Manuscript accepted on :23-03-2020
Published online on: 18-07-2020
Plagiarism Check: Yes
Reviewed by: Muhammad Ajmal Shah
Second Review by: Mohammad Rizki Fadhil Pratama
Final Approval by: Dr. Beatrice O. Ondondo
Dudhamal Tukaram Sambhaji1 , Ajmeer Ahmad Shahan2 and Vijay Kumar3
, Ajmeer Ahmad Shahan2 and Vijay Kumar3
1Department of Shalyatantra, Institute for Post graduate Teaching and Research in Ayurveda (I.P.G.T. and R.A.) Gujarat Ayurved University, Jamnagar, Gujarat-361 008, India.
2Maharshi Hospital, Mount Lavinia, Colombo, Sri Lanka
3SRF all India Institute of Ayurveda, New Delhi, India
Corresponding Author E-mail : drtsdudhamal@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1994
Abstract
To evaluate antimicrobial and antioxidant activity of leaf and bark of Securinega leucopyrus for anti-microbial activity against clinically important bacterial and fungal strains using agar disc diffusion method and for antioxidant activity. The methanolic extracts of leaf and stem bark were tested for antimicrobial activity against four gram positive bacteria, four gram negative bacteria, antifungal activity and for their antioxidant activity using total antioxidant capacity, total reducing power, ferric reducing antioxidant power and DPPH free radical scavenging activity. Securinega leucopyrus (Willd) Muell. is traditionally used for the management of boils and wounds in Sri Lanka and India. This shrub is clinically evaluated for wound healing as case reports and some clinical trials also showed encouraging results in diabetic wounds. So it is need of an hour to evaluate its antimicrobial as well as antioxidant activity for scientific validation. The highest antimicrobial activity was exhibited by 250µg/ml methanolic extracts of both leaf and bark. The extracts at the 250µg/ml concentration shows maximum antifungal activity against Aspergillus flavus used in the study. Methanolic extracts of leaves exhibited highest radicals scavenging activity, that is, 88.14 percent. Similar results were observed in total antioxidant capacity, total reducing power and ferric reducing assay determination. Methanolic extract of leaf and stem bark at different concentrations showed in-vitro antimicrobial and antioxidant activity.
Keywords
Anti-Microbial; Antioxidant; Free Radical Scavenging; Humarai; Katupila; Securinega leucopyrus
Download this article as:| Copy the following to cite this article: Sambhaji D. T, Shahan A. A, Kumar V. In-Vitro Antimicrobial and Antioxidant Activity of Securinega leucopyrus (Willd) Muell. Biomed Pharmacol J 2020;13(3). |
| Copy the following to cite this URL: Sambhaji D. T, Shahan A. A, Kumar V. In-Vitro Antimicrobial and Antioxidant Activity of Securinega leucopyrus (Willd) Muell. Biomed Pharmacol J 2020;13(3). Available from: https://bit.ly/398pe8t |
Introduction
Plant derived drugs are generally nontoxic, effective at low concentrations, easily affordable and environment friendly. It has been reported that some fruits, vegetables and herbs are effective for certain chronic diseases because they may show their bactericidal, antiviral, analgesic, anti-inflammatory, anti-carcinogenic, and/or antioxidant actions.1,2 Traditional medicines have a long history of serving the people all over the world. The use of medicinal plants to maintain public health and treat diseases in many countries with cultures of different nations is highly prevalent.3, 4 Nowadays, bioactive photochemical found in traditional plants have gained significant consideration because these compounds retard the lipids degradation, prevent microbial deterioration and improve the food quality.5, 6
Medicinal plants are rich sources of antimicrobial agents. Many infectious diseases have been known to be treated with herbal extracts. The clinical efficacy of many existing antibiotics is being threatened by the emergence of multidrug-resistant pathogens. Although many plant species have been tested for antimicrobial properties, the majority of them have not been sufficiently evaluated7. As an alternate source to the existing antibiotics, there is an urgent need to discover new antimicrobial compounds from various medicinal plants which can be used to treat many infectious diseases.
Reactive oxygen species (ROS) are class of highly reactive molecules derived from the metabolism of oxygen. Rapid production of free radicals may lead to oxidative damage to bio-molecules and results in disorders such as degenerative disorders, cancer, diabetes, neural disorders and ageing.8 These free radicals occur in the body during an imbalance between ROS and antioxidants. Many medicinal plants have large amount of antioxidants such as Vitamin C, Vitamin E, polyphenols etc. Natural antioxidants increase the antioxidant capacity of the plasma and reduce the risk of certain diseases like heart disease, cancer.9 There are many synthetic antioxidants but they have side effects, hence there is a need for more potent and less toxic antioxidants.10
Securinega leucopyrus (Willd) Muell. belonging to family Euphorbiaceae known as Katupila (in Sri Lanka), Thumri (Sanskrit name), Humari (in Hindi), Shinavi (in Gujarat) and also called as “Spinous fluggea” in English.11 Tribal people in Sri Lanka used various parts of this plant against cancer, wounds and ulcers in diabetes mellitus.12 S. leucopyrus leaves act as an antiseptic and its paste is used in folklore to extract any extraneous materials from body tissues. Some clinical case studies reported the wound healing potential of this herb,13-15 However no reports are available on its antimicrobial activity and antioxidant activity. Hence the study was planned to evaluate the in-vitro antimicrobial activity of S. leucopyrus leaf and bark.
Materials and Method
Collection of Plant Materials
The plant was identified from its natural habitat of Kaduwela, Colombo, Sri Lanka and its morphological characters and comparing them with the characters mentioned in various floras.11,16 The stem bark was collected, washed properly under running water, to make them free from foreign matter like sand, soil etc. and dried under shade. Herbarium voucher No. Phm 6126/2013/14 was also prepared and submitted to Pharmacognosy museum of IPGT & RA, Jamnagar, for future reference.
Sample Preparation
For analysis S. leucopyrus leaf and stem bark was coarsely powdered to a sieve of 60 mesh size and then tests were performed.
Antimicrobial Activity
Extract Preparation
Methanol extract was used for the study, for this 1g of material was extracted in 50 ml of methanol by sonicating it for 10 min and then keeping it overnight. Next day after filtration, methanol evaporated, then by taking weight of residue 5 different concentrations 5 µg/ml, 25 µg/ml, 50 µg/ml, 100µg/ml, 250µg/ml of each sample prepared. These are used for determination of antimicrobial activity.
Culture Conditions
The antimicrobial efficacy was tested on 9 different strains, 4 Gram positive bacteria namely Bacillus subtilis (NCIM 2063), Staphylococcus aureus (NCIM 2079), Bacillus cereus (NCIM 2106) and Staphylococcus epidermidis (NCIM 2493) and 4 Gram negative bacteria Escherichia coli (NCIM2065), Klebsiella pneumoniae (NCIM2719), Salmonella typhi (NCIM2501) and Proteus vulgaris (NCIM2857). One fungal strain namely Aspergillus flavus (NCIM 1028) was also tested. All cultures were obtained from NCL, Pune. 24 hour old cultures of all these organisms were inoculated in sterile broths and incubated till 0.5 Mcfarland standard turbidity obtained and then used for assay.
Antimicrobial Assay
Sterile soybean casein digest agar (25 ml/plate) used for antibacterial activity and sterile sabouraud agar (25ml per plate) used for antifungal activity17. Medium obtained from Himedia laboratories. Sterile 20 ml medium poured in sterile plates aseptically and let them solidified. Then inoculate 0.5 ml of culture in 5 ml sterile melted and cooled medium and poured them on solidified agar plates aseptically. After solidification made well with the help of cup borer and inoculate 0.3 ml of each sample in the well and for antibiotic discs there is no need to make wells and directly place disc on agar surface aseptically. For diffusion purpose plates were placed in refrigerator for 20-25 minutes. Then Incubate plates at 370 C for 24 hrs except sabouraud agar plates and plates containing K. pneumoniae organism, they incubated at 300 C for 24-48 hrs. After incubation zone of inhibition was measured with Himedia antibiotic zone scale- c.
Antioxidant Activity
Total Antioxidant Capacity
10% w/v methanolic extracts of both leaves and stem bark samples prepared by macerating overnight, diluted further with methanol were used for estimation of total antioxidant activity18. An aliquot of 0.1 ml of sample solution containing a reducing species in methanol was combined in an Eppendorf tube with one ml of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The tubes were capped and incubated in a thermal block at 950C for 90 min. After those samples were cooled to room temperature, the absorbance of the solution was measured at 695 nm against a blank. A typical blank solution contained 1 ml of reagent solution and the appropriate volume of same sample used for the sample, and it was incubated at the same conditions as the rest of the samples. Ascorbic acid solution (concentration range: 20 to 80 µg) was taken as the known standard. Total antioxidant capacity of the samples calculated in terms of ascorbic acid µg equivalents corresponding to 1 mg of original sample using the standard curve.
Total Reducing Power
10% w/v methanolic extracts of the samples prepared by macerating overnight, diluted further with 1 ml methanol, were mixed with 2.5 mL of 0.2 M phosphate buffer (pH 6.6) and 2.5 mL of potassium hexacyanoferrate solution (1% w/v)19. The mixture was incubated at 500C for 20 min, 1.5 mL of trichloroacetic acid (10% w/v) was added to the mixture and filtered. 2.5 mL of filtrate was mixed with equal volume of distilled water and 0.5 mL of ferric chloride solution (0.1% w/v) and the absorbance was measured at 700 nm after 40 min. Ascorbic acid solution (concentration range: 10 to 40 µg) was taken as the known standard. Increased absorbance of the reaction mixture indicates stronger reducing power. A calibration curve was plotted for absorbance of ascorbic acid standard against its concentration in micrograms and the equivalent values for samples were extrapolated from the slope equation of line against their respective absorbance. The results were expressed in microgram equivalents of ascorbic acid with respect to the original sample. Total reducing power of the samples calculated in terms of ascorbic acid µg equivalents corresponding to 1 mg of original sample using the standard curve.
Ferric Reducing Antioxidant Power (FRAP Assay)
FRAP assay was performed according to the methods of Benzie and Strain (1999) with slightly modification20. It is based on the principle of reduction of Fe3+-TPTZ to Fe2+-TPTZ complex at low pH which gives blue colour and can be measured at 593 nm. 0.1 ml of 10% w/v methanolic extracts of the samples prepared by macerating overnight, diluted further with methanol was added to 3.0 ml of FRAP working reagent, mixed well and absorbance was measured after 10 minutes. Freshly prepared aqueous ascorbic acid solution (0.1 mg/ml) was used as standard. Different volumes of ascorbic acid solution (equivalent to 2-8 µg) were used in same manner for calibration of standard curve and quantification was done in terms of mg equivalents of ascorbic acid. The blank was prepared by using distilled water in place of sample/standard.
DPPH Free Radical Scavenging Activity (DPPH Assay)
This assay measures the free radical scavenging capacity of the extract under investigation. DPPH is a molecule containing a stable free radical21-23. In the presence of an antioxidant, which can donate an electron to DPPH, the purple colour which is typical for free radical decays and change in absorbency at 517 nm was measured using PerkinElmer Lambda 25 UV-Visible spectrometer. 0.3 mM DPPH solution was prepared in methanol (freshly prepared), for standard Ascorbic acid solution – 1 mg/ml in methanol was prepared. Different volumes (100, 200, 250 and 300 µg) of test samples/standard were taken in a set of test tubes and methanol was added to make the volume to 3 ml. To this, 1 ml of DPPH reagent was added mixed thoroughly and absorbance was recorded at 517 nm after 30 minutes incubation in dark at room temperature. 1 ml of DPPH reagent diluted to 4 ml with methanol was taken as reagent blank. The radical scavenging activity was calculated from the equation:
Radical scavenging activity (%): (Abscontrol – Abssample)/ Abscontrol×100
Result and Discussion
Antimicrobial Activity
Global burden of infectious diseases caused by bacterial agents is a serious threat to public health.24 Antibiotic treatment is a preferred choice to treat bacterial infections; however, emergence of antimicrobial resistance and toxicity issues subside the use of antibacterial agents.25, 26 Safety- and efficacy-related limitations to antibiotics augment biological research on the antimicrobial role of plants due to comparable toxicity and efficacy.24
In the present study, we have investigated the antibacterial activity of different concentrations (25, 50, 100 & 250 µg/ml) of dried methanolic extract of leaf and stem bark of S. leucopyrus prepared in dimethyl sulfoxide and 0.3 ml of each was used for assay. The antimicrobial activity was exhibited against 8 different strains, 4 Gram-positive bacteria (Table-1) and 4 Gram-negative bacteria (Table-2).The zone of inhibition around the disc impregnated with plant extract over the lawn of bacterial and fungal culture plates determined the antimicrobial activity as quantitatively. The result showed that the antimicrobial activities of plant extracts were increased with increasing concentration. Both, sample- A which was methanolic extract of leaves and sample- B which was methanolic extract of bark, at 250µg/ml showed highest inhibition of growth of all four Gram-positive bacteria with inhibition zone ranging from 11 to 17 mm and all four Gram-negative bacteria with inhibition zone ranging from 13 to 18 mm. Gentamycin (10 µg/ml), Cifpodoxime (10 µg/ml) and Streptomycin (30 µg/ml) were used as a positive control which showed highest inhibition against Bacillus subtilis with inhibition zone of 28 mm, Staphylococcus aureus with inhibition zone of 23 mm and Bacillus subtilis with inhibition zone of 27 mm respectively. The reason accounting for antimicrobial activity of methanolic extracts might be firstly due to nature of biological active compounds (terpenoids, tannins, flavonoids etc.) which might be enhanced in presence of methanol. Secondly, the stronger extraction capacity of methanol might have produced a greater number of active constituents responsible for antimicrobial activity27. The greater activity of methanolic extract against bacteria gave further indication of sterilization capabilities of leaf and stem bark. The antibacterial activity may be attributed to the inhibition of enzymatic function within the cell28. In antifungal analysis the methanol extracts at different concentration (25, 50, 100 & 250 µg/ml) were compared with standard Amphotericin B, Fluconazole and Clotrimazole. Both extracts at 250µg/ml concentration shows maximum antifungal activity against Aspergillus flavus used in the study (Table-3). Antifungal activity exhibited may attributes to the presence of these secondary metabolites. These compounds can combat with pathogens by different mode of action.29
Table 1: Antibacterial activity of Securinega leucopyrus against gram positive organism
| Sample | Conc. (µg/ml) | Zone of inhibition (mm) | |||
|
|
Bacillus subtilis (NCIM2063) | Staphylococcus aureus (NCIM2079) | Bacillus cereus (NCIM2106) | Staphylococcus epidermidis (NCIM2493) | |
| Sample A | 25 | 10 | 10 | 10 | NI* |
| 50 | 11 | 11 | 12 | NI* | |
| 100 | 12 | 12 | 13 | 10 | |
| 250 | 15 | 16 | 17 | 11 | |
| Sample B | 25 | 11 | NI* | 10 | NI* |
| 50 | 12 | NI* | 11 | 10 | |
| 100 | 13 | NI* | 12 | 11 | |
| 250 | 15 | 13 | 14 | 12.5 | |
| Gentamycin | 10 | 28 | 25 | 26 | 26 |
| Cifpodoxime | 10 | 22 | 23 | 19 | 14 |
| Streptomycin | 30 | 27 | 17 | NI* | NI* |
* NI: No inhibition
Table 2: Antibacterial activity of Securinega leucopyrus against gram negative organism
| Sample | Conc. (µg/ml) | Zone of inhibition (mm) | |||
|
|
Escherichia coli (NCIM2065) | Klebsiella pneumoniae (NCIM2719) | Salmonella typhi (NCIM2501) | Proteus vulgaris (NCIM2857) | |
| Sample A | 25 | 11 | 14 | 11 | NI* |
| 50 | 12 | 15 | 11 | NI* | |
| 100 | 14 | 16 | 12 | 11 | |
| 250 | 16 | 18 | 15 | 13 | |
| Sample B | 25 | 10 | 12 | 12 | 11 |
| 50 | 11 | 13 | 13 | 12 | |
| 100 | 12 | 14 | 14 | 13 | |
| 250 | 15 | 16 | 16 | 16 | |
| Gentamycin | 10 | 22 | 24 | 24 | 21 |
| Cifpodoxime | 10 | 21 | 19 | 16 | 20 |
| Streptomycin | 30 | 17 | 24 | 15 | NI* |
* NI: No inhibition
Table 3: Antifungal activity of Securinega leucopyrus against Aspergillus flavus
| Sample | Conc.
(µg/ml) |
Zone of inhibition (mm) Aspergillus flavus (NCIM 1028) |
| Sample A | 25 | 10 |
| 50 | 12 | |
| 100 | 12.5 | |
| 250 | 14 | |
| Sample B | 25 | 11 |
| 50 | 12 | |
| 100 | 13 | |
| 250 | 15 | |
| Amphotericin B | 50 | 14 |
| Fluconazole | 30 | NI* |
| Clotrimazole | 30 | 24 |
* NI: No inhibition
Antioxidant Activity
In recent years much attention has been devoted to natural antioxidants and their health benefits. Antioxidant-based drug formulations are used for the prevention and treatment of many complex diseases. Plants are a major source of natural antioxidants; they produce a wide range of secondary metabolites with antioxidative activities that have therapeutic potential. Polyphenols are the most abundant antioxidant compounds of plant raw material. Their antioxidant activity is based on to their redox properties, which facilitate their activity as reducing agents, hydrogen donors, singlet oxygen quenchers, metal chelators and reductants of ferryl hemoglobin. The reducing ability is generally associated with the presence of reductants which exert antioxidant action through breaking the free radical chain by donating a hydrogen atom or preventing peroxide formation.30 Medicinal plant tissues are commonly rich in phenolic compounds such as flavonoids, phenolic acids, stilbenes, tannins, coumarins, lignans and lignins. These compounds have multiple biological effects including antioxidant activity.31,32 The methanol extracts of plant showed a high effective total antioxidant capacity, high effective free radical scavenging in DPPH assay, total reducing power and Ferric reducing antioxidant power.
Total Antioxidant Capacity
Total antioxidant capacity is based on the reduction of Mo (VI) to Mo (V) by the extract and subsequent formation of green phosphate/Mo (V) complex at acid pH. TAC of the phosphomolybdenum model evaluates both water-soluble and fat-soluble antioxidant capacity (total antioxidant capacity). The results indicate a total antioxidant capacity in both samples (Table-4). It means that the methanol extracts of leaf and stem bark will have to contain as much quantity of antioxidants compounds as equivalents of ascorbic acid to effectively reduce the oxidant in the reaction matrix33. Antioxidant capacity of ascorbic acid has been used as a reference standard from which plant extracts with potential antioxidant activity are compared (Figure-1).
Table 4: Total antioxidant capacity, total reducing power and ferric reducing antioxidant power of Securinega leucopyrus
| Sample ID | Replicate | Total antioxidant capacity | Total reducing power | Ferric reducing antioxidant power |
| Ascorbic acid µg equivalent/mg of sample | ||||
|
Sample A |
1 | 115.33 | 39.86 | 52.52 |
| 2 | 96.92 | 45.42 | 51.24 | |
| 3 | 103.28 | 43.86 | 52.04 | |
|
Sample B |
1 | 50.41 | 7.72 | 28.13 |
| 2 | 46.92 | 6.73 | 29.68 | |
| 3 | 48.72 | 9.88 | 30.54 | |
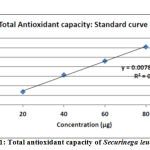 |
Figure 1: Total antioxidant capacity of Securinega leucopyrus |
Total Reducing Power
For the measurements of the reductive ability it has been investigated from the Fe3+ to Fe2+ transformation in the presence of extract samples using the method described by 0yaizu (1986) .19 The data shows that leaves of S. leucopyrus have a moderate antioxidant activity as far as this in vitro method is concerned (Table-4). The values are expressed in ascorbic acid microgram equivalents with respect to original sample (Figure-2). The reducing properties are generally associated with the presence of reductones, which have been shown to exert antioxidant action by breaking the free radical chain or by donating a hydrogen atom.34
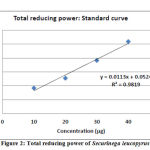 |
Figure 2: Total reducing power of Securinega leucopyrus |
FRAP Assay
FRAP assay method depends upon the reduction of ferric tripyridyltriazine [Fe (III)-TPTZ] complex to ferrous tripyridyltriazine [Fe (II)-TPTZ] by a reductant at low pH. Fe (II)-TPTZ has an intensive blue colour and can be monitored at 593 nm. FRAP values are obtained by comparing the absorbance change at 593 nm in test reaction mixtures with those containing range with antioxidant mixtures.35 Ferric reducing antioxidant power of the samples calculated in terms of ascorbic acid µg equivalents corresponding to 1 mg of original sample using the standard curve.21 The ascorbic acid standard curve for FRAP at a concentration range of 2 to 8 µg (Figure-3). Methanolic extracts of leaves showed mean 51.93 ascorbic acid µg equivalent/mg of sample and stem bark showed mean 29.45 Ascorbic acid µg equivalent/mg of sample (Table-4).
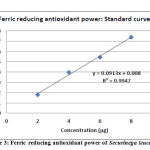 |
Figure 3: Ferric reducing antioxidant power of Securinega leucopyrus |
DPPH Assay
The DPPH assay has been largely used as a quick, reliable and reproducible parameter to search for the in-vitro antioxidant activity of pure compounds as well as plant extracts. It was observed that leaves of S. leucopyrus had higher free radical scavenging activity than that of stem bark. At a concentration of 300 µg, the scavenging activity of methanol extract of the leaves reached 88.14 %, while at the same concentration, that of the stem was 47.25 %. The usual trend to show scavenging activity is by indicating IC50 (Half-maximal Inhibitory concentration). IC50 for leaf was 153.66 µg and for stem bark 308.89 µg (Table-5). The effect of antioxidants on DPPH is thought to be due to their hydrogen donating ability.36, 37 The study showed that the extracts have the proton-donating ability and could serve as free radical inhibitors or scavengers, acting possibly as primary antioxidants.38
Table 5: DPPH Free radical scavenging activity of Securinega leucopyrus
| Sample ID | Concentration (µg*) | % Scavenging activity | IC50 in µg* |
|
Sample A |
100 | 35.15 |
153.66 |
| 200 | 64.73 | ||
| 250 | 73.28 | ||
| 300 | 88.14 | ||
|
Sample B |
100 | 23.02 |
308.89 |
| 150 | 29.66 | ||
| 300 | 47.25 | ||
| 400 | 62.89 |
*of original sample
Conclusion
The result obtain in the present study are in agreement to a certain degree with the traditional use of the plant. In this screening work, the test drug extract at different concentrations was found to be comparatively highly effective against all organisms such as Gram positive, Gram negative and single fungal strain. From the above results the activity of all extracts showed significant antibacterial and antifungal activity, however the results shown by leaf extract were better than stem bark. The present study justified the claimed ethnic uses of Katupila (Securinega leucopyrus) leaf and stem bark externally in ringworms, scurvy, snakebite, sprains, bruises, rheumatic swelling, wounds and to treat various infectious diseases caused by the microbes.
Acknowledgement
The authors wish to thank Director Prof. AB Thakar, Institute of Postgraduate Teaching and Research in Ayurveda, Gujarat Ayurved University, Jamnagar for providing facilities and consulting staff.
Conflict of Interest
There is no conflict of interest.
Funding Source
There is no funding source
References
- Kahkonen M. P, Hopia A. I and Heino M. Berry phenolics and their antioxidant activity. Agric Food Chem., 2001; 49: 4076-4082.
CrossRef - Bakkali F, Averbeck S, Averbeck D and Idaomar M. Biological effects of essential oils. Food Chem Toxicol., 2008; 46: 446-475
CrossRef - Asgary S, Sahebkar A, Afshani MR, Keshvari M, Haghjooyjavanmard S and Rafieian-Kopaei M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother Res., 2014; 28: 193-199.
CrossRef - Bahmani M, Banihabib E, Rafieian-Kopaei M and Gholami-Ahangaran M. Comparison of disinfection activities of nicotine with copper sulphate in water-containing Limnatis nilotica. Kafkas Univ Vet Fak Derg., 2015; 21: 9-11.
- Osorio E, Flores M, Hernandez D, Ventura J, Rodriguez R and Aguilar CN. Biological efficiency of polyphenolic extracts from pecan nutsshell (Carya illinoensis), pomegranate husk (Punica granatum) and creosotebushleaves (Larrea tridentata) against plant pathogenic fungi. Ind Crop Prod., 2010; 31: 153-157.
CrossRef - Sofia R, Mushtaq A, Muhammad Z, Asad A, Shazia S, Shahzadi T and Sidra N. A. Ethnopharmacological evaluation and antioxidant activity of some important herbs used in traditional medicines. Journal of Traditional Chinese Medicine, 2016; 36: 689-694.
CrossRef - Balandrin M. F, Kjocke A. J. and Wurtele E. Natural plant chemicals: sources of industrial and mechanical materials. Science, 1985; 228: 1154-1160.
CrossRef - Hyun D. H, Hernandez J. O, Mattson M. P and De C. R. The plasma membrane redox system in aging. Aging Res. Rev., 2006; 5: 209-220.
CrossRef - Prior R. L and Cao G. Antioxidant phytochemicals in fruits and vegetables. Diet and health implications. Sci., 2000; 35: 588-592.
CrossRef - Ito N, Fukushima S, Hagiwara A, Shibata M and Ogiso T. Carcinogenicity of butylated hydroxyanisole in F344 rats. Natl. Cancer. Inst., 1983; 70: 343-347.
- Santhapu H. Humari (Securinega leucopyrus). In: Plants of Saurashtra a Preliminary Rajkot: S.J.F.N.I. Saurashtra Research Society; 1953.
- Ajmeer A. S, Harisha C. R, Dudhamal T. S and Gupta S. K. Micromorphological and micrometric evaluation of Securinega leucopyrus (willd.) Muell. Leaf and stem-unexplored drug. International Journal of Science Invention Today, 2013; 2: 140-149.
- Ajmeer A. S, Dudhamal T. S, Gupta S. K and Mahanta V. D. Case Report: Katupila (Securinega leucopyrus) as a potential option for diabetic wound management. Journal of Ayurved and Integrative Medicine, 2014; 5: 60-63
CrossRef - Ajmeer A. S, Dudhamal T. S, Gupta S. K and Mahanta V. D. Topical application of Katupila (Securinega leucopyrus) in Dushta Vrana (chronic wound) showing excellent healing effect: A case study. AYU, 2014; 35: 175-78.
CrossRef - Ajmeer A. S, Dudhamal T. S. and Gupta S. K. Management of Madhumehajanya Vrana (diabetic wound) with Katupila (Securinega leucopyrus [Willd] Muell.) Kalka. Short Communication. AYU, 2015; 36: 353-55
CrossRef - Illustrations of the forest flora of North-West and Central India: Dietrich Brandis; 1874.
- Dorman H. J. and Deans S. G. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J. Appl. Microbiol, 2000; 88: 308-316.
CrossRef - Prieto P, Pineda M and Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Analytical biochemistry, 1999; 269: 337-344.
CrossRef - Oyaizu M. Studies on products of browning reaction prepared from glucosamine. J. Nutr, 1986; 44: 307-15
CrossRef - Benzie I. F. and Strain J. J. The Ferric Reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Analytical Biochemistry, 1996; 239: 70-76.
CrossRef - Deore S. L, Khadabadi S. S, Baviskar B. A, Khadabadi S. S, Khangenbam R. A, Koli U. S, Daga N. P, Gadbail P. A and Jain P. A. In vitro antioxidant activity and phenolic content of Croton caudatum. International Journal of Chem Tech Research, 2009; 1: 174-176.
- Namjooyan F, Azemi M. E. and Rahmanian V. R. Investigation of antioxidant activity and total phenolic content of various fractions of aerial parts of Pimpinella barbata (DC.) Boiss. Jundishapur. Journal of Natural Pharmaceutical Products, 2010; 5: 1-5.
- Molyneux P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Sci. Technol, 2004; 26: 212.
- Eggleston K, Zhang R and Zeckhauser R. J. The global challenge of antimicrobial resistance: insights from economic analysis. Int J Environ Res Public Health, 2010; 7: 3141–3149.
CrossRef - Malini M, Abirami G, Hemalatha V and Annadurai G. Antimicrobial activity of ethanolic and aqueous extracts of medicinal plants against waste water pathogens. Inte J Res Pure Appl Microbiol, 2013; 3: 40–42.
- Zhang R, Eggleston K, Rotimi V and Zeckhauser RJ. Antibiotic resistance as a global threat: evidence from China, Kuwait and the United States. Global Health, 2006; 2: 6.
CrossRef - Tanti B, Buragohain A. K, Gurung L, Kakati D, Das A. K. and Borah S. P. Assessment of antimicrobial and antioxidant activities of Dendrocnide sinuate (Blume) Chew leaves-A medicinal plant used by ethnic communities of North East India. Indian Journal of Natural Products and Resources, 2010; 1: 17-21.
- Mundt S, Kreitlow S and Jansen R. Fatty acids and antimicrobial activity from cyanobacterium Oscillatoria redekei Journal of Applied Phycology, 2013; 15: 263-267.
CrossRef - Khan Z. S. and Nasreen S. Phytochemical analysis, antifungal activity and mode of action of methanol extracts from plants against pathogens. Journal of Agricultural Technology. 2010; 6: 793-805
- Kumaran A and Karunakaran R. J. Antioxidant and free radical scavenging activity of anaqueous extract of Coleus aromaticus. Food Chem, 2006; 97: 109-114.
CrossRef - Chen J. H. and Ho C. T. Antioxidant activities of caffeic acid and its related hydroxycinnamic acid. J Agric Food Chem, 1997; 45: 2374-2378.
CrossRef - Rubio L, Motilva M. J. and Romero M. P. Recent advances in biologically active compounds in herbs and spices: a review of the most effective antioxidant and anti-inflammatory active principles. Crit Rev Food Sci Nutr, 2013; 53: 943–953.
CrossRef - Aderogba M. A, Okoh E. K and Idowu T. O. Evaluation of the Antioxidant Activity of the Secondary Metabolites from Piliostigma reticulatum (D.C.) Hochst. Biol. Sci., 2005; 5: 239-242.
CrossRef - Raghavendra H, Lakshmanashetty V. B, Nagaraj M. G. and Hiremath V. K. In vitro Antioxidant Activity of Vitex negundo L. Leaf Extracts. Chiang Mai Journal of Science, 2010; 37: 489-497.
- Kaushik A, Jijta C, Kaushik J. J, Zeray R, Ambesajir A and Beyene L. FRAP assay and effect of Diplazium esculantum (Retz) Sw. (A green vegetable of North India) on central nervous system. Indian Journal of Natural Products and Resources, 2012; 3: 228-231.
- Burda S, Oleszek W. Antioxidant and antiradical activities of flavonoids. Agric. Food. Chem, 2001; 49: 2774-2779.
CrossRef - Ara N. and Nur H. In vitro antioxidant activity of methanolic leaves and flowers extracts of Lippia alba. J. Med. Med. Sci., 2009; 4: 107-110.
- Adedapo A. A, Jimoh F. O, Afolayan A. J and Masika P. J. Antioxidant Properties of the Methanol Extracts of the Leaves and Stems of Celtis Africana. Rec. Nat. Prod, 2009; 3: 23-31.







