properties.
Miana leaves extraction (MLE) is done by using ethanol, antioxidant compounds such as anthocyanin (pelargonidin-3 routines and cyanidin-3-O-glucoside compounds).Flavonoid compounds play a role in producing antioxidant effects, and this compound will have 5 equilibrium forms depending on the pH conditions, namely cation flavilium, carbinol bases, chalcons, quinonoidal bases, and anionic quinonoidals.39 The antioxidant activity of ethanol extract of miana leaves is effective in an acidic atmosphere with a citric buffer with citrate-phosphate buffer.Methanol extraction produces compounds such as alkaloids, tannins, flavonoids, saponins and terpenoids. In the chromatograph analysis showed 7 chemical compounds identified in Miana leaf extract, namely (2, 4, 4, 4, 16, 16-D6) -3.alpha., 17.beta.-dihydroxy-5.beta.-androstane, (E,E) – 3,7,11-trimethyl-2,6,10-dodecatrien-1-olacetate, 1,8-Bis (3,4-dicyanophenyl) anthracene, 23-R-methylcholesterol, Stigmasterol, Stigma- 8 (14) – en-3.beta.-ol, and alpha -Amyrin acetate.
The antibacterial mechanism of flavonoids in plants is to inhibit the nucleic acid synthesis and cause damage to the permeability of bacterial cell walls, microsomes, and lysosomes. The mechanism includes interactions between flavonoids and bacterial DNA, forming complex compounds with extracellular proteins, dissolvingin the bacterial cell membranesand destroying it, then mixingit with intracellular compounds. Other studies suggest the mechanism of flavonoids inhibits cell membrane function by disrupting the permeability of cell membranes and inhibiting enzyme bonds such as ATPase and phospholipase.404142In 2018Anita et al.43conducted a study to determine the flavonoid content of miana leaves. Based on the results of quantitative testing on the levels of miana leaf flavonoids (Coleus atropurpereus) that was performed using a UV-Vis spectrophotometer, it showed that an average total flavonoid of 8.59 mg/gram extract was found.Its flavonoids have a relatively high antioxidant activity, namely IC50 in the ethanol extract of Miana leaves 48.04 ppm. A study conducted by Khotimah et al. found that IC50 obtained from measurement of antioxidant activity in days 0, 1, 3, 7, and 14 were 70,13 ppm, 57,91 ppm, 50,91 ppm, 48,43 ppm, and 56,10 ppm respectively which were considered very strong antioxidant activity.1644
These are the compound that can be obtained from the extraction of miana leaves.3845
Flavonoid
Of the various components that can be extracted from miana leaves, flavonoids are the most critical results because they contain antioxidant properties. The group of compounds that play a role in eliminating worms and bacteria is known because of the presence of the properties of the group of flavonoid compounds contained therein which can systemically act as immune stimulators that can enhance the body’s response to various parasitic and bacterial infections.
Flavonoid also hasan antibacterial mechanism by inhibiting the nucleic acid synthesis and cause damage to the permeability of bacterial cell walls, microsomes, and lysosomes. The interactions between flavonoids and bacterial DNA form complex compounds with extracellular proteins and dissolving them so that they can damage bacterial cell membranes and be followed by intracellular compounds. Other studies suggest the mechanism of flavonoids inhibits cell membrane function by disrupting the permeability of cell membranes and inhibiting enzyme bonds such as ATPase and phospholipase.404242 In 2018, Anita et al. conducted a study to determine the flavonoid content of miana leaves. Based on the results of quantitative testing on the levels of miana leaf flavonoids (Coleus atropurpereus) that was performed using a UV-Vis spectrophotometer, it showed that an average total flavonoid of 8.59 mgextract was found.42Its flavonoids have a relatively high antioxidant activity, namely IC50 in the ethanol extract of miana leaves 48.04 ppm. A study conducted by Khotimah et al. found that IC50 obtained from measurement of antioxidant activity in days 0, 1, 3, 7, and 14 were 70,13 ppm, 57,91 ppm, 50,91 ppm, 48,43 ppm, and 56,10 ppm respectively which were considered very strong antioxidant activity.44
Tanin
The mechanism of action of tannin as an antibacterial is protein agglutination. Tanin has an antibacterial activity related to its ability to activate microbial cell adhesin, activate enzymes, and interfere with protein transport in the inner cell layer. Tanin also has a target on cell wall polypeptides so that the formation of cell walls becomes less perfect.4647
Saponin
The mechanism of action of saponins as an antibacterial is that it can cause leakage of proteins and enzymes from within the cell. Saponins can be antibacterial because the surface-active agents are similar to detergents. As a result, saponins will reduce the surface tension of bacterial cell walls and damage membrane permeability. Saponins diffuse through the outer membrane and vulnerable cell walls and then bind to the cytoplasmic membrane so that it interferes with and reduces cell membrane stability.48
Alkaloids
The mechanism of action of alkaloids as an antibacterial is by disrupting the constituent components of peptidoglycan on bacterial cells so that the cell wall layer is not formed intact and causes cell death. Other mechanisms of alkaloid components are known as DNA intercalators and inhibit bacterial cell topoisomerase enzymes.49
Steroids
The mechanism of steroids as an antibacterial is related to lipid membranes and sensitivity to steroid components that cause liposome leakage. Steroids can interact with cell phospholipid membranes and cause fragile cell membranes and lysis.48The mechanism of steroids as an antibacterial is related to lipid membranes and sensitivity to steroid components that cause liposome leakage.
Alkaloid, steroid, flavonoid, saponin, and tannins are used for antibacterial,and phytols are used for antifungals.50Active substances can dilate blood vessels and fibroblasts which act as anti-inflammation.51As well as the flavonoidthat has anti-inflammation properties.52Lastly, quercetin has an antioxidantproperties.53
Molecular and Immunology Mechanism of Infectious Disease
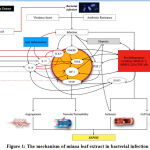 |
Figure 1: The mechanism of miana leaf extract in bacterial infection |
Microorganisms such as bacteria that penetrate the epithelial surfaces of the body initially are met immediately by cells and molecules that can mount an innate immune response.54 Virulence factors play a huge role in stimulating natural immune systems through the pathway of high-mobility group box 1 (HMGB1).17There is a plethora of classification of virulence factors which includes exotoxins (secreted by Corynebacterium diphtheria, Vibrio cholera, and Clostridium tetani), modulins such as bacterial lipopolysaccharides which can damage a host by eliciting inflammatory responses and cascades55, enzymes such as proteases, neuraminidases, and phospholipases 56, attachments such as Gal/GalNAc lectin in Entamoeba histolytica, lipoteichoic acids and M protein in Streptococcus pyogenes, and flagellae in Aeromonas spp.57and E.coli58motility such as actin-based motility to propel themselves forward in Shigella spp., Listeria monocytogenes and Rickettsiae for cell-to-cell spread59, capsules such as polysaccharide capsules in Streptococcus pneumoniae, Neisseria meningitidis, Cryptococcus neoformans, and Haemophilus influenzae which protect microbes from phagocytosis and intracellular killing60, complement evasion proteins such as C5a peptidase which inhibits leukocyte recruitment from Group A and B streptococci.61
AMR facilitatesthe pathogenicity of microorganisms by evading antimicrobial action. There are 2 basic AMR. There wasa genetic and mechanistic mechanism. In genetic, there is a mutation in horizontal gene transfer that plays a role in developing AMR. The mechanistic properties work inthe antibiotic molecule, decreased antibiotic penetration and efflux, changes in target sites, and resistance isowing to global cell adaptations.62
Innate immunity cells respond to various stimuli using pattern-recognition receptors (PRRs) like the Toll-Like Receptors (TLRs) and the NOD-like receptors (NLRs). The pathogen-associated molecular patterns (PAMPs) can be detected by both TLRs and NLRs.at the same time, damage-associated molecular patterns (DAMPs) can be seen by NLRs. Damage-Associated Molecular Patterns s are molecules that are released from damaged or necrotic host cells which include HMGB1 protein.63
NF-κB is especially important in inducing pro-inflammatory genes encoding TNF-α, IL-1β, IL-6, IL-12p40, and cyclooxygenase-2.64 NF-κB does that with the help of TLR4 to mediate the differentiation of macrophages towards the M1 phenotype.65 M1 promotes the production of pro-inflammatory cytokines and the differentiation of inflammatory T cells which both lead to inflammation.66In contrast to the M1 macrophages, the M2 phenotype produces anti-inflammatory cytokines such as IL-10 and IL-13, which are crucial to inhibit inflammation and aid faster-wound healing.67
High-mobility group box 1 can amplify the changes in immune response towards multiple organ dysfunction syndromes (MODS) and death. Structures such as TLR2, TLR4, and TLR9 recognize the bacterial structure and HMGB1, including the receptor for advanced glycation end (RAGE) such that bacteria can activate the adaptive immune system. Also, TLR4 and HMGB1 interact to activate nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) and then the hypoxic inducible factor (HIF)-1α.6814 NF-κB is especially important in inducing pro-inflammatory genes encoding TNF-α, IL-1β, IL-6, IL-12p40, and cyclooxygenase-2.64 NF-κB alongside TLR4 helps the differentiation of macrophages towards the M1 phenotype.65
Upon the onset of infection, pro-inflammatory cytokines such as tumour necrosis factor-α (TNFα), matrix metalloproteinases (MMPs), interleukin 2 (IL-2), and interleukin 6 (IL-6) were produced. TNF-α regulates several critical cell functions, including cell proliferation, survival, differentiation, and apoptosis. Aberrant TNF-α production and TNF receptor signalling have been associated with sepsis.69
Host-derived MMPs facilitate leucocyte recruitment, cytokine and chemokine processing, defensin activation, and matrix remodelling, which are essential for pathogen clearance from the host. However, excessive MMP activities may, in turn, lead to tissue damage, favouring pathogen dissemination or persistence, by breaking down barriers to spread or by creating an immune-privileged site that is poorly accessed by host immune cells, therefore causing immunopathology instead.70
Interleukin 6 (IL-6) also contributes to aid host defence in times of infection by stimulating acute phase responses, hematopoiesis, and immune reactions. Dysregulated continual synthesis of IL-6 results in pathological conditions of chronic inflammation and autoimmunity.71
A unique pathway these cytokines use to activate its action is by communication with signal transducer and activator of transcription 3 (STAT3). STAT3 is a transcription factor activated by many cytokines and growth factors. It plays a crucial role in cell survival, proliferation, and differentiation. Thereby, the Kupfer cells, the ligation of IL-10 with IL-10R1 and IL-10R2 leads to prolonged activation of STAT3, inhibiting inflammatory responses. In contrast, the ligation of IL-6 with IL-6R and gp130, which are expressed at high levels on Kupffer cells, leads to transient activation of STAT3, followed by the induction of inflammatory responses.72
Both neutrophils and macrophages have low HIF-1α in an oxygen-rich environment. When recruited to an inflamed site that is relatively hypoxic, the cellular level of HIF-1α is increased and will activate effector pro-inflammatory and bactericidal genes. This increase will induce phagocytotic activities that will be assisted with antimicrobial peptides such as cathelicidins and protein granules with direct antimicrobial activities. Nitric oxide synthase (NOS) which is generated by the increase in HIF-1α will produce nitric oxide (NO) that has antimicrobial properties which in turn will stabilize HIF-1α and enhance innate immunities. The increase in HIF-1α levels will ultimately increase the level of vascular endothelial growth factor (VEGF).73
Inflammation stimulates the production of cyclooxygenase (COX) 1 and COX 2, which will induce the production of prostaglandin E2 (PGE2). Prostaglandin E2,together with HIF-1α, will increase the production of VEGF.74 Vascular endothelial growth factor, CXCL12, endothelial monocyte activating peptide-II (EMAPII), and Angiopoietin-2 (Ang2) mediate extravasation of monocytes under hypoxic conditions. Moreover, VEGF plays a role in infectious disease by mediating angiogenesis and vasodilation while VEGF works together with histamine to increase vascular permeability during inflammation which causes plasma exudation.75
There are anti-inflammatory cytokines that serve to regulate inflammatory reactions such as IL-10 and IL-37. Interleukin-10 inhibits NF-κB by blocking IκB kinase activity and also interferes NF-κB in the nucleus76 while IL-37 suppresses NF-κBand blocking TLR4 from binding tothe host cell.77 Interleukin-37 is known to promote IL-10 production after cytokine secretion. Moreover, IL-37 upregulated STAT3 expression at genomic and proteomic. IL 37 reduces STAT3 phosphorylation.78 Interferon-γ is involved in regulating the immune response in autoimmune disease by suppressing the inflammatory response.79
Flavonoids in Miana leaf contains antibacterial, antioxidant, and anti-inflammatory effects1451 in which they increase the T lymphocytes, CD4 T cells, IFN-γ level, and TNF-α.34 Flavonoids can inhibit bacterial growth and kill microorganisms, especially gram-positive bacteria. The mechanism isinhibiting nucleic acid synthesis, destroying cell membrane, microsomes, and lysosomes, and increase cell permeability resulting in the elimination of the pathogens.11Syamsuri et al. in 2018 found that flavonoid has antibacterial activities by suppressing TLR4.14
Meanwhile, flavonoid effects on HIF-1α and VEGF are still not apparent. One study found that homoisoflavone-type methyl ophiopogonanone B (MOB), a flavonoid compound, is effective in inhibiting HIF-1α and regulating VEGF under hypoxic conditions.80
Miana Leaf Extract In Vitro Studies
MLE had a similar antibacterial activity with different concentrationsat 80% and 100% concentrations. MLE was sufficient enough to treat a broadspectrum of bacteria, such as Staphylococcus sp.and Streptococcus sp.toEscherichia coli and Pseudomonas aeruginosa.3481
Leaf Extract In Vivo/Animal Studies
Table 1: Summary of Studies for Miana Leaf Extract in Vivo/Animal Model
| Study | Animal used and exposure | Outcome | Results |
| Tari et al. 2013 82 | Rabbit incised on the right and left back | Wound healing | On the day 14, the wound that were given Miana leaves showed faster healing process and shorter wound lengths when compared with control group with difference in the wound lengths varied between 0,5 – 1,1 cm favoring miana leaves. |
| Marpaung et al. 2014 51 | Rabbit incised 1,5 cm on the back until subcutaneous tissue and then Staphylococcus aureus bacterial suspension 0.2 mL were given to each location. | Wound healing | On day 8, the wound that were given MLE (SEDM) 20%, 40% and 80% were all already perfectly closed/healed while the wound on gentamycin ointment still have 0.2 – 0.3 cm wound length. |
| Pakadang et al. 2015 34 | Adult male Wistar rats infected with Mycobacterium tuberculosis | Number of T-lymphocytes, CD4 T-Cells, IFN-γ and TNF- α levels
|
Administration of 510mg/kg BW of EDM to male rats caused a significant increase in T-lymphocyte, CD4 T-Cells proliferation and IFN-γ |
| Palette et al. 2017 84
|
Mice model infected with Mycobacterium tuberculosis
|
IL-10 mRNA expression
|
MLE was able to decrease IL-10 mRNA expression with mean difference (MD) of 0.951 and was statistically significant (P = 0.001) |
| Karo, 2018 77 | BALB/c mice
Infected with Candida albicans |
Fungi load and IL37 | Administration of 750 mg/kg BW of EDM to BALB/c mice show similar fungi load suppression and elevation of IL 37 as it compares to Ketoconazole group |
| Karo, 201885 | BALB/c mice infected with Candida albicans | IgM antibody | IgM antibody is decreased in MLE compared to the control group |
| Syamsuri, 2018 14 | BALB/c mice infected with Salmonella typhi | mRNA TLR4 | Administration of 510 mg/kg BW of Miana to BALB/c suppression of mRNA TLR4 expression |
| Amsyah, 2018 83 | Adult Wistar rats infected with A. actinomycetemcomitans | mRNA IL 10 | Administration of 510 mg/kg BW of Miana to rats elevate the expression mRNA IL 10 |
Table 1 summarizes the evidence regarding the benefit of MLE from in vivo/animal studies. Wound healing seems to also benefit from MLE application on the wound. A study by Tari et al.82 that by the 14th day since the incision, MLE applied to the wound showed a faster healing process and shorter wound lengths when compared with thecontrol group with the difference in the wound lengths varied between 0,5 – 1,1 cm favouringMLE. The wound which was given MLE 20%, 40% and 80% reveals a faster healing rate than gentamycin ointment.51
The first animal study of MLE on infection was published by Pakadang et al.2015. The study looked into the effect of MLE on intratracheal infection of M. tuberculosis in the Wistar rat model. 510mg/kg BW of EDM were able to increase the proliferation and the activation of CD4 T cells which in turn elevates IFN-γ production at the early stage of tuberculosis infection, leading to immunomodulation of the host.34
Another similar study on M. tuberculosis-infected rats found that Miana extract was able to decrease IL-10 mRNA expression. This finding contradicts another study by Amsyah 2018, which found Adult Wistar rats infected with A. actinomycetemcomitans experience elevation of mRNA IL-10 when Miana extract was given.8384
MLE has another potential immunomodulatory role as it playsan essential effect on IL-37. This was a study in BALB/c mice infected by Candida albicans model. MLE significantly increases IL-37 mRNA expression in the mouse model. The mechanism of action of IL-37 is still not exact, but IL-37 expression has been shown to suppress pro-inflammatory cytokines in experimental animals, it also exerts anti-inflammatory activities. A study by Syamsuri et al. on BALB/c mice infected with Salmonella typhi detects suppression of mRNA TLR4 expression, potentially reducing the prevalence of gram-negative infections.14
Miana Leaves Extract in Human Studies
To this day, there is no research on human have been published on the effects of MLE treating infectious disease. Therefore, there is plenty of opportunities to investigate its efficacy and safety in human studies.
Future Direction
There are several molecular mechanisms in the immune response that may be modulated by MLE. Firstly, we should investigate the effect of miana in sepsis condition as it compares to the standard antibiotic or its adjuvant role. Secondly, the strategic modulation through HIF-1a activation needs to be investigated whether Miana can alter HIF-1a expression in its role in adjuvant therapy to the antibiotic in infection treatment. Thirdly, the follow-up of cellular and tissue reaction following the expression of VEGF after HIF-1a activation should be explored. Then, we should recognize the active substance in MLE that play a critical role in the intervention. The translation research from animal to human studies need to be conducted immediately. There is a lot more research that needs to be done to explore thetherapeutic mechanism,optimal dosage, side effects, and adverse reaction to make MLE a standard herbal medication.
Conclusion
Infectious disease always becomesa health problem. Antimicrobial resistance is a great challenge in managing infectious disease. The role of antibiotics is limited, and the discovery of novel antibiotics has been obstructed in the last decade. The understanding of immune response atthe molecular level has created opportunities to enhance the correct immune response. Herbal medicine has been used for ages and empirically show benefit. Miana has demonstrated a potential role in modulating the immune system through enhancing CD 4 cell. Miana increases the production of IL 37 and IL 10 as anti-inflammatory cytokines. Furthermore, Miana alters TLR 4 expression in response to LPS on gram-negative bacteria. Therefore, Miana has a significant potential role in infectious disease management, whether as an alternative or adjuvant to the antibiotic. However, thereis plenty of other molecular mechanismsin various infection model that need to be explored.
Acknowledgement
I am very grateful to all those who have helped in the implementation of this review, especially Prof. Dr.dr. Eka JuliantaWahjoepramono, DR. dr.Jeno Wibisono, SpOG, Sp.BS, my colleges, laboratory staff, and my students, Gilbert Sterling, Ivan, Karunia, VikaDamay, Felix Kwenandar.
Conflict of Interest
There is no conflict of interest declared.
Funding Source
The authors received no specific grants from any funding agency in the public, commercial, or non-profit sector
References
- Camargo KR de. Closing the gap in a generation: Health equity through action on the social determinants of health. Glob. Public Health6:102–5 (2011).
CrossRef - Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. J. Am. Med. Assoc.281:61–6 (1999).
CrossRef - Hay SI,et al. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet390: 1260–1344 (2017).
- Jamilah J,Hatta M, Natzir R, Umar F, Sjahril R, Agus R,et al. Analysis of existence of multidrug-resistant H58 gene in Salmonella enterica serovar Typhi isolated from typhoid fever patients in Makassar, Indonesia. NewMicrobes and New Infections, 38, C,November 2020:1-6 (2020) doi:10.1016/j.nmni.2020.100793.
CrossRef - Amann S, Neef K, Kohl S. Antimicrobial resistance (AMR). Eur. J. Hosp. Pharm. 26: 175–7 (2019).
CrossRef - Nasution RA,Islam AA, Hatta M, Prihantono, Turchan A, Nasrullah, et al. Role of CAPE in reducing oxidative stress in animal models with traumatic brain injury. Ann. Med. Surg.57, 118–22 (2020) doi:10.1016/j.amsu.2020.07.036.
CrossRef - Nasution RA, Islam AA, Hatta M, Prihantono. Decreased neutrophil levels in mice with traumatic brain injury after cape administration. Ann. Med. Surg.54: 89–92 (2020). doi:10.1016/j.amsu.2020.04.015.
CrossRef - Kamelia, E. et al. Beta Amyloid Peptide Role in Animal Modeling Trial of Alzheimer’s Disease. Int. J. Sci. Basic Appl. Res.28: 90–9 (2016).
- Kamelia E, Miko H, Karo MB, Islam AA, Hatta M. Clinical Neurology and Neuroscience The Effect of Leaf Extract of Centella Asiatica on Neurogenesis and Bdnf Level in Hippocampus Cell Culture in Young Mice. Clin. Neurol. Neurosci.1, 14–9 (2017).
- Kamelia, E., Asadul Islam, A., Hatta, M., Miko, H. & Karo, M. B. R. Evaluation of the activity of F2-isoprostane in Alzheimer’s disease rats given banana extract. Pakistan J. Med. Heal. Sci.14(2):1459-564 (2020).
- Syamsuri F.Hatta M, Natzir R, Alam G, Massi MN, Dwiyanti Ret al. A review: Worldwide medicinal plants for typhoid fever. Indian J. Public Heal. Res. Dev.9:1461–465 (2018).DOI: 10.5958/0976-5506.2018.00938.5
CrossRef - Syarif LI, Junita AR, Hatta M, Dwiyanti R, Kaelan C, Sabir M,et al. A mini review: Medicinal plants for typhoid fever in Indonesia. Syst. Rev. Pharm.11(6):1171–80 (2020).doi:10.31838/srp.2020.6.170
- Karo MB,Tambaip T, Hatta M, Simanjuntak T, Irmawaty L, Rina T, et al. A mini review of Indonesian medicinal plants for Vulvovaginal candidiasis. Rasayan J. Chem.10 (4): 1280–8 (2017).
- Syamsuri F, Hatta M, Natzir R, Alam G, Massi MN, Bahar Bet al. Expression of TLR-4 in Salmonella typhi-Induced Balb/c Mice Treated by Miana Leaves (Coleus scutellaroides (L) Benth. Indian J. Public Heal. Res. Dev.9 (12): 1449–54 (2018).
CrossRef - Febriza A, Kasim VNA, Idrus HH, Hatta M. The effects of curcumin and vitamin D combination as inhibitor toward Salmonella typhi bacteria growth in vivo. Int. J. Appl. Pharm.11(5):116–20 (2019).DOI: http://dx.doi.org/10.22159/ijap.2019.v11s5.T0093
CrossRef - Djais, A. I. et al. Effect of the combination of demineralization freeze dried dentin matrix (DFDDM) and Moringa oleifera Lam on nuclear factor kapa B as a marker of bone. Syst. Rev. Pharm.11(4): 515–22 (2020).doi:10.31838/srp.2020.4.78
CrossRef - Idrus HH, Hatta M, Febriza A, Kasim VNA. Antibacterial activities of sapodilla fruit extract inhibiting Salmonella typhi on mice BALB/c. Int. J. Appl. Pharm.11(5): 121–6 (2019).http://dx.doi.org/10.22159/ijap.2019.v11s5.T0095
CrossRef - Simanjuntak T, Hatta M, Tahir AM, Sirait RH, Karo MB, Tambaib T, et al. Analysis of anti-toxoplasma immunoglobulin G and immunoglobulin M antibody levels after intervention with Curcuma Longa extract on early pregnant mice with acute toxoplasmosis. J. Glob. Infect. Dis.11: 25-9 (2019).DOI: 10.4103/jgid.jgid_28_18
CrossRef - Simanjuntak TP, Hatta M, Rauf S, Yusuf I, Tahir M. Forkhead box P3 messenger-RNA expression after Curcuma longa extract intervention in early pregnant mice with toxoplasmosis. Res. J. Immunol.11: 1-6 (2018) doi:10.3923/rji.2018.1.6.
CrossRef - SiraitLI, MassiMN, Hatta M, Prihantono. The effects of extract andaliman fruit ( Zanthoxylum acanthopodium Dc ) to CAMP mRNA expression and bacterial load in mice balb-C after Gardnerella vaginal Infection. Indian J. Public Heal. Res. Dev.9(11): 607-611 (2018). DOI: 10.5958/0976-5506.2018.01525.5
CrossRef - Tambaip T, Karo MB, Hatta M, Dwiyanti D, Natzir R, Massi MN,et al. Immunomodulatory Effect of Orally Red Fruit (Pandanus conoideus) Extract on the Expression of CC Chemokine Receptor 5 mRNA in HIV Patients with Antiretroviral Therapy. Res. J. Immunol.11: 15–21 (2018).DOI: 10.3923/rji.2018.15.21
CrossRef - Kamelia E, Islam AA, Hatta M, Kaelan C, Patellongi I. The Effect Of Administration Of Ethanol Extract From Musa Paradisiaca L. (MPL) Fruin On The Caspase-3 MRNA Gene Expression In Rat Amyloid Beta Induced An Alzheimer’s Disease Model. Asian J. Pharm. Clin. Res.11(4): 298-302 (2018).DOI: http://dx.doi. org/10.22159/ajpcr.2018.v11i4.24103
CrossRef - Bayan L, Koulivand PH, Gorji A. Garlic: a review of potential therapeutic effects. Avicenna J. phytomedicine4:1–14 (2014).
- Febriza A, Hatta M, Natzir R, Kasim VNA, Idrus HH. Activity of Antimicrobial Peptide; Cathelicidin, on Bacterial Infection. Open Biochem. J.13: 45–53 (2019).DOI: 10.2174/1874091X01913010045
CrossRef - Wijaya I,Taslim NA, Natzir R, Aman AM, Hatta M, Patellongi I,et al. Molecular and Immunological Mechanisms of Channa striata in Diabetic Wound Healing. Int. J. Pharm. Res.12(2): 279-89 (2020).DOI: https://doi.org/10.31838/ijpr/2020.SP2.046
CrossRef - Hudson JB. Applications of the Phytomedicine Echinacea purpurea (Purple Coneflower) in Infectious Diseases. J. Biomed. Biotechnol.2012: 1–16 (2012).
CrossRef - Royani I, As’ad S, Mappaware NA, Hatta M, Rabia. Effect of Ajwa Dates Consumption to Inhibit the Progression of Preeclampsia Threats on Mean Arterial Pressure and Roll-Over Test. Biomed Res. Int. (2019) doi:10.1155/2019/2917895.
CrossRef - Rosyidi RM, Januarman, Priyanto B, Islam AA, Hatta M, Bukhari A. The effect of snakehead fish (Channa striata) extract capsule to the albumin serum level of post-operative neurosurgery patients. Biomed. Pharmacol. J.12(2):893-99 (2019) doi:10.13005/bpj/1714.
CrossRef - Chavan SS.Damale, MG.Devanand B. Antibacterial and Antifungal Drugs from Natural Source: A Review of Clinical Development. in Natural Products in Clinical Trials.1:114–64 (2018).
CrossRef - David B, Wolfender JL, Dias DA. The pharmaceutical industry and natural products: historical status and new trends. Phytochem. Rev.14: 299–315 (2015).
CrossRef - Mulyawan E, Ahmad MR, Islam AA, Massi MN, Hatta M, Arif SK. Analysis of GABRB3 Protein Level After Administration of Valerian Extract (Valeriana officinalis) in BALB/c Mice. Pharmacogn. J.12(4): 821-27 (2020).DOI : 10.5530/pj.2020.12.118
CrossRef - Newman DJ, Cragg GM. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod.79: 629–661 (2016).
CrossRef - Karo MB, Kamelia E, Miko H, Simanjuntak TP, Hatta M. Effects of Herbal Plants on Candidiasis Vulvovaginalis Therapy. Am. J. Lab. Med.1: 65–68 (2016).
- Pakadang R, S. et al. Immunomodulator Potential of Miana Leaves (Coleus scutellarioides (L) Benth) in Prevention of Tuberculosis Infection. Am. J. Microbiol. Res.3:129–134 (2015).
- Lukhoba CW, Simmonds MSJ, Paton AJ. Plectranthus: A review of ethnobotanical uses. Journal of Ethnopharmacology (2006) doi:10.1016/j.jep.2005.09.011.
CrossRef - Suva M, Patel A, Sharma N. Coleus Species : Solenostemon scutellarioides. Inven. Journals2015:1–5 (2016).
- Tag H, Das AK, Loyi H. Anti-inflammatory plants used by the Khamti tribe of Lohit district in eastern Arunachal Pradesh, India. Nat. Prod. Radiance6: 334–340 (2007).
- Ridwan Y. et al. Efektivitas Anticestoda Ekstrak Daun Miana (Coleus blumei Bent) terhadap Cacing Hymenolepis microstoma pada Mencit Eff ectivity of Anticestode of Painted Ne le Extract (Coleus blumei Bent) Againts Hymenolepis microstoma in Mice.Media Peternakan.33: 6–11 (2010).
- Hardiyanti Y, Darwis D, Santoni A. Ekstraksi dan Uji Antioksidan Senyawa Antosianin dari Daun Miana (Coleus scutellarioides L. (Benth).) Serta Aplikasi pada Minuman. J. Kim. Unand2: 44–50 (2013).
- Li H, Wang Z, Liu Y. Review in the studies on tannins activity of cancer prevention and anticancer]. J. Chinese Med. Mater.26: 444–8 (2003)
- Hendra R, Ahmad,S, Sukari A, Shukor MY, Oskoueian E. Flavonoid analyses and antimicrobial activity of various parts of Phaleria macrocarpa (Scheff.) Boerl fruit. Int. J. Mol. Sci.12: 3422–3431 (2011).
CrossRef - Cushnie TPT, Lamb AJ. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents26: 343–356 (2005).
CrossRef - Anita, Basarang. Uji Daya Hambat Ekastrak Daun Miana (Coleus atropurpureus) Terhadap Escherichia coli Inhibiting Activity of Miana Leave (Coleus atropupureus) on Escherichia coli. J. Media Anal. Kesehat.10: 72–78 (2019).
CrossRef - Khotimah H, Agustina R, Ardana M. Pengaruh Lama Penyimpanan Terhadap Aktivitas Antioksidan Ekstrak Daun Miana (Coleus atropurpureus L. Benth). Proceeding Mulawarman Pharm. Conf.8: 1–7 (2018).
CrossRef - Nugroho AE. Manggis (Garcinia mangostana L.) : dari Kulit Buah yang Terbuang menjadi Kandidat Suatu Obat. Univ. Gadjah Mada12:1–9 (2007).
- Akiyama H, Fujii K, Yamasaki O, Oono T, Iwatsuki K. Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother.48: 487–491 (2001).
CrossRef - Cowan MM. Plant products as antimicrobial agents. Clinical Microbiology Reviews.12: 564–582 (1999).
CrossRef - Madduluri S, Babu RK, Sitaram, B. In vitro evaluation of antibacterial activity of five indigenous plants extract against five bacterial pathogens of human. Int. J. Pharm. Pharm. Sci.5: 679–684 (2013).
- Karou D. et al. Antibacterial activity of alkaloids from Sida acuta. African J. Biotechnol.4: 1452–1457 (2005).
- Sangi M, Runtuwene MRJ, Simbala HEI. Analisis Fitokimia Tumbuhan Obat Di Kabupaten Minahasa Utara. Anal. Fitokimia Tumbuh. Obat Di Kabupaten Minahasa Utara1:47–53 (2008).
- Marpaung PNS, Wullur AC, Yamlean PVY. Uji Efektivitas Sediaan Salep Ekstrak Daun Miana (Coleus Scutellarioides [L] Benth.) Untuk Pengobatan Luka Yang Terinfeksi Bakteri Staphylococcus Aureus Pada Kelinci (Oryctolagus Cuniculus). Pharmacon3: 2493 (2014).
- Levita J, Sumiwi SA, Pratiwi TI, Ilham E, Sidiq SP. Pharmacological Activities of Plectranthus scutellarioides (L.) R.Br. Leaves Extract on Cyclooxygenase and Xanthine Oxidase Enzymes. J. Med. Plants Res.10: 261–269 (2016).
- Triantafyllou A. et al. The flavonoid quercetin induces hypoxia-inducible factor-1α (HIF-1α) and inhibits cell proliferation by depleting intracellular iron. Free Radic. Res.41: 342–356 (2007).
CrossRef - Janeway CAJ, Travers P, Walport M. The major histocompatibility complex and its functions. Immunobiol. Immune Syst. Heal. Dis. Chapter 5.9-5.18 (2001).
- Henderson B, Poole S, Wilson M. Bacterial modulins: A novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol. Rev.60: 316–341 (1996).
CrossRef - Cox GM, Mukherjee J, Cole GT, Casadevall A, Perfect JR. Urease as a virulence factor in experimental cryptococcosis. Infect. Immun.68: 443–448 (2000).
CrossRef - Kirov SM, Castrisios M, Shaw JG. Aeromonas Flagella (Polar and Lateral) Are Enterocyte Adhesins That Contribute to Biofilm Formation on Surfaces. Infect. Immun.72: 1939–1945 (2004).
CrossRef - Pratt LA, Kolter R. Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol.30: 285–293 (1998).
CrossRef - Gouin E, Welch MD, Cossart, P. Actin-based motility of intracellular pathogens. Curr. Opin. Microbiol.8: 35–45 (2005).
CrossRef - Vecchiarelli A. Immunoregulation by capsular components of Cryptococcus neoformans . Med. Mycol.38: 407–417 (2000).
CrossRef - HillHR,et al. Group B streptococci inhibit the chemotactic activity of the fifth component of complement. J. Immunol.141: 3551–6 (1988).
- Munita JM, Arias, CA, Unit, A. R. & Santiago, A. De. HHS Public Access Mechanisms of Antibiotic Resistance. HHS Public Access4: 1–37 (2016).
- Dorak MT. Basic Immunology: Functions and Disorders of the Immune System. American Journal of Epidemiology.155 (2002).
CrossRef - Wang,N, Liang H, Zen K. Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Frontiers in Immunology. 5: (2014).
CrossRef - Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine.. 42: 145–151 (2008).
CrossRef - Sica A, Mantovani A. Macrophage plasticity and polarization: In vivo veritas. Journal of Clinical Investigation.122: 787–795 (2012).
CrossRef - Mosser DM. The many faces of macrophage activation. J. Leukoc. Biol.73,:209–212 (2003).
CrossRef - Splichal I. et al. High mobility group box 1 and TLR4 signaling pathway in gnotobiotic piglets colonized/infected with L. Amylovorus, L. mucosae, E. Coli nissle 1917 and S. Typhimurium. Int. J. Mol. Sci.20: 6294 (2019).
CrossRef - Parameswaran N, Patial S. Tumor necrosis factor-a signaling in macrophages. Critical Reviews in Eukaryotic Gene Expression. 20: 87–103 (2010).
CrossRef - Elkington PTG, O’Kane CM, Friedland JS. The paradox of matrix metalloproteinases in infectious disease. Clin. Exp. Immunol.142: 12–20 (2005).
CrossRef - Tanaka T, Narazaki M, Kishimoto T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol.6: 1–16 (2014).
CrossRef - Wang H. Lafdil F, Kong X, Gao B. Signal transducer and activator of transcription 3 in liver diseases: A novel therapeutic target. International Journal of Biological Sciences.7: 536–550 (2011).
CrossRef - Vink A. et al. HIF-1alpha expression is associated with an atheromatous inflammatory plaque phenotype and upregulated in activated macrophages. Atherosclerosis195: e69–e75 (2007).
CrossRef - Amrite AC, Ayalasomayajula SP, Cheruvu NPS, Kompella UB. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Investig. Ophthalmol. Vis. Sci.47: 1149–1160 (2006).
CrossRef - Karaman S, Leppänen VM, Alitalo K. Vascular endothelial growth factor signaling in development and disease. Dev.145: 142–60 (2018).
CrossRef - Driessler F, Venstrom K, Sabat R, Asadullah K, Schottelius AJ. Molecular mechanisms of interleukin-10-mediated inhibition of NF-κB activity: A role for p50. Clin. Exp. Immunol.135: 64–73 (2004).
CrossRef - Karo MB, Hatta M, Salma W, Patellongi I, Natzir, R. Effects of miana (Coleus scutellariodes (L) Benth) to expression of mRNA IL-37 in Balb/c mice infected Candida albicans. Pharmacogn. J.10(1): 16–19 (2018).DOI : 10.5530/pj.2018.1.3
CrossRef - Wang L, Quan Y, Yue Y, Heng X, Che F. Interleukin-37: A crucial cytokine with multiple roles in disease and potentially clinical therapy (Review). Oncol. Lett.15: 4711–4719 (2018).
CrossRef - Lee SH, Kwon JY, Kim SY, Jung KA, Cho ML. Interferon-gamma regulates inflammatory cell death by targeting necroptosis in experimental autoimmune arthritis. Sci. Rep.7: 2–10 (2017).
CrossRef - Hasebe Y. et al. Specific inhibition of hypoxia-inducible factor (HIF)-1α activation and of vascular endothelial growth factor (VEGF) production by flavonoids. Biol. Pharm. Bull.26: 1379–1383 (2003).
CrossRef - Karo MB, Salma W, Kamelia E, Patellogi I, Natzir R, Bintang M, Hatta M. Effects of ethanolic extract of Miana (Coleus scutellariodes [L] Benth) leaf on IgM profile in Balb/c mice with systemic of vulvovaginal candidiasis. Der Pharm. Lett.9(1): 6–11 (2017).
- Tari R, Posangi J, Wowor PM. Uji Efek Daun Iler (Coleus atropurpureus [L.] Benth.) Terhadap Penyembuhan Luka insisi Pada Kulit Kelinci (Oryctolagus cuniculus). J. e-Biomedik (2013) doi:10.35790/ebm.1.1.2013.4602.
CrossRef - Amsyah UK, Hatta M, Tahir H, Alam G, Asmawati A. Expression of IL-10 in A.actinomycetemcomitans induced rat treated by Purple Miana Leaves. Biomed. Pharmacol. J.12(4): 2099–21 (2019).doi : http://dx.doi.org/10.13005/bpj/1845
CrossRef - Palette T, Hatta M, As’ad S, Alam, G. Effect of Purple Miana Leaf (Coleus Scutellorioide (L) Benth) from Tana Toraja District on IL-10 mRNA Expression in Mice Induced Mycobacterium Tuberculosis. Int. J. Sci. Basic Appl. Res. Int. J. Sci. Basic Appl. Res.34: 111–115 (2017).
- Karo MB, Hatta M, Patellongi I, Natzir R, Tambaip T. IgM antibody and colony fungal load impacts of orally administered ethanol extract of plectranthus scutellarioides on mice with systemic candidiasis. J. Pharm. Pharmacogn. Res.6(1): 27–34 (2018).







