Manuscript accepted on :8-Dec-2020
Published online on: --
Plagiarism Check: Yes
Reviewed by: Avtanski, Dimitar B
Second Review by: Ankur Singh Bist
Final Approval by: Dr. Josphert Ngui Kimatu
Hani Wendmu1, Peter Etim Ekanem1* , Birhane Alem1, Adhanom Gebreslassie1, Nigus Abrha1, Yohannes Tekle Asfaw2 and Anne Caroline Kendi Nyaga3
, Birhane Alem1, Adhanom Gebreslassie1, Nigus Abrha1, Yohannes Tekle Asfaw2 and Anne Caroline Kendi Nyaga3
1Department of Anatomy, College of Health Sciences, Mekelle University, P.O. Box 1871, Mekelle, Ethiopia.
2 Department of Pathology, College of Veterinary Medicine, Mekelle University, P.O. Box 1871, Mekelle, Ethiopia
3Department of Pediatrics and Child Health, College of Health Sciences, Mekelle University, P.O. Box 1871, Mekelle, Ethiopia.
Corresponding Author Email:etimakpan@gmail.comDOI : https://dx.doi.org/10.13005/bpj/2076
Abstract
Introduction:Aloe megalacanthaBaker is an endemic plant growing in Ethiopia. It is commonly used by traditional healers in the eastern and northern parts of the country to treat various ailments. Aim: The present study was aimed at investigating the effects of Aloe megalacanthaBaker leaf latex on testicular histopathology and hormonal profiles of adult male Sprague Dawley rats. Methodology:Adult male Sprague Dawleyrats were randomly divided into four groups of six rats each. GroupI received 0.5ml distilled water. Groups II, III, and IV were treated with doses of 200mg, 400mg,and 600mg per kilogram body weight per dayofAloemegalacanthaleaf latex orally using gavage for 28 days(sub-acute treatment). Assessments of testicular histopathology, sperm analysis, and hormonal assays were performed to evaluate the contraceptive effect of the leaf latex. Results: Thisstudy revealed thatAloe megalacanthaBaker leaf latex induces vascular, cellular, and structural changes in the testesat all doses. The mean values of testosterone and luteinizing hormones weresignificantly decreased in rats treated at 400mg/kg and 600mg/kgof leaf latex compared with the control group. The concentration of follicle-stimulating hormone levels also decreased significantly at 600mg/kg/daydosing of the leaf latex when compared with the control group. Increased morphological abnormality of sperm cells accompanied by a dose-dependent significant reduction of sperm count and motility were also observed in the study. Conclusions:Aloe megalacanthaBaker could affect male rats by altering histoarchitecture of the testes, lowering hormone levels, increasing abnormal sperm morphology, reducing sperm concentration, and decreasing sperm motility. It could, therefore, act asa contraceptive or antifertility agent.
Keywords
Aloe Megalacantha; Contraceptive; Hormonal Profile; Testicular Histopathology
Download this article as:| Copy the following to cite this article: Wendmu H, Ekanem P. E, Alem B, Gebreslassie A, Abrha N, Asfaw Y, Nyaga A. C. K. Evaluation of Aloe megalacantha Baker Leaf Latex on Testicular Histopathology and Hormonal Profile of Sprague Dawley Rats. Biomed Pharmacol J 2020;13(4). |
| Copy the following to cite this URL: Wendmu H, Ekanem P. E, Alem B, Gebreslassie A, Abrha N, Asfaw Y, Nyaga A. C. K. Evaluation of Aloe megalacantha Baker Leaf Latex on Testicular Histopathology and Hormonal Profile of Sprague Dawley Rats. Biomed Pharmacol J 2020;13(4). Available from: https://bit.ly/34yRekF |
Introduction
In the developing world, seventy-four million unintended pregnancies occur annually [1]. Of these, thirty percent are caused by contraceptive failures among women using some form of contraception [1]. This, in turn, results in an increment in intentional and unintentional abortions[2]. Male contraceptive techniques account for only 14 percent of all birth control methods [3]. The term contraceptive in males, refers to a chemical agent that regulates fertility through numerous ways such as:a) suppression of sperm cell production, b) disruption of sperm cell maturation, c) interruption of sperm cell transport,d) alteration of hormonal levels, e) disruption of histoarchitecture of testes among others [4,5].
Nature has been a supplier of therapeutic agents for a long time. A remarkable number of modern drugs have been isolated from natural sources [6]. Traditional medicine is a cost-effective birth control method that reduces overdependence on allopathic drugs. Thus, the World Health Organization (WHO) has suggested that its practice and usage for the control of fertility be encouragedto complement synthetic drugs [5]. For decades, efforts have been made to develop safe and effective contraceptives from natural sources [7].Medicinal plants are one of the important sources of new agents in current investigations. According to different studies,different plant species of the genus Aloe have been tested for their male antifertility effects [8-11].
Aloe megalacanthaBaker (AM) is an endemic plant growing in Ethiopia, commonly used by traditional healers in theeastern and northern parts of the country. The leaf latex of the plant is used topically for the treatment of itches, dandruff, wounds and in systemic multiple diseases including malaria, diabetes, and ascariasis [12, 13].
Several authors have reported the potential male antifertility effect of Aloe species. A study by Oyewopoet al.[10] suggested that Aloe Barbadensis(commonly known asAleovera) has an anti-fertility effect in males by significantly decreasing sperm count, sperm motility, and testicular weight. Moreover, Asgharzadeet al.[8] showed the potential effect of Aloevera in the reduction of testicular weight, serum testosteronelevels as well as a sperm count in male rats. Due to the harmful effect of Aloe on sperm cell characteristics and morphology,Oyeyemi and Ajani also concluded that Aloe vera has great potential in precipitating infertility in male Wistar albino rats[11]. Another study by Karimiet al.[9] showed the possible effect of Aloe vera in the reduction of serum level of luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone.
Most studies have been conducted to elucidate the effect of AM on sperm mobility, and hormonal variations. The present study, however, was aimed at evaluating the effect of AM, found in Ethiopia, on the testicular histopathology and hormonal concentrations of testosterone, luteinizing hormone (LH), and follicle-stimulating hormones(FSH) in adult male Sprague Dawley rats.
Materials and Methods
Study Design
Experimental study design to investigate the effects of leaf latex of AM on testicular histopathology, hormonal levels, and seminal parameters of adult male rats was conducted.
Plant Collection and Extraction
The leaves of Aloe megalacanthaBaker were collected from Klte Awulaelo district, locally called Genfel, which is located 47.7 km north of Mekelle city, the regional capital city of Tigray Regional State, Northern Ethiopia [12]. The plants were identified and authenticated by the Department of Ethno-botany, College of Computational Sciences, Mekelle University; Addis Ababa University and National herbarium, Department of Biology, and were deposited with the voucher number of GH001 and LG001. Fresh leaves of AM were collected and cleaned with brushes and water. The leaves were cut transversely near the base and were inclined in plastic material to collect the yellow latex.
Experimental Animals
Twenty-four adult male Sprague Dawley rats aged 12-14 weeks old and withan average weight of 200±20g were used for this study. The animals were given a two week acclimatization period to ensure physiological, psychological, and nutritional stabilization before their use. Human-animal interaction is important in animal experimentation; both for the welfare of the animal and the outcome of the experiment; for this reason, animals in this study were allowed to adapt to different experimental activities like handling, restraining, and dosing among others.
Individual body weights were also recorded and detailed physical examinations were performed periodically during the acclimatization/pretest period to ensure the use of healthy animals.The animals were kept in plastic cages under standard laboratory conditions (temperature of 22°C ± 3°C). They were also exposed to photoperiods of 12 hour light/dark cycles for the entire duration of the experiment and fed with a conventional rodent laboratory diet (pellets)with an unlimited supply of drinking water (Ad libitum)[13].
Administration of the AM leaf latex
All animals were weighed and randomly grouped into one control and three treatment groups, witha total of 6 rats per groupin separate cages. Control(GP I), received distilled water at 0.5ml/kg/day orally.Treatment groups II, III, and IV received 200mg/kg/day, 400mg/kg/day, and 600mg/kg/day dosesrespectively of aqueous AM leaf latex; diluted in distilled water according to the concentration of each dose. Oral administration was conducted daily using an intragastric tubefor 28 consecutive days.
Data Collection Techniques
Weight Measurements and Hormonal Assays
The bodyweights of all the animals were measured using a digital electronic balance, Entris® – Sartorius (China). Weights were taken on the first day before administration of the leaf latex and weekly till the last day of administration.At the end of the experiment, all animals were fasted overnight, anesthetized using diethyl ether, and blood samplesdrawn by cardiac puncture.Blood samples were collected in separate test tubesafter animal sacrifice for hormonal assay.The collected blood was centrifuged and the serum was assayed for testosterone, FSH, and LH using ahormone analyzer, mini Vidas®(France). Comparisonsof the results were made between the control and treatment groups.
Animal Dissection and Tissue Collection
A midline incision was made on the lower abdominal walldown to the pubic symphysis to exposethe abdominal cavity and contents. The caudal epididymis wascarefullyremoved,weighed, and placed in 5ml of 10% neutral buffered formaldehyde (NBF).
Semen Analysis
Sperm count was carried out using the new improved Neubauer`s counting chamber (Haemocytometer). The procedure was Akang et al.[14] protocol for sperm count. The sperm concentration was then calculated and multiplied by 106 and expressed as X × 106 /ml, where “X” is the number of sperm in a 16-celled square [14].
All sperm cells without movement at all were considered to be nonmotile.The rest, which displayed flagellar movements were considered motile[10].
A drop of diluted semen was stained withGiemsa stain, mounted on a slide, and examined under a light microscope (EVOS XI, China) with an automated built-in digital photo camera. Morphological abnormalities such asdamage to the head, mid-piece, or tail were noted.These parameters were also comparedbetween the control and treatment groups.
Histological Processing
Processed sections of the testes were cut into ribbons at a thickness of 5μm using a Leica rotary microtome (Leica RM 2125RT Nussloch GmbH, Germany). The ribbons were collected using forceps, floated, mounted on pre-cleaned slides, and placed in an oven ata temperature of 40 0c for about 20 minutes. The slides were then stained with routine hematoxylin and eosin (H and E)[15].
Stained tissue sections from the treated groups were examined for any histopathological changes and compared with the control. Photomicrographs of selected slides from both the treated and control groups were taken at magnifications of 40X objective using an EVOS XI (China) microscope with an automated built-in digital photo camera.
Data Processing and Analysis
Data were represented in numerical form, entered and analyzed using SPSS version 20 statistical software. Results were presented in tables and photomicrographs. Tabular parameters were expressed as Mean±SEM(standard error of the mean). One-way analysis of variance (ANOVA) was used to compare treatments over time between control and treated groups followed by Tukey’s multiple comparison tests. The level of significance was considered at p<0.05.
Ethical Considerations
Ethical clearance was obtained from the Animal Ethics Experimentation Committee of the College of veterinary medicine, Mekelle University (Reference number: AEEC 08/2019). The animals were protected from pathogens and placed in an appropriate environment. They were also kept from any unnecessary painful and terrifying situations with the use of appropriate anesthesia before and during surgical procedures[13].
Results
Physical and Behavioral Signs of Toxicity
Cage side observations of the animals before and after administration of both distilled water and AM leaf latex were carried out daily to study physical and behavioral signs of toxicity. In the first week of the experiment, there were no physical and behavioral signs of toxicity. In the second up to the last week of leaf latex administration, piloerection, shivering, low locomotion, deep sleep, loose stool, and urination were observed in all treatment groups. No mortality was observed at all doses.
Effects of the Latex on Organ and Body Weight
The sub-acute effects of AMleaf latex on the general body and specific organ weights are summarized in Tables 1 and 2. The results showed a gradual increase in body weight in the treated groups at all doses of leaf latex compared with thecontrol throughout the treatment period. Statistically significant increments were observed from the second week of administration in all treated groups compared with the control(p<0.05). Asshown in table 2, testicular weightsshowed no significant changes over the treatment period.
Table 1: The mean body weights of rats treated with Aloe megalacantha Baker leaf latex compared with control.
| Weeks Group Mean Weight in grams | ||||
| Control | 200mg/kg | 400mg/kg | 600mg/kg | |
| 1 | 195.3±3.6 | 199.6±5.4(.894) | 200.9±4.7(.790) | 209.6±2.8(.117) |
| 2 | 206±4.06 | 214.5±3.2(.390) | 212.4±4.3(.621) | 223.5±2.9(.160) |
| 3 | 199±4.1 | 222.8±5.2(.012) | 232.1±6.7(.001) | 231.3±2.4(.001) |
| 4 | 223±3.7 | 241.1±1.9(.026) | 255.3±6.2(.000) | 255.8±2.9(.000) |
| 5 | 229.6±3.3 | 260.1±2.9(.000) | 255.9±3.8(.000) | 271.4±3.6(.000) |
| Each value represents the mean± SEM of (n=6) rats per group. Thefiguresin brackets indicate the calculated pvalues of the treatment groups as compared with thecontrol. Results in bold indicate values found to be statistically significant between treatment and control groups at p<0.05 and p<0.01 using posthoc Tukey HSD (Honestly Significant Difference). | ||||
Table 2: Mean testicular weights of rats treated with Aloe megalacantha leaf latex compared with the control rats.
|
Group |
Treatment (mg/kg/day)
|
Testicular weight (g)
|
|
II |
200 |
1.28±.05(.720) |
| II | 400 | 1.30±.01(.996) |
|
III
|
600 | 1.33±.1 (.401) |
| I /Control | 0.5ml/kg/day DW | 1.18±.03 |
| DW: distilled water
Each value represents the Mean± SEM of (n=6) rats per group. The figuresin brackets indicate the calculated pvalues of the treatment groups as compared with the control |
||
Semen Parameters
Sperm Count and Motility
The sub-acute effects of Aloe megalacanthaleaf latex on sperm count and motility are summarized in Table 3. Rats that received 400 mg/kg/day and 600mg/kg/day showed a significant reduction in sperm counts compared with the controls(p<0.05). On the contrary, no significant change was observed inrats that received 200 mg/kg/day of theleaf extractcompared withthe controls. A highly significant reduction (p<0.01) in sperm motility was observed in rats treated with 400mg/kg/day and 600mg/kg/day compared with the controls.
Table 3: Sperm count and motility of rats treated with Aloe megalacantha leaf latex compared with the control rats.
| Treatment (mg/kg/day)
Group |
Sperm- Count (×106cells/ml) | Sperm motility %] |
|
I (Control)0.5ml/kg DW II 200 III 400 IV 600 |
130.8±4.4 119.2±9.2(.594) 45.5±5.09(0.00) 40.7 ±6.1(0.00) |
77.6±3.8 60.3±5.2(.350) 55.8±2.2(.007) 50.3±4.6(.001) |
Values are given as Mean±SEM. For each ratssub group.N=6. DW: distilled water The figures in parent heses indicate the calculated p values of the treatment groups when compared with the control. Results in bold indicate significant differences between treatment and control groups at p<0.05 and p< 0.01 using posthoc Tukey HSD. DW: dextrose water.
Hormonal Assay
Serum testosterone, FSH, and LH levels were determined and the results are summarized in Table 4. The mean value of testosterone hormone levels was significantly decreased in rats treated with 400mg/kg/day and 600mg/kg/day of leaf latex compared with the control group(p<0.05 andp<0.01). Though the concentration of testosterone decreased in the 200 mg/kg/day treatment group compared with the control group; thedifference was not statistically significant(p =0.150).
The concentration of FSH levels also showed dose-dependent reduction compared with the control group, with a significant difference observed in rats treated with 600mg/kg/day (p=0.002). Furthermore, the concentration of serum luteinizing hormone also decreased significantly at the treatment doses of 400mg/kg/day and 600mg/kg/day(p =0.013 and p = 0.003 respectively) compared with the controls.LH concentration was also decreased at 200 mg/kg group compared with the control group, but not significantly (p =0.110).
Table 4: Summary of levels of serum testosterone, follicle-stimulating and luteinizing hormones following administration of Aloe megalacantha leaf latex.
| Parameter | Control
I (N=6) |
Groups
II (N=6) |
III (N=6) |
IV (N=6) |
| Testosterone | 6.1±..27 | 5.2±.33 (.150) | 4.3±.28(.001) | 2.9±.20(.000) |
| FSH | .71±.02 | .66±.01(.440) | .63±.03(.110) | .56±.02(.002) |
| LH | .50±.01 | .42±.01(.110) | .39±.02(.013) | .37±.06(.003) |
N = number of Rats
The figuresin brackets indicate the calculated p values of the treatment groups as compared with the control. Significant differences are indicated in bold at p<0.05 and p< 0.01 using posthoc Tukey HSD. FSH: follicular stimulating hormone; LH: luteinizing hormone.
Effect of the latex on the seminiferous tubules and sperm morphology.
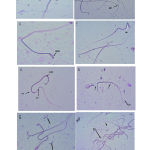 |
Figure 1: Photomicrograph of semen stained with Giemsa at 40x magnification. |
A: Control shows normal morphology of a sperm head (SH), sperm mid-piece (SMP), and sperm tail (ST) [White arrows]. B: Double-Headed and Tailed sperm (DHTS), Bent Tail (BT) at 200mg/kg/day). C: Curved Mid-piece (CMP) and Curved Tail (CT) at 200mg/kg/day). D: shows Broken Sperm (arrowed) with Coiled Tail (CT) at 400mg/kg/day.E: Presence of Hook-less Head (HLH) at 400mg/kg/day.F:Bent Head (BH =white arrow), at400mg/kg/day.G: ShowsDecapitated Head (DH), and Headless Sperm (HS=white arrow) at 600mg/kg/day.H: shows severe damage of the sperm at the level of the head, mid-piece, and tail (arrow) at 600mg/kg/day.
Effects Of Amleaf Latex on Histology of the Testis.
Microscopic examinations of rat testes administered with the different dose levels of the leaf latex exhibited histopathological changes. Generally, vascular, cellular, and structural damage to the testicular organization was observed in the three treatment groups. Normal histology of seminiferous tubules was observed in the control group (Figure 2). In normal testes, histological examinations demonstrated a normal arrangement of cellular components as seen in the photomicrograph A. Changes observed in the treatment groups were cell vacuolation, testicular damage, cell depletion, and degeneration, disruption of the basement membrane, interstitial edema, etc. These findings were also dose-dependent.
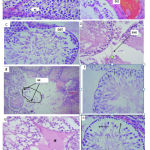 |
Figure 2: Photomicrographs of H&E stained testes sections at magnification 40X. |
(A) Control showsa seminiferous tubule with a clear lumen (L) and a normal arrangement of cellular types: late spermatids (LS), early-spermatids (ES), Sertoli-cell (SC),Leydig cells(LG), and spermatogonia(SG).(B)Shows sub-capsular congestion(SCC), presence of degenerated germ cells (DGC), at 200mg/kg/day.(C)reveals detachment of round spermatids (DST) indicated by a circle, at 200mg/kg/day (D) depicts disrupted basement membrane (BMD)at 200mg/kg/day). (E)shows severe testicular damage evidenced by the presence of atrophied and irregularly structured seminiferous tubule, loss of germinal epithelium, basement membrane damage, and presence of cell debris and inflammatory cells (circled area) at 200mg/kg/day. (F): Sertoli cell vacuolation, germ cell vacuolation, vacuolation of spermatogonia, and thinness of the basal membrane at 200mg/kg/day.(G)depicts interstitial edema (IE), vacuolation, loss of germ cells, large intercellular space at, 400mg/kg/day.(H): depletion of germ cells in the seminiferous tubule, and loss of basement membrane (arrowed areas), at 600mg/kg/day leaf latex administration.
Discussion
The present study showed that Aloe megalacanthaBaker possesses agents that could produce contraceptive effects in male rats. It does this by significantly lowering hormonal levels, altering normal testicular histology, increasingabnormal sperm morphology, decreasing sperm concentration, and reducingsperm motility. Phytochemistry of AM leaf latex has revealed varying amounts of polysaccharides, coumaric acids, phenols, saponins, flavonoids, and Aloe-emodins[16,17]. The changes in hormonal, histopathological, and seminal parameters observed in this study could be linked to these agents.
Dose-dependent increments in body and organ weights were recorded in this study. This is in line with the findings of Naftal et al.[18], and Mahdavi et al. [19]. They reported that Aloe vera polysaccharide supplementation resulted in increased body weight, due to the nutritional value of the extract and also has an effective influence on growth performance as a promoter and appetite stimulator.
A significant decrease in sperm count and motility at 400mg/kg/dayand 600mg/kg/day observed corresponded to similar findings observed by Oyeyemi and Ajani [11]after the administration of Aloe verato rats for 10 consecutive days. Farnsworth and Waller [20] screened a large number of plants for spermicidal properties.They established that the majority of plant-derived spermicides were saponins of several structural types, flavonoids, and phenol compounds. Saponins are said to have astringent actions on the cell surfaces of sperm cells, disrupting the cell membrane and could result, consequently, in the reduction of sperm motility, as well as the inhibition of specific enzymes necessary for sperm synthesis [20]. Therefore, the reason for the significant reduction of sperm count and motility observed in this study could be attributed to the individual or multiple actions of phytochemical constituent(s). Moreover, a reduction in the epididymal sperm count in AM treated rats could have resulted from the disruption of testicular tissue. This is evidenced by damages to the Leydig and Sertoli cells which are directly involved in the production of testosterone and androgen binding proteins [21].
Sperm morphological alterations at the level of the head, mid-piece, and tail were observed in all treatment groupsof the experimental animals. This finding agreeswith Oyeyemi and Ajani [11] who reported increased morphological abnormalities of spermatozoa.Studies involving hypophysectomy, castration, and androgen-replacement therapy have further strengthened the observed results in this study because androgens have been shown to be essential for the physiological maturation and survival of spermatozoa in the epididymis[22,23]. The observed morphological abnormalities of sperm cells in this study might be due to alteration in the epididymal milieu, probably due to androgen deficiency consequent to the anti-androgenic property of AM leaf latex [24]. Low sperm count, reduced motility, and increased percentage of abnormal spermatozoa have been associated withreduced fertility [11].
Significantly decreased testosterone secretion at 400mg/kg/day and 600mg/kg/day of AM leaf latex was also observed. The finding is comparable to a study conducted by Karimi et al. [9] who demonstrated a significant reduction in testosterone levels after 30 days of Aloe veraadministration. Compounds in Aloe vera, such as coumaric acids, which are also present in AMare said to stimulate the activity of testicular macrophages to produce nitrous oxide[25]. Studies have reported that Leydig cell steroidogenesis appears to be highly sensitive to the paracrine nitrous oxide. This is because it suppresses the conversion of cholesterol into pregnenolone through inhibition of the heme-containing steroidogenic enzyme CYP17A1, thereby inhibiting testosterone production [26].
AM leaflatex,in this study,significantly lowered the serum levels of FSH at 600mg/kg and LH at 400mg/kg and 600mg/kg, respectively. These results are consistent with Shariati et al.[27] who observed that the administration of Aloe vera to rats for 21 days led to a significant drop in FSH and LH levels.The presence of active compounds in Aloe Vera extract, including aloe-emodin, which is also found in AM, have been shown to have a direct effect on gonadotropin receptors or the pituitary gland. These could affect the levels of LH and FSH seen our results[28].
Histologically, cellular and structural damage of spermatogenic elements and sloughing of germinal epithelium from the basement membrane were demonstrated in all treatment groups. A detachment of round spermatids from the Sertoli cells was observed at 200mg/kg/day whilereduced nuclear area and lower number of mature Leydig cells were seen at 400mg/kg/day Induced vacuolation at the level of the interstitium and seminiferous epithelium,sub-capsular, and inter-tubular congestion of blood vessels with surrounding inflammatory cells were observed at 200mg/kg/day and 600mg/kg/day doses,respectively. These findings are consistent with the works of Joshi [29], Aladakatti and Nazeer [30], Gupta et al.[31], Tolba and Mandour [32], Han et al.[33]respectively. Reduction in the number of spermatogenic elements viz. spermatogonia, spermatocytes, and spermatids in the testis could be attributed to the decreased availability of pituitary FSH and LH [34]. Devoid or reduced numberof Leydig cells also could decrease the production of testosterone known to be responsible for the normal testicular architecture [35].Disruption of intercellular bridges between germ cells and Sertoli cells due to the anti-androgenic properties of the plantcould have resulted inthe premature detachment of round spermatids from Sertoli cells and seminal epithelium [30]. A direct effect of saponins on Leydig cells due to decreased availability of pituitary LH has been reported to result in the degeneration and depletion of the total volume of Leydig cells [36]. The epithelium of the male reproductive tubular organs may respond to injury by sloughing away from the basement membrane due to increased movement of the smooth muscle cells and inflammation [32]. Donohue et al.[37] suggested that inflammation could alter ion channel activity and expression or activation of intracellular messengers or transcription factors that regulate genes which subsequently impacts smooth muscle function. According to Johnson[38], seminiferous epithelium vacuolation is a relatively common histopathological observation associated with Sertoli cell injury after exposure to testicular toxicants with various modes of action. It has been reported that vacuolization occurs as a non-specific response of Sertoli cells to androgen deprivation [39] or as a direct consequence of germ cell phagocytosis by the Sertoli cells. These in turn give rise to an accumulation of lipid droplets in the cytoplasm of Sertoli cells [40].
Studies have indicated that the presence of interstitial inflammation interferes with the transport of oxygen which subsequently results in an increased ratio of oxygen need and oxygen supply that stimulates an increased production rate of the vasodilator, adenosine [41,42]. This leads to dilatation of the vessels and increased blood flow to restore the oxygen ratio to the normal level [41]. Besides, diffuse hemorrhage and increased interstitial fluid, which suggests a vascular mediated lesion, was observed at all treatment groups. Those changes in the blood flow to the testis by chemicals damaging the vascular endothelium are likely to reduce oxygen and nutrient movement [43].
Conclusion
Aloe megalacant haBaker leaf latex exerted adverse alterations in testicular histoarchitecture and sperm morphology, lowered hormonal levels, reduced sperm, Leydig and Sertoli cellconcentrations, besidessperm motility in dose-dependent treated groups compared with the control. Thus, it could be useful in the development of a male contraceptive agent. Comprehensive investigations involvingthe effect of Aloe megalacanthaBaker on the histopathology of the pituitary gland, hypothalamus, and epididymis would be desirable to authenticate its possible anti-fertility potentials.
Study limitations
This study did not examine whether the pathological changes of testicular and epidydimal tissues correlated with changes in other reproductive organs like in the prostate, seminal vesicles, and others which may also contribute to other findings in the study.
Acknowledgments
The authors would like to appreciate the staff of the various departments that assisted in the actualization of this study.
Conflict of Interest
The authors have no conflict of interest to declare.
Funding Source
This study was funded by Mekelle University after reviewing and approving the study topic and design (Reference number: AEEC 08/2019). They, however, did not have any direct role in the research process.
References
- Singh S, Darroch J. E, Ashford L. S. Adding it up: the costs and benefits of investing in sexual and reproductive health. Guttmacher Institute.,2014; 1-58
- Halvaei I, Roodsari H. R. S, Harat Z. N. Acute effects of Ruta graveolens L. on sperm parameters and DNA integrity in rats. JRI., 2012;13(1):33.
- Page S. T, Amory J. K, Bremner W. J. Advances in male contraception. Endocr. Rev., 2008;29(4):465-93.
CrossRef - Hyacinth A. A, Terzungwe A, Owoicho O. D, Mathias A. A. Evaluation of spermicidal property of aqueous ethanolic extract of Lawsonia inermis linn. Leaves. Ann. Biol. Res., 2012;3(8):3846-8.
- Sewani-Rusike C, Gundidza M. Antifertility effects of oldenlandia affinis in male rats-a preliminary study. Afr. J. Tradit. Complement. Altern. Med., 2011;8(4).
CrossRef - Cragg G. M and Newman D. J. Medicinals for themillennia. NY Acad. Sci., 2001;953:3-25.
CrossRef - De Kretser D. Fertility regulation in the male. Bulletin of the World Health Organization., 1978;56(3):353.
- Asgharzade S, Rafieian-Kopaei M, Mirzaeian A, Reiisi S, Salimzadeh L. Aloe vera toxic effects: expression of inducible nitric oxide synthase (iNOS) in testis of Wistar rat. IJBMS., 2015;18(10):967.
- Karimi J. H. H, Najmadini N, Hooshmand F. Effect of alcoholic extract of Aloe vera plant on serum testosterone and gonadotropin levels in rats. J Jahrom UnivMed Sci., 2012;10(2):1-8.
CrossRef - Oyewopo A, Oremosu A, Akang E, Noronha C, Okanlawon A. Effects of Aloe Vera (Aloe barbadensis) aqueous leaf extract on testicular weight, sperm count and motility of adult male Sprague-Dawley rats. Am. J. Sci., 2011;7(4):31-4.
- Oyeyemi M. O, Ajani O. S. Haematological parameters, semen characteristics and sperm morphology of male albino rat (Wistar strain) treated with Aloe vera gel. J. Med. Plant Res., 2015;9(15):510-4.
CrossRef - Gebremeskel L, Bhoumik D, Sibhat G. G, Tuem K. B. In vivo wound healing and anti-inflammatory activities of leaf latex of aloe megalacantha baker (Xanthorrhoeaceae). Evid Based Complement Alternat Med., 2018.
CrossRef - (OECD/OCDE). OfEC-oaD. OECD guidelines for repeated dose 28-day oral toxicity study in rodents, 407. 2008.
- Akang E. N, Oremosu, A. A, Dosumu O. O, Noronha C. C and Okanlawon A. O. The effect of fluted pumpkin (Telfairia occidentalis) seed oil (FPSO) on testis and semen parameters. ABJNA. 2010;1:697-703
- Ayele M. Evaluation of acute and subchronicic toxicity of aqueous leaves extracts of maytenus gracilipes celastraciae (kombolcha)on some blood parameters and histopathology of liver and kidney in Swiss albino mice on some blood parameters and histopathology of liver and kidney in Swiss Albino Mice. 2015. http://localhost:80/xmlui/handle/123456789/459
- Sahu P. K,Giri D. D, Singh R, Pandey P, Gupta S, Shrivastava A. K, Kumar A, Pandey K. D. Therapeutic and medicinal uses of Aloe vera: a review. J. Pharm. Pharmacol., 2013; 4(08):599.
CrossRef - Steenkamp V and Stewart M. J. Medicinal applications and toxicological activities of Aloe Products. Pharm. Biol., 2007; 45(5):411-20.
CrossRef - Gabriel N. N, Wilhelm M. R, Habte-Tsion H-M, Chimwamurombe P, Omoregie E, Iipinge L. N, et al. Effect of dietary Aloe vera polysaccharides supplementation on growth performance, feed utilization, hemato-biochemical parameters, and survival at low pH in African catfish (Clarias gariepinus) fingerlings. Int. Aquat. Res., 2019;11(1):57-72.
CrossRef - Mahdavi M, Hajimoradloo A, Ghorbani R. Effect of Aloe vera extract on growth parameters of common carp (Cyprinus carpio). World Journal of Medical Sciences. 2013;9(1):55-60.
- Farnsworth N. R, Waller D. P. Current status of plant products reported to inhibit sperm. Res Front Fertil Regul., 1982;2(1).
- Obianime A. W, Aprioku J. S, Esomonu C. T. Antifertility effects of aqueous crude extract of Ocimum gratissimum L. leaves in male mice. J. Med. Plant Res., 2010; 4(9):809-16.
CrossRef - Dyson A, Orgebin-Crist M. C. Effect of hypophysectomy, castration and androgen replacement upon the fertilizing ability of rat epididymal spermatozoa. Endocrinology. 1973;93(2):391-402.
CrossRef - Setty B. S, Riar S. S, Kar A. B. Androgenic control of epididymal function in rhesus monkey and rabbit. Fertil Steril., 1977; 28(6):674-81.
CrossRef - Ahmed M, Ahamed R. N, Aladakatti R. H, Ghodesawar M. A. G. Effect of benzene extract of Ocimum sanctum leaves on cauda epididymal spermatozoa of rats. Iran J Reprod Med., 2011;9(3):177.
- Chrousos G. P. The gonadal hormones and inhibitors. Katzung BG, ed Basic and Clinical Pharmacology 9th ed New York, NY, USA: McGraw-Hill. 2004:669-72.
- Pathak N. D, Lal B. Paracrine role of macrophage produced-nitric oxide (NO) in Leydig cell steroidogenesis in a teleost, Clarias batrachus: impact of gonadotropin, growth hormone and insulin on NO production by testicular macrophages. Gen Comp Endocrinol., 2009;160(1):12-8.
CrossRef - Shariati M, Mokhtar M, Rastgar S. Effect of Aloe vera extract on testosterone and gonadotropin hormone changes in rats. J Sabzevar Univ M Sci., 2009;16(1):12-7.
- Mazur W and Adlercreutz H. Natural and anthropogenic environmental estrogens: the scientific basis for risk assessment. Pure Appl Chem., 1998;70(9):1759-76.
CrossRef - Joshi A. R, Ahamed R. N, Pathan K. M, Manivannan B. Effect of Azadirachta indica leaves on testis and its recovery in albino rats. Indian J. Exp. Biol., 1996; 34(11):1091-4.
- Aladakatti R. H, Ahamed R. N, Ghosewar M. G. Azadirachta indica A. Juss induced changes in spermatogenic pattern in albino rats. J. Nat. Med., 2006;6(1):62-72.
- Gupta R. S, Chaudhary R, Yadav R. K, Verma S. K, Dobhal M. P. Effect of Saponins of Albizia lebbeck (L.) Benth bark on the reproductive system of male albino rats. J. Ethnopharmacol., 2005; 96(1-2):31-6.
CrossRef - Tolba A. M, Mandour D. A. Histological effects of bisphenol-A on the reproductive organs of the adult male albino rat. Eur J Anat., 2018;22(2):89-102.
- Han X. D,Zhi G. T, Gong Y, Shen S. N, Wang X. Y, Kang L. N, Hou Y. Y, Chen J. X. The toxic effects of nonylphenol on the reproductive system of male rats. Reprod Toxicol. 2004; 19(2):215-21.
CrossRef - Ahmed M, Al-Daghri N, Alokail M, Hussain T. Potential changes in rat spermatogenesis and sperm parameters after inhalation of Boswellia papyrifera and Boswellia carterii incense. Int. J. Environ. Res. Public Health., 2013;10(3):830-44.
CrossRef - Eik-nes K. Synethesis and Secretion of Andostetiedione and Tesosterones 1970.
- Hall P. F. Testicular steroid synthesis: organization and regulation. Physiology and Reproduction. 1994; 2: 1335–1362
- Shea‐Donohue T, Notari L, Sun R, Zhao A. Mechanisms of smooth muscle responses to inflammation. Neurogastroenterol Motil., 2012; 24(9):802-11.
CrossRef - Johnson K. Testicular histopathology associated with disruption of the Sertoli cell cytoskeleton. Spermatogenesis., 2014; 4(2):e979106.
CrossRef - Krueger P. M, Hodgen G. D, Sherins R. J. New Evidence for the Role of the Sertoli Cell and Spermatogonia in Feedback Controlof FSH Secretion in Male Rats. Endocrinology., 1974; 95(4):955-62.
CrossRef - Soliman H. M, Wagih H. M, Attia G. M, Algaidi S. A. Light and electron microscopic study on the effect of antischizophrenic drugs on the structure of seminiferous tubules of adult male albino rats. Folia Histochem Cyto. 2014;52(4):335-49.
CrossRef - Huether S. E and McCance K. L. Understanding pathophysiology 6th Ed. St. Louis Mo.: Mosby/Elsevier., 2008.
- Gossman W,Faysal F, Berim I. Anoxia (Hypoxic Hypoxia)StatPearls Publishing. 2019; Available from https://www.ncbi.nlm.nih.gov/books/NBK482316/.
- Creasy D. M. Evaluation of testicular toxicity in safety evaluation studies: the appropriate use of spermatogenic staging.Toxicol. Pathol., 1997; 25(2):119-31.
CrossRef







