Manuscript accepted on :24-Nov-2020
Published online on: 04-01-2021
Plagiarism Check: Yes
Reviewed by: Dr. H. B. Nayaka
Second Review by: Manon Mani
Final Approval by: Ayush Dogra
Nokhanyo M. Ntshanka1 , Ikechukwu P. Ejidike1,2*
, Ikechukwu P. Ejidike1,2* , Fanyana M. Mthunzi1,3
, Fanyana M. Mthunzi1,3 , Makwena J. Moloto1
, Makwena J. Moloto1 , Kalenga P. Mubiayi4
, Kalenga P. Mubiayi4
1Department of Chemistry, Vaal University of Technology, Private Bag X021, Vanderbijlpark, 1911, South Africa.
2Department of Chemical Sciences, Anchor University, P.M.B. 001, Ipaja, Lagos, Nigeria.
3Institute of Chemical and Biotechnology, Vaal University of Technology Southern Gauteng Science and Technology Park, Sebokeng, 1983, South Africa.
4School of Chemistry, University of the Witwatersrand, Private Bag 3, Wits, 2050, South Africa.
Corresponding Author E-mail : iejidike@aul.edu.ng
DOI : https://dx.doi.org/10.13005/bpj/2043
Abstract
The use of herbal plants in recent years have increased tremendously owing to their user-friendliness, accessibility, and affordability. In this study, the plant species Combretum molle and Acacia mearnsii were phytochemical screened for the existence of active organic compounds, the content of total phenols, flavonoids and antioxidants using different solvents were investigated. The functional groups existing in the plants extracts were identified using FT-IR. The total phenol contents varied from 53.74 ± 5 – 97.29 ± 3 GAE mg/g and 53.74 ± 7 – 98.58 ± 2 GAE mg/g in the extraction powders for C. molle and A. mearnsii. In C. molle, the content of total flavonoids ranged from 76.90 ± 2 – 114.54 ± 0.18 QE mg/g, while 89.40 ± 3 – 105.45 ± 0.15 QE mg/g was found in A. mearnsii. The radical scavenging activities of the solvent extracts against DPPH and the controls revealed that acetone extract of C. molle displayed 99.64% DPPH inhibition, while A. mearnsii showed a maximum activity of 85.53% at 5 µg/ml. The antimicrobial activity of the two plant species was evaluated using diffusion disk and broth dilution methods. The activity by broth dilution of ethanol, methanol and acetone extracts of C. molle exhibited MIC values (39.06 and 78.13 mg/µL) against P. aeruginosa and E. coli strains respectively, while ethanol and chloroform extracts of A. mearnsii showed (39.06 and 78.13 mg/µL) against P. aeruginosa and E. faecalis respectively. Results from this study showed that the leave extracts constitute healthy supplements with antioxidant and antibacterial potentials that could be useful in traditional medicine for the treatment of numerous infectious ailments.
Keywords
Acacia Mearnsii; Antibacterial; Broth Dilution; Combretum Molle; Flavonoids; Folk Medicine
Download this article as:| Copy the following to cite this article: Ntshanka M. N, Ejidike I. P, Mthunzi F. M, Moloto M. J, Mubiayi K. P. Investigation into the Phytochemical Profile, Antioxidant and Antibacterial Potentials of Combretum Molle and Acacia Mearnsii Leaf Parts. Biomed Pharmacol J 2020;13(4). |
| Copy the following to cite this URL: Ntshanka M. N, Ejidike I. P, Mthunzi F. M, Moloto M. J, Mubiayi K. P. Investigation into the Phytochemical Profile, Antioxidant and Antibacterial Potentials of Combretum Molle and Acacia Mearnsii Leaf Parts. Biomed Pharmacol J 2020;13(4). Available from: https://bit.ly/3b7KnTo |
Introduction
Herbal medication is the utmost common form of medication used by large populace in the world, particularly people who cannot afford luxurious medications or have no direct access to good health care facilities some of the rural areas. Hence, it forms the foundation of all medication, the mother of all medication in use today. Medicinal plants exploited as traditional remedy alongside their therapeutic potential is well documented1,2. From previous studies, many African plants have demonstrated good antimicrobial activities against both gram-negative and gram-positive Multiple Drug Resistance (MDR) bacteria3. Interestingly, numerous plant species have been used for the treatment or served as a prophylaxis against several forms of both communicable and non-infectious diseases in South African traditional medicine4,5.
Species of the genus Combretum molle and Acacia mearnsii are some of the medicinal plants that are rich in natural bioactive compounds with protective or disease preventive properties such as primary and secondary metabolites. Such secondary metabolites include alkaloids, steroids, coumarins, essential oils, flavonoids, terpenoids, and anthraquinones1,2,5,6. These compounds are known to possess wide-ranging spectrum of biological potentials including anticancer, anti-inflammatory, antiprotozoal, antibacterial, anthelmintic, analgesic, antimycobacterial, antifungal and antioxidant1,5-9. C. molle and A. mearnsii indicate the availability of secondary metabolites having diverse activities in the plants. The different parts of these plant species have also been used for hookworm, fever, snake bite, menstrual disorders, leprosy, stomach pains, general body swelling and abortion, anti-inflammation, hypertension, and diarrhea, they also possess antibacterial and antioxidant properties8,9. Anthelmintic activity in lambs disease-ridden with Haemonchus contortus by faecal egg count reduction test have been exhibited by aqueous methanol extract from the stem-barks 8,9.
Medicinal plant extracts contain lot of flavonoids, alkaloids, starch, proteins, sugar, phenols, antioxidant molecules, quinones, non-soluble compounds like condensed tannins, lignin’s, and cell-wall bound hydroxycinnamic acids which all bearing the important compounds such as hydroxyl and carbonyl10. Phenolics are of importance owing to their wide range of ecological effects from organisms to ecosystem level10,11. Their most significant characteristic is their involvement in redox reactions and neutralization of active oxygen species12. Their free radicals scavenging abilities owing to the hydroxyl group highlights their significance. This contribute directly to their antioxidant action. The phenolic oxidants also provide for their anti-inflammatory, antimicrobial, spasmolytic and neuroprotective actions13,14,15. The World Health Organization (WHO) approximates that about 80% of the unindustrialized nations population uses herbs for some aspect of therapeutic purposes and ailment control traditional medicine2,16.
Free radicals and active oxygen species cause oxidation which contributes to additional one hundred syndromes in humans as well as cell damage, ischemia, reperfusion injury of many tissues, atherosclerosis, gastritis, central nervous system injury, arthritis, cancer and AIDS1,2,13. Antioxidants are constituents that are found in plants possessing free radical chain reaction breaking properties10,17. Antioxidant are classified as enzymatic and non-enzymatic. Naturally occurring antioxidants (obtained from plants) such as ascorbic acid, carotenoids, and phenolic compounds are of great benefit and more effective than the synthetic14,17. Their use doesn’t persuade side effects, while synthetic (or artificial) antioxidants have been found to possess genotoxic effects17,18. They are known to inhibit lipid peroxidation, to scavenge free radicals and active oxygen species6,15.
In this study, the plant species of C. molle and A. mearnsii were phytochemically screened for the presence of active organic compounds, the content of total phenols, flavonoids and antioxidants using different solvent extracts were investigated. The functional groups existing in the medicinal plant extracts were documented using FT-IR.
Materials and Method
Methanol (99.5%), Acetone (99.8%), Ethanol, Chloroform, Acetone (99.8%), 2.2-diphenyl-1-picrylhydrazyl (DPPH), Quercetin (95.5%), Folin-Ciocalteu reagent, Sodium Carbonate, Gallic acid (97.5%), Aluminum chloride (AlCl3) (97%), Ascorbic acid (99.5%), Vanillin, Ethyl acetate, p-iodonitrotetrazolium chloride (INT), Sulphuric acid were obtained from Aldrich-Sigma and Merck (Johannesburg, South Africa). Muller-Hinton Agar (MHA), Muller-Hinton broth (MHB), Malt-Extraction broth (MEB), Malt-Extraction Agar (MEA), Neomycin, Amphotericin, were analytical grade, and were purchased from Neogene. Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Candida albicans, and Enterococcus faecalis were obtained from Anatech. Combretum molle and Acacia mearnsii plant leaves were collected at Vaal University of Technology, Vanderbijlpark Campus in the province of Gauteng, South Africa.
The leaves of (C. molle and A. mearnsii) collected were washed with running water; air dried and crushed to a fine dust with a laboratory blender. The plant powder material was stored in a sample container at room temperature in the dark for additional examination. The plant material (2.0 g) was transferred to four different beakers and 200 ml of solvents with various polarities (chloroform, methanol, acetone and water/ ethanol) was added to each beaker, respectively, and stored in a dark place at room temperature. After 24 h, infusions were sieved through Whatman No. 1 sieve paper and the deposit was re-extracted with equal capacity of solvents to ensure maximum extraction of organic compounds. After 24 h the procedure was repeated, and the combined supernatants evaporated to dryness under fume hood. The obtained dry materials were kept in sample vials and stored in the refrigerator for the characterization of phenols, flavonoids, and antioxidant.
The crude methanolic extract of the two plants was re-dissolved in methanol. Few drops of each extract (C. molle and A. mearnsii) were spotted on the line near the bottom of Thin Layer Chromatography (TLC) plates. The TLC plate was then placed on a shallow pool of different mobile solvent systems: ethyl acetate: methanol: water (EMW, 30:4.05:3, v/v/v)16,19 in a developing chamber such that only the bottom of the plate is in the liquid and the chamber was closed. The plate was removed from the developing chamber, air dried and squirted with the vanillin-sulphuric acid reagent (0.1 g vanillin, 1 ml sulphuric acid, 28 ml methanol) for the discovery of higher alcohols, phenols, essential oils, and steroids. The plate was air dried again then heated in oven at 105 ºC for 5 – 10 min. The process was repeated again but this time instead of using vanillin spray, DPPH was used. The plate was sprayed with DPPH spray (0.1 g of DPPH in 100 ml of methanol), heated at 105 ºC until the colour of chromatograms were optimally developed. The presence of antioxidant ingredients was distinguished by the colour change. The DPPH radical scavenging potentials of the plant samples were also enumerated spectrophotometrically at wavelength of 517 nm (T80+, PG Instruments Ltd) following standard method2,16.
The phenols concentration in plant extracts was determined by means of Folin-Ciocalteu reagent method2,20. Concentration of 1 mg/ml for the methanolic solution of each plant extracts used for the analysis. The reaction blend was ready by mixing 1 ml of (0.1 mg/ml) phenolic solution of the extract, 1.5 ml of Folin-Ciocalteu reagent, and 1.5 ml of sodium carbonate (0.05 mg/ml). The sample was vortexed and thereafter hatched for 1 h at room temperature, in the dark. The absorbance of entirely test samples was measured using UV-spectrophotometer at continuous wavelength 750 nm (T80+, PG Instruments Ltd). Samples preparation were carried out in triplicate for each analysis, then after, the mean value of absorbance was estimated. The standard curve of Gallic acid solution was prepared by dissolving 3 mg of Gallic acid in methanol (10 ml) to produce a concentration of 300 mg/l. The calibration curve was prepared using a working concentration of 0, 10, 20, 30, 50, 80, and 100 mg/L in methanol. The concentration of phenols was read from the calibration curve and articulated in terms of Gallic acid equivalent (mg of GAE/g of plant materials). The obtained results were stated as mean values ± standard deviation (mean ± SD), n = 3.
Aluminum chloride colorimetric technique was utilized for determining the concentration of flavonoids in plant extracts2,5,6. The concentration (0.1 mg/ml) of plant extract solution was used for each plant in the analysis. The 3 ml of the sample was mixed with 0.2 ml of 10 % aluminum chloride (0.25 into 10 ml), 5.6 ml of distilled water, and 0.2 ml of 1 M potassium acetate. The samples were vortexed and thereafter incubated for 30 min at room temperature. The absorbance of the reaction mixtures was measured at 420 nm (T80+, PG Instruments Ltd). Samples preparation were carried out in triplicate for each analysis, then after, the mean value of absorbance was estimated. The standardization curve was obtained following the preparation of quercetin solution at concentrations 0 to 100 mg/l in methanol. The concentration of flavonoids was determined from the standardization curve and expressed by quercetin equivalent (mg of QE/g of extract). The obtained results were stated as mean values ± standard deviation (mean ± SD), n = 3.
The capability of the plant extract to eliminate 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals was measured by adopting standard technique with the appropriate modification2,16,21. The stock solution of species extracts was prepared for each solvent to attain the concentration of 100 ppm. The stock solution was used to prepare the working solution to attain (0.1, 0.2, 0.3, 0.4, 0.5 mg/ml) concentrations in a 50 ml flask. Working concentrations (3 ml) of plant extracts were vortexed with 3 ml of DPPH (2 mg/100 ml CH3OH). Ascorbic acid and Gallic acid (Sigma–Aldrich, South Africa), was employed as the positive control. The incubation of the mixtures was carried out for 30 min at room temperature together with the standards in a dark place. The absorbance was recorded at 517 nm by means of spectrophotometer (T80+, PG Instruments Ltd). The control sample contained entirely the components except the extract. Percentage inhibition was estimated from % inhibition = Scavenged DPPH % = [(A0 – A1) / A0] × 100% where, A0 is the control absorbance, A1 is the extract absorbance, whilst IC50 values were obtained from the % inhibition versus concentration plot, by means of a non-linear regression algorithm. The obtained results were stated as mean values ± standard deviation (mean ± SD), n = 3.
The antimicrobial study of the C. Molle and A. Mearnsii extracts was carried out by testing these against Gram (+ve) bacteria (S. aureus and E. faecalis), Gram (-ve) bacteria (P. aeruginosa and E. coli) and fungal (C. albicans). The disk diffusion technique was first used for the antimicrobial activity screening, followed by Minimal Inhibitory Concentration method, while Neomycin served as a positive control for bacterial and Amphotericin for fungi.
Maintenance of the stock cultures was observed at 4 °C on slopes of nutrient agar. Active cultures (S. aureus, E. coli, C. albicans, E. faecalis, and P. aeruginosa) for the test were made ready by the transfer of a loop full of cells from the standard cultures to a flask containing a Muller-Hinton broth (MHB), Muller-Hinton Agar (MHA), and incubation at 37 °C for 24 h. Disk diffusion pates for bacterial test were prepared by weighing 10 g of Malt-Extraction Agar (MEA) into 500 ml flask and addition of 200 ml distilled water was. The solute was dissolved using a microwave, thereafter autoclaved was cooled to room temperature for 1 h. The plates were prepared by transferring the molten media (15 ml) into a sterile disk plates under sterilised fume hood. Disk diffusion plates for fungal test follows same process but 7.6 g of Muller-Hinton Agar (MHA) was used in place of Malt-Extraction Agar. The disk plates were left for 10 min. to solidify. Uniformly swabbed inoculum suspension was allowed to set for 5 min. A well of 6 mm was created on a disk and 50 µL (0.06 mg/L) of the samples each was added. The samples were permitted to diffuse for 5 min., then incubated at 37 °C for 24 h. In general, antimicrobial agent diffuses into agar during the incubation period and prevents the growth of the test microorganisms, and the diameter of their inhibition zones are measured.
Minimum inhibitory concentration (MIC) of the plant agents were assessed using the micro dilution bioassay as described2,16. Overnight cultures (incubated at 37 ℃) of S. aureus, E. coli, C. albicans, E. faecalis, and P. aeruginosa strains were each diluted with sterile Mueller-Hinton broth (MHB) to attain a final inoculum of ≈ 106 CFU/ml. 60 mg of plant extracts were each dissolved in 3 ml of 0.17% ethanol solution to achieve a concentration of 20.0 mg/ml. 100 microliters of each solution was diluted serially in two-fold with sterilized Mueller-Hinton broth in a 96-well microliter plate for individual bacterial strains. Neomycin (20 mg/ml) was two-fold diluted as the positive control, while distilled water was applied as negative control. 100 microliters of each bacterial culture were added to individual well. Growth of bacterial was observed via the addition of 0.2 mg/ml p-iodonitrotetrazolium chloride (50 µl), followed by plates coverage and incubation at 37 ℃ for 24 h. Subsequently, the colorless tetrazolium salt was biologically reduced to a red product owing to the existence of energetic organisms. The MIC values were determined as the concentration in the last wells with no colour alteration detected. In the wells, bacterial growth was designated by a reddish-pink colour. Two replicates per assay was carried out for this assay.
Results and Discussion
The functional groups of Combretum molle and Acacia mearnsii have been identified using Fourier Transform Infrared spectroscopy (Figure 1). The vibrations include ν(C=C) which is observed at 1594 cm-1, stretching vibration of the phenolic ν(C-O) observed at 1012 cm-1, ν(C=O) at 1705 cm-1, while 2915 cm-1 corresponding to symmetric and asymmetric aliphatic ν(CH2/CH3) and ν(O-H) showed a broadband at 3228 cm-1, an indication of hydroxyl group of the C. molle extracts. Similar vibrations were observed in A. mearnsii at 1605 cm-1, 1015 cm-1, 1696 cm-1, 2994 cm-1, 2918 cm-1 and 3280 cm-1 corresponding to ν(C=C), stretching vibration of the phenolic ν(C-O), ν(C=O), ν(CH2/CH3) and a strong broad band ν(O-H) respectively (Table 1). Following the above identifications, C. molle and Acacia mearnsii can be said to contain phenols, flavonoids, antioxidants, tannins, etc.2,6,20.
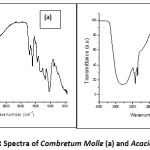 |
Figure 1: FT-IR Spectra of Combretum Molle (a) and Acacia Mearnsii (b) |
Table 1: Functional Groups Present in Leave Extracts of Combretum Molle and Acacia Mearnsii
| Functional groups of interest | Wavenumber (cm-1) | |
| Combretum mole (a) | Acacia mearnsii (b) | |
| ν(O-H) | 3228 | 3280 |
| ν(CH2/CH3) | 2915 | 2994, 2918 |
| ν(C=O) | 1705 | 1696 |
| ν(C=C) | 1594 | 1605 |
| ν(C-O) | 1012 | 1015 |
The mobile phase systems used for this study was the ethyl acetate/water/methanol (EWM, 30:3:4.05). The phase showed a good separation (Figure 2). The TLC plates developed was squirted with vanillin-sulphuric acid reagent and heated at 105 °C in order to allow for colour development indicating the presence of chemical constituents in the plant extracts. Visualization of the separated compounds was accomplished by natural colour in hours of daylight or by fluorescent quenching on 254 nm or 366 nm2,16,19.
The characteristic blue and green fluorescence indicates the existence of flavonoids while yellow colour after spraying with DPPH indicates the antioxidant capabilities of the test samples16,22. Also, stilbenes are indicated by bright red to dark pink colour, while proanthocyanins is pink ion colour2,16,19. C. molle and A. mearnsii demonstrated various characteristic colour of the compounds of interest and confirmed the presence of phenols, flavonoids and antioxidant capabilities, respectively. Masoko and Eloff23 observed that acetone and methanol constituents of some South African species exhibited good scavenging activities afterward spraying the TLC chromatogram with 2,2-diphenyl-1-picrylhydrazyl (DPPH). Flavonoids are known to possess pharmacological and biochemical effects including antiviral, anti-inflammatory, diuretic, anti-allergic, and antispasmodic1,2,7.
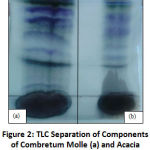 |
Figure 2: TLC Separation of Components of Combretum Molle (a) and Acacia Mearnsii (b) Methanolic Plant Extracts. |
According to literature, presence of phenolic compounds is responsible for the antioxidant activity of plants1,10. The plant constituent samples with flavonoids and phenolic compounds possess biological potentials including antiatherosclerosis, endothelial function improvement, and protection of the cardiovascular alongside the angiogenesis inhibition and cell antiproliferation potentials1,2,10,16. The biological activities of flavonoid compounds are connected to their antioxidant activity as suggested by some evidence. Also, bioactive flavonoids have also been investigated for potent anti-viral, anti-fungal, and anti-bacterial activity1,13,17. Phytochemicals such as tannins and saponins possess reported anti-inflammatory effects, cholesterol lowering, allelopathic, and anticancer properties1,2,6.
In this report, phenolic and flavonoids compounds total content in C. molle and A. mearnsii were investigated to estimate their antioxidant activity. The phenolic content of C. molle and A. mearnsii plants was determined using Folin-Ciocalteu reagent also known as Gallic Acid Equivalence technique and therefore Gallic acid served as the reference material for the determination of phenols. The results obtained were expressed as mg Gallic acid Equivalents (GAE) per gram of plant material. Aluminum chloride colorimetric technique was utilized for the assessment of flavonoids and quercetin was used as a reference because they are natural flavonoids that are found abundantly in plants. This is expressed as Quercetin Equivalence QE method. The relationship of total flavonoid and phenol contents with antioxidant activities have been previously reported2,10,16,19.
The crude plant extracts of both C. molle and A. mearnsii were tested for the total concentration of phenols using solvents with different polarities. Calibration of instrument was ascertained, and the calibration curve for phenols obtained using the standard curve equation and regression: y = 0.0031x + 0.0424, r2 = 0.9967 (Figure 3a). The phenolic content results of C. molle and A. mearnsii extracts for each solvent are presented in Table 2. For C. molle, chloroform, methanol, acetone and water extracts gave phenol contents of 89.87 ± 2.0, 84.07 ± 3.0, 97.29 ± 5.0 and 53.74 ± 3.0 mg GAE/g respectively. In A. mearnsii, chloroform extract gave the phenolic content of 53.74 ± 7.0 mg GAE/g, methanol extract gave a concentration of 89.87 ± 3.0 mg GAE/g. Acetone extract showed the phenol contents of 98.58 ± 2.0 mg GAE/g, the highest content followed by water extract with a phenolic content of 96.65 mg GAE/g. The results revealed that both C. molle and A. mearnsii acetone extracts gave the highest concentration of phenol. The lowest content of phenols in C. molle was observed in methanol extract whereas in A. mearnsii, it was observed in chloroform extract. Zlotek et al.24 conveyed that C. molle acetone extracts gave the highest phenolic content. The solvents polarities and extraction procedures can be accountable for the solubility of the endogenous constituents of the plants, thereby giving various polar or non-polar components2,10,16.
The methanol extract gave the least phenolic concentration in comparison to the other three solvents of extracts with a significant difference value of >13 mg GAE/g. Chloroform gave the lowest concentration of phenols with a significant difference value of >3 mg GAE/g. These significant values show that the total concentration of phenols highly depends on the solvent used for extraction. A. mearnsii produced slightly higher phenolic concentration than C. molle. Phenolic compounds are significant plant ingredients exhibiting redox potentials accountable for antioxidant activities. They are important owing to their function as scavengers of the free radicals in the human systems and support to the maintenance of a healthy body as reactive oxygen species (ROS) are being scavenged or removed2,10. The obtained data obtain for all extracts in this report show that C. molle and A. mearnsii leave extracts are comparatively good basis of antioxidant activity.
Table 2: Total Phenol and Flavonoid Contents in Combretum Molle and Acacia Mearnsii
| Plant name | Extracts | Total Phenolic (mg GAE/g) | Total Flavonoids (mg QE/g) |
| Combretum mole | Chloroform | 89.87 ± 2.0 | 89.68 ± 1.0 |
| Methanol | 84.07 ± 3.0 | 114.54 ± 0.2 | |
| Acetone | 97.29 ± 5.0 | 103.40 ± 0.2 | |
| Water | 53.74 ± 3.0 | 76.90 ± 2.0 | |
| Acacia mearnsii | Chloroform | 53.74 ± 7.0 | 89.40 ± 3.0 |
| Methanol | 89.87 ± 3.0 | 95.93 ± 0.9 | |
| Acetone | 98.58 ± 2.0 | 105.45 ± 0.2 | |
| Water | 96.65 ± 3.0 | 90.24 ± 1.0 |
Flavonoids have been reported to exhibit anti-viral, anti-fungal, and anti-bacterial activities1,17. Flavonoids are natural antioxidants. The concentration of flavonoids in C. molle and A. mearnsii plant extracts were determined with following the aluminum (III) chloride colorimetric technique. The results were obtained from the standard curve equations and regression: y = 0.0072x + 0.0023, r2 = 0.9976 (Figure 3b). The flavonoid content results of the plant extracts for each solvent are presented in Table 2. The results showed that content of flavonoids in C. molle varied from 76.90 ± 2.0 to 114.54 ± 0.18 mg QE/g. In C. molle, the solvents extracts: chloroform, methanol, acetone and water gave the flavonoids contents of 89.68 ± 1.0, 114.54 ± 0.2, 103.40 ± 0.2 and 76.90 ± 2.0 mg QE/g, respectively. Methanol extract gave the highest flavonoid content among the various extracts, while the least quantities of flavonoids originate water extract of C. molle (76.90 ± 2.0 mg QE/g). In A. mearnsii, the content of flavonoids varied from 89.40 ± 3.0 to 105.45 ± 0.15 mg QE/g. Chloroform, methanol, acetone and water had their flavonoid content to be 89.40 ± 3.0, 95.93 ± 0.9, 105.45 ± 0.2 and 90.24 ± 1.0 mg QE/g, respectively. Acetone had the highest flavonoid content followed by methanol. The trend of solvent extract for total flavonoid contents in C. molle can be in the range: methanol > acetone > chloroform > water; while A. mearnsii ranged as follows: acetone > methanol > water > chloroform.
The solvent extract trends observed agrees with the previous studies of Velicković et al.25 and Motsumi et al.16, signifying the importance of organic solvents and medicinal plant materials relationships. Flavonoids are secondary metabolites with biological activities including antiprotozoal, analgesic, antibacterial, anticancer, antioxidant, anti-inflammatory. Their activities depend on the position, position, and the number of free OH groups2,7,10. C. molle and A. mearnsii exhibited the availability of secondary metabolites owing to diverse activities in the plants. The high contents of these phytochemicals in these plant extracts further support their radical scavenging activity.
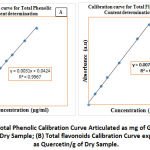 |
Figure 3: Total Phenolic Calibration Curve Articulated as mg of Gallic Acid/g of the Dry Sample; (B) Total flavonoids Calibration Curve expressed as Quercetin/g of Dry Sample. |
An easy, quick and sensitive technique for determining the content of antioxidant in plant extracts was the radical scavenging assay (RSA) by means of 2,2-diphenyl-1-picrylhydrazyl (DPPH) stable radical spectrophotometrically. When the antioxidants are present, the DPPH radical obtain one electron from the plant extract and this led to a decrease in absorbance2,5,6,16,21. A known stable organic free radical is DPPH, that loses its absorption band in the range of 515 – 518 nm by accepting an electron or a radical species2,10.
The DPPH radical scavenging assay was carried out using extracts from C. molle and A. mearnsii of different solvents including methanol, ethanol, acetone, and chloroform. The data are presented in form of line graphs together with ascorbic acid and Gallic acid which were used as reference standards. The antioxidant capacity of the leave extracts of the test plants were utilized to assess the bioactive extracts as determined by the TLC-DPPH technique. It is interesting to note that methanolic extract of C. molle displayed 99.54% inhibition of the radical scavenging activity at 0.5 mg/ml and 99.3% at 0.1 mg/ml, while ascorbic acid presented the lowest radical scavenging activity of 99.37% at 0.1 mg/ml and maximum activity of 99.52% at 0.5 mg/ml (Figure 4a). This is an indication of a high scavenging capacity. Meanwhile, Figure 4b displayed the results of methanolic extract of A. mearnsii, revealing that the maximum DPPH scavenging activity of this extract was observed at 99.39% for 0.5 mg/ml and 99.22% at 0.1 mg/ml while ascorbic acid showed a maximum activity of 99.52% at 0.5 mg/ml and a minimum activity of 99.37% at 0.1 mg/ml.
Methanol, acetone, chloroform and ethanol exhibited DPPH scavenging activities (IC50, mg/ml) to be 82.12, 43.99, 57.21 and 46.83 respectively in C. molle (Table 4). The extract fractions inhibited DPPH radical (IC50) in the range 43.99 – 82.12 mg/mL for C. molle and 3.79 – 111.75 mg/mL for A. mearnsii, with methanol (111.75 mg/ml), acetone (23.79 mg/ml), Chloroform (48.11 mg/ml) and ethanol (33.94 mg/ml). The IC50 values of the plant extracts represent the concentration of the test extracts where the inhibition of test sample activity at 50%. These results disclosed that extracts of acetone, being a polar solvent exhibited good radical scavenging activities trailed by ethanol than other solvents. Though, the potential displayed by the plant extracts in this study are low as compared to those of the standard agents: ascorbic acid (IC50 = 17.56 mg/ml) and Gallic acid (IC50 = 14.99 mg/ml). DPPH radical scavenging activity of solvents on C. Molle showed the order: Gallic acid > ascorbic acid > ethanol > acetone > chloroform > methanol and for A. Mearnsii, showed the order: Gallic acid > ascorbic acid > acetone > ethanol > chloroform > methanol.
The low radical scavenging potentials of the DPPH assay can be connected with the amount of the phenolic compounds present in the extract fractions and also the solvent types5,10. Therefore, both plants showed their highest scavenging activity in acetone extracts. The highest phenolic content in acetone extracts for both plants explains the highest scavenging activities of DPPH by both plant extracts. Acetone extract also showed the highest flavonoids content in A. mearnsii and this might have contributed to its highest scavenging activity2,10.
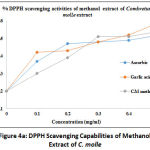 |
Figure 4a: DPPH Scavenging Capabilities of Methanol Extract of C. Molle |
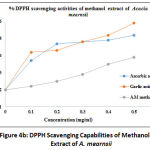 |
Figure 4b: DPPH Scavenging Capabilities of Methanol Extract of A. Mearnsii |
Table 3: DPPH Radical Scavenging Potentials of C. Molle and A. Mearnsii Solvent Fractions (IC50)
| Name of Plant | Solvent of Extracts | IC50 (mg/ml) |
| Combretum mole | Chloroform | 82.12 |
| Methanol | 46.83 | |
| Acetone | 57.21 | |
| Water | 43.99 | |
| Acacia mearnsii | Chloroform | 111.75 |
| Methanol | 23.79 | |
| Acetone | 48.11 | |
| Water | 33.94 |
The anti-bacterial and anti-fungal potentials of the C. molle and A. mearnsii different solvent extracts were investigated against bacterial strains: gram-positive bacteria (E. faecalis and S. aureus), gram-negative bacteria (P. aeruginosa and E. coli) and fungal (C. albicans).
A disk diffusion and MIC methods have been used to test for the anti-bacterial and anti-fungal of the extracted active ingredients of C. Molle and A. Mearnsii. The sensitivity determination was carried out by measuring the inhibition zone which is the area of media where bacteria are unable to grow due to their sensitivity towards the antibacterial agent. A large zone of inhibition is an indication of high sensitivity of bacteria to the antibiotic drug. The results of plants extract according to their solvents are listed in Table 5. Amongst the antibacterial agents that were used, E. coli showed more sensitivity towards the acetone extracts of C. Molle with the highest inhibition zone of 24 mm. P. aeruginosa, showed maximal sensitivities towards ethanol and methanol extracts of A. Mearnsii with inhibition zone of 23 mm followed by acetone extract of C. Molle with inhibition zone of 22 mm against S. aureus and P. aeruginosa. E. Faecalis showed more sensitivity towards acetone extracts of C. Molle with inhibition zone of 20 mm. C. albicans had more sensitivity towards ethanol extracts of C. Molle with inhibition zone of 32 mm.
After the screening of the antimicrobial activity via zone of inhibition, a more accurate and quantitative method (MIC) was used to determine the lowest concentration of the antimicrobial agent to inhibit bacterial activity. The results are displayed in Table 6. It was observed that all the microorganism was susceptible to the ethanol, acetone, chloroform and methanol extracts of C. Molle and A. Mearnsii. Gram-negative (E. coli) was found to be resistant microorganism towards the extracts, this can be due to their cell membrane composition2,3. Ethanol, methanol and acetone extracts of C. Molle showed the highest antimicrobial activity towards E. coli with minimum inhibition concentration of 78.13 mg/µL. This gives an indication that the fractions possess broad range of antimicrobial activity2,10,16. Gram-negative (P. aeruginosa) showed high sensitivity towards ethanol, methanol, chloroform and acetone extracts of C. Molle; ethanol, acetone, and chloroform extracts of A. Mearnsii with a minimum inhibition concentration of 39.06 mg/µL. Studies have shown that herbal extracts with low MIC values have recognized a good frontline of bioactive constituents possessing antimicrobial power1,2,7,25.
E. Faecalis showed high resistance towards the selected antimicrobial agents, especially the chloroform extracts of C. Molle which requires a minimum concentration of 10 000 mg/µL to inhibit its growth. The low activities of C. Molle and A. Mearnsii extracts against the investigated strains might be accredited to the bacterial high resistance and cell wall thickness shaped by repeating units of peptidoglycan coatings, as a result of an additional external membrane in their cell wall which acts as hindrance to the antimicrobial agents such as phenolic compounds existing in plant extracts5,10,16. Nonetheless, this microorganism showed sensitivity towards the ethanol and acetone extracts of A. Mearnsii with a MIC of 78.13 mg/µL. Only ethanol, chloroform and methanol extracts of A. Mearnsii showed the highest antimicrobial activity towards C. albicans with MIC of 78.13 mg/µL. It was observed that C. Molle and A. Mearnsii extracts possess moderate to good anti-bacterial and anti-fungal activity owing to their activity against the investigated pathogenic strains. The in vitro antibacterial potentials of the investigated extracts in this present study demonstrated substantial activities against Gram(+) bacteria and Gram(-) bacteria. The activities might be associated with the soluble phenolic, flavonoid, and polyphenolic compounds found efficient as antimicrobial constituents against a varied collection of microorganisms investigated in vitro5,14,15,17,18,26.
Table 4: Agar Disk-Diffusion for Screening the Activities of C. Molle and A. Mearnsii Extracts
| Zone of Inhibition (mm) | ||||||
| Extracts | Solvent for Extraction | E. faecalis | S. aureus | P. aeruginosa | E. coli | C. albicans |
| Combretum molle | ethanol | 15 | 17 | 11 | 12 | 32 |
| Combretum molle | methanol | 14 | 19 | 14 | 16 | 28 |
| Combretum molle | acetone | 20 | 22 | 22 | 24 | 23 |
| Combretum molle | chloroform | 14 | 16 | 8 | 10 | 23 |
| Acacia mearnsii | ethanol | 12 | 12 | 23 | 11 | 18 |
| Acacia mearnsii | methanol | 6 | 7 | 23 | 14 | 22 |
| Acacia mearnsii | acetone | 16 | 13 | 11 | 20 | 11 |
| Acacia mearnsii | chloroform | 12 | 13 | 8 | 6 | 20 |
Table 5: Minimum Inhibitory Concentrations (MICs) Determination of C. Molle and A. Mearnsii Extracts by Broth Dilution
| Minimum Inhibitory Concentrations (MICs) (mg/µL) | ||||||
| Extracts | Solvent for Extraction | E. faecalis | S. aureus | P. aeruginosa | E. coli | C. albicans |
| Combretum molle | ethanol | 312.5 | 156.3 | 39.06 | 78.13 | 156.3 |
| Combretum molle | methanol | 1250 | 156.3 | 39.06 | 78.13 | 156.3 |
| Combretum molle | acetone | 1250 | 156.3 | 39.06 | 78.13 | 156.3 |
| Combretum molle | chloroform | 10000 | 156.3 | 39.06 | 10000 | 156.3 |
| Acacia mearnsii | ethanol | 78.13 | 625 | 39.06 | 2500 | 78.13 |
| Acacia mearnsii | methanol | 312.5 | 78.13 | 156.3 | 156.3 | 78.13 |
| Acacia mearnsii | acetone | 78.13 | 156.3 | 39.06 | 312.5 | 625 |
| Acacia mearnsii | chloroform | 625 | 156.3 | 39.06 | 10000 | 78.13 |
Conclusion
Herbal products are well acknowledged natural precursors for numerous diseases treatment since time immemorial. The information and remarks obtained from the study demonstrated that Combretum molle and Acacia mearnsii signpost the availability of secondary metabolites possessing diverse activities in the plants. The different parts of these plant species have also been used for snake bite, menstrual disorders. The two medicinal plants were studied for their phenolic, flavonoids and antioxidant activity by extracting the active ingredient using different solvents. It was discovered that in both plants, acetone extracts showed the highest phenolic content. When the two plants were studied for flavonoids content, methanol showed a highest content in C. Molle, while acetone showed highest content in A. Mearnsii. Radical (DPPH) scavenging activities of the solvent fractions of showed that ethanol extract of C. molle exhibited the highest scavenging, while acetone extract of A. mearnsii showed the highest scavenging activity. When these plants extracts were tested for antibacterial activities according to their solvent, ethanol extracts from both plants showed the highest sensitivity towards all the microorganisms studied. The minimum inhibitory concentration values demonstrated that the leaves extract exhibit potential for treatment of infections ranging from bacterial to fungal pathogens. The assays mechanistic action justified the use of this medicinal plants for use in folk medicine for the treatment of infections such as leprosy, stomach pains, hookworm, fever, anti-inflammation, anti-hypertension, anti-diarrhea, antibacterial and antioxidant agents. Therefore, the study recommends future isolation of the bioactive constituents of the plants for proper evaluation of the pharmacological properties and toxicity studies.
Acknowledgments
The authors acknowledge the support of Directorate of Research, Vaal University of Technology Vanderbijlpark Campus.
Conflict of Interest
The authors declare that they have no competing interests.
References
- Batta AA review on phytochemicals and their activities. J. Res. Med. Sci. 2(1): 20-8 (2016).
- Mtunzi FM, Ejidike IP, Matamela T, Dikio ED, Klink MJ. Phytochemical profiling, antioxidant and antibacterial activities of leaf extracts from Rhus leptodictya. J. Pharmacogn. Phytochem. Res. 9(8): 1090-9 (2017).
CrossRef - Nguedia AJC, Shey ND. African medicinal plant derived products as therapeutic arsenals against multidrug resistant microorganisms. Pharmacogn. Phytother. 6(5): 59-69 (2014).
- Semenya SS, Potgieter MJ. Bapedi traditional healers in the Limpopo Province, South Africa: Their socio-cultural profile and traditional healing practice. Ethnobiol. Ethnomed. 10: 4 (2014).
CrossRef - Mtunzi FM, Ejidike IP, Ledwaba I, Ahmed A, Pakade VE, Klink MJ, et al. Solvent-solvent fractionations of Combretum erythrophyllum (Burch.) leave extract: Studies of their antibacterial, antifungal, antioxidant and cytotoxicity potentials. Asian Pac. J. Trop. Med. 10(7): 670-9 (2017).
CrossRef - Fankam AG, Kuiate JR, Kuete V. Antibacterial and antibiotic resistance modifying activity of the extracts from Allanblackia gabonensis, Combretum molle and Gladiolus quartinianus against gram-negative bacteria including multi-drug resistant phenotypes. BMC Complement. Altern. Med. 15(1): 206 (2015).
CrossRef - Mohammed AEI. Phytoconstituents and the study of antioxidant, antimalarial and antimicrobial activities of Rhus tripartite growing in Egypt. Pharmacogn. Phytochem. 4(2): 276-81 (2015).
- Suleiman MM, Simon MK, Ajanusi OJ, Idris AL, Abubakar MS. In vitro anthelmintic activity of the stem-bark of Combretum molle Br. x. G. Don (Combretaceae) against Haemonchus contortus. J. Med. Plants Res. 7(15): 952-6 (2013).
- Simon MK, Ajanusi OJ, Abubakar MS, Idris AL, Suleiman MM. The anthelmintic effect of aqueous methanol extract of Combretum molle (R. Br. x. G. Don) (Combretaceae) in lambs experimentally infected with Haemonchus contortus. Parasitol. 187: 280-4 (2012).
CrossRef - Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants-Basel 8(4): 96 (2019).
CrossRef - Chirisa E, Mukanganyam S. Evaluation of in vitro anti-inflammatory and antioxidant activity of selected Zimbabwean plant extracts. Herbs Spices Med. Plants 22(2): 157-72 (2016).
CrossRef - Fadipe VO, Mongalo NI, Opoku AR. In vitro evaluation of the comprehensive antimicrobial and antioxidant properties of Curtisia dentata (Burmf) C.A. Sm: toxicological effect on the Human embryonic kidney (HEK293) and Human hepatocellular carcinoma (HepG2) cell lines. J. 14: 971-83 (2015).
- Khumalo BM, Qwebani-Ogunleye T, Ejidike IP, Mtunzi FM, Pinkoane M. Evaluation of immune booster formulation by traditional health practitioners: Phytochemical, antioxidant and mineral elements studies. J. Pharma Bio Sci., 9(2): 29-37 (2018).
CrossRef - Kannan M, Kumar TS, Rao MV. Antidiabetic and antioxidant properties of Waltheria indica, an ethnomedicinal plant. Int. J. Pharma Res. Health Sci. 4(5): 1376-84 (2016).
CrossRef - Chukwujekwu JC, van Staden J. In vitro antibacterial activity of Combretum edwardsii, Combretum krausii, and Maytenus nemorosa and their synergistic effects in combination with antibiotics. Pharmacol. 7: 208 (2016).
CrossRef - Motsumi PT, Qwebani-Ogunleye T, Ejidike IP, Mtunzi FM, Nate Z. Teedia lucida root extracts by ultrasonication and maceration techniques: Phytochemical screening, antimicrobial and antioxidant activity. Rasayan J. Chem. 13(1): 423-33 (2020).
CrossRef - Anand David AV, Arulmoli R, Parasuraman S. Overviews of biological importance of quercetin: A Bioactive flavonoid. Rev. 10(20): 84-9 (2016).
CrossRef - Lokesh T, Sarada B, Swarnalatha K, Kareem MA, Varadacharyulu NCH. Antihaemolytic activity of phytochemical aqueous extracts of Pterocarpus Santalinus and Phyllanthus Emblica in red blood cells of human subjects receiving chronic alcohol and cigarette smoking. J. Pharm. Sci. Res. 7: 3857-64 (2016).
- Kotze M, Eloff Extraction of antibacterial compounds from Combretum microphyllum (Combretaceae). South Afr. J. Botany 68(1): 62-7 (2002).
CrossRef - Maslennikov PV, Chupakhina GN, Skrypnik LN. The content of phenolic compounds in medicinal plants of a botanical garden (Kaliningrad oblast). Bull. Russ. Acad. Sci. 14: 133-8 (2014).
CrossRef - Shah MD, Hossain MA. Total flavonoids content and biochemical screening of the leaves of tropical endemic medicinal plant Merremia borneensis. J. Chem. 7(6): 1034-8.
CrossRef - Gwatidzo L. Dzomba P. Mangena M. TLC separation and antioxidant activity of flavonoids from Carissa bispinosa, Ficus sycomorus, and Grewia bicolarfruits. Nutrire 43(1): 3 (2018).
CrossRef - Masoko P, Eloff JN. Screening of twenty-four South African Combretum and six terminal species (Combretaceae) for antioxidant activities. J. Tradit. Complement. Altern. Med. 4(2): 231-9 (2007).
CrossRef - Zlotek U, Mikulska S, Nagajek M, Swieca M. The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum) extracts. Saudi Jour. Bio. Sci. 23(5): 628-33 (2016).
CrossRef - Velicković JM, Dimitrijević DS, Mitić SS, Mitić MN, Kostić DA. The determination of the phenolic composition, antioxidative activity and heavy metals in the extracts of Calendula officinalis Advanced technologies 3(2): 46-51 (2014).
CrossRef - Pérez-Ramíreza IF, Castaño-Tostadoa E, León JAR, Rocha-Guzm´an NE, Reynoso-Camacho R. Effect of stevia and citric acid on the stability of phenolic compounds and in vitro antioxidant and antidiabetic capacity of a roselle (Hibiscus sabdariffa) beverage. Food Chem. 172: 885-92 (2015).
CrossRef







