Manuscript accepted on :25-Sep-2020
Published online on: --
Plagiarism Check: Yes
Reviewed by: Marilena Stoian
Eman A . Elghoroury1, Esmat E. Abdelghaffar1, Aliaa Ahmed wahby1, Soha A Nasr1, Mohamed A. Hussein2, Walaa S. Nazim3, Gamila S.M. El-Saeed4, Eman Refaat Youness4* amd Hisham A. Orban4
1Clinical Pathology Department, National Research Centre Cairo Egypt.
2Internal Medicine Department, Cairo University, Egypt.
3Genetic Biochemistry Department, National Research Centre, Cairo Egypt.
4Medical Biochemistry Department, National Research Centre. Cairo Egypt.
Corresponding Author E-mail: hoctober2000@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1982
Abstract
In Egypt the incidence of HCC is increasing because of the high incidence of HCV infection. HCC starts silently with mild clinical and pathological deviation from chronic liver disease. Detection of specific and sensitive marker to help early prediction of such deviation to afford proper treatment was our aim.120 subjects were enrolled in the study, 40 patients suffering from chronic viral hepatitis C,40 developed hepatocellular carcinoma after HCV chronic hepatitis and 40 healthy controls. Liver functions,α-Fetoproteins (αFP), homocysteine(Hcy). Heat shock protein 70 (HSP 70) were done for all the subjects, MicroRNAsRQ26a and RQ27a were done using the RT-PCR method. Our results showed a significant difference between each of HCV & HCC group and the controls (P<0.05), while the difference between HCC and HCV group was highly significant (P<0.001) Except HSP70. RQ26a and RQ27a were down regulated in HCC group when compared to HCV group. It was previously shown that there was an inverse correlation between (RQ26a and RQ27a) results and each of, α FP, homocysteine and HSP70 within the HCC group. Again the same inverse correlation exists with Hcy. In conclusion, MicroRNAs (RQ26a and RQ27a) group, either solely or in combination with homocysteine might actually be used to classify sections with respect to progression to HCC and liver function, helping to reach treatment protocols and reside prognostic criteria.
Keywords
α-Fetoprotein; Hepatitis; Hepatocellular carcinoma; Homocysteine; MicroRNAs; Viral hepatitis.
Download this article as:| Copy the following to cite this article: Elghoroury E. A, Abdelghaffar E. E, Wahby A. A, Nasr S. A, Hussein M. A, Nazim W. S, El-Saeed G. S. M, Youness E. R, Orban H. A. Evaluation of Homocystein and Micro RNA as Diagnostic Markers for Hepatocellular Carcinoma in Virus Hepatitis C Egyptian Patients. Biomed Pharmacol J 2020;13(3). |
| Copy the following to cite this URL: Elghoroury E. A, Abdelghaffar E. E, Wahby A. A, Nasr S. A, Hussein M. A, Nazim W. S, El-Saeed G. S. M, Youness E. R, Orban H. A. Evaluation of Homocystein and Micro RNA as Diagnostic Markers for Hepatocellular Carcinoma in Virus Hepatitis C Egyptian Patients. Biomed Pharmacol J 2020;13(3). Available from: https://bit.ly/3j4lmZw |
Introduction
Hepatitis C virus (HCV) has prevalence approximately 3% worldwide 1. This dangerous viral infection could propagate to liver cirrhosis, fibrosis, chronic hepatitis and hepatocellular carcinoma (HCC)2. In Egypt, chronic HCV was the cause of 94% of HCC cases that resembles about 13% of all cancer cases in 2010. Also it is the second most frequent cancer in males with 6000–7000 deaths/year 1.
Diagnostic criteria of HCC were defined by the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL). Criteria mainly depend on finding some typical radiological changes in dynamic contrast-enhanced imaging 3. Biomarkers may resemble a useful diagnostic tool in cases with suspicious lesion in liver 4. Elevated serum α-fetoprotein (α-FP) to significantly high levels in patients with cirrhotic liver having suspicious liver mass >2 cm, was assured to diagnose HCC 5. Nowadays, this rationale has been cancelled because α-FP was found to be less sensitivity and specificity. Α-FP level didn’t elevate in about 80% of early HCC cases 3. Thus, studies are still searching for new non- invasive biomarkers that could help early diagnosis of HCC and improve the therapeutic approach of these patients 2.
Homocysteine (Hcy ) is known as an intermediate protein in methionine metabolic pathway, that takes place in the liver. Deterioration of liver function leads to alteration of methionine and homocysteine metabolic pathway;
Hcy level gradually elevates from normal tissue to cirrhotic liver and malignant tissue in HCC6.
There is a close relation between hyperhomocystinemia and malignancy. First, increased levels of homocysteine in plasma were noticed among patients with malignant tumors, also venous thromboembolism (VTE) is considered the second leading reason of doom in patients with malignancy. Second, many polymorphisms in the Hcy detoxification pathways enzymes (trans-sulfuration and remethylation) have major impact in many cancer types. Third, folate, with its main role in cell proliferation, inversely correlated with Hcy 7.
Heat shock protein 70 (HSP70) have been studied in cases with HCC 8.HSP 70 as a stress response protein functions for cells protection and their repair enhancement. It is overexpressed stress conditions, including carcinogenesis. Overexpression of HSP70 in HCC leads to promoting tumour growth and metastasis 9.
Recent studies have growing attention towards microRNA (miRNA) in the last decade 10. MiRNAs are non-coding small RNAs which function to regulate mRNA expression 11.
Also, microRNAs may function both intracellular through regulating the expression of a target molecules or extracellular after their release from the original cell in the form of free molecules or protein bound molecules 12. Studies have shown many advantages of miRNAs over other types of RNA like they are relatively stable against degradation represent the original cell and easily detected in all types of human body fluids 13, 14. Many studies have linked altered levels of miRNAs in different malignancies including HCC 15. MiRNAs including miR-192, miR-21, miR-27a, miR-223, miR-122, miR-801, and miR-26a have shown the highest accuratediagnosisfor the HCC especially on top of viral hepatitis from these mi-RNAs 15. Some of these miRNAs acting as oncogenes and others are acting as tumor suppressors as miR-26a & miR-27a16.
In our study the aim was to assess Hcy levels, correlate it with the expression of miR-26a and 27a and other biomarkers including HSP70 in liver cirrhosis and HCV infection-associated HCC patients.Our study also highlights the interaction of the Hcy in HCV infection-associated HCCdevelopment. Understanding this process could elucidate new therapeutic protocols to HCV infection-associated HCC patients.
Ethical Consideration
Subjects participated in this study were recruited from the outpatient clinic of both the National hepatology and the tropical medicine research institute and National Research Centre in Cairo. A written consent was taken from each subject enrolled in the study according to the ethical committee of National Research Centre.
Subjects and Methods
Study design
The inclusion criteria included: both sexes with age ranges from 18 to 70 years, cases of primary HCCafter chronic viral hepatitis Cand cases with liver cirrhosis caused by HCV. While the exclusion criteria included: ages below 18 or above 70, patients with metastatic malignancies in the liver, patients presented with tumors in any organs other than liver and patients possessing viral hepatitis B and chronic debilitating diseases.
The study included 120 subjects who were classified into three groups: first group comprises 40 patients with HCC (18 females & 22 males) aged from 50 to 63 (mean= 56.4) all of cases had chronic HCV infection.
Second group consists of 40 patients with liver cirrhosis second to chronic HCV infection (18 females and 22 males), aged from 50 to 76 (mean= 59.4).
Third group consists of 40 healthy controls with no apparent disease (21 males and 19 females) their ages ranged from 36 to 70 (mean= 53.9).
Full clinical assessment and laboratory investigations were done to all controls and patients including:
Clinical Examination: An itemized history taking comprising history of alcohol intake, smoking, drug intake, previous diseases, occupational exposure to chemicals. A thorough clinical valuation and total body examination through abdominal ultrasound and/or CT scan if needed to identify tumor extension and size. 5 ml blood sample was taken from each subject, divided, processed & stored until needed.
Study Investigations
Liver function tests
Total bilirubin, serum albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT) was measured using an automatic biochemistry analyzer (Olympus America Inc., Center Valley, Pennsylvania, USA).
Estimation of α- fetoprotein (α-FP)
Estimated using enzyme linked immune assay technique (ELISA) according to directions of Immunospec kits for α-FP 15
Determination of Heat shock protein 70 (HSP 70)
HSP 70 were estimated by Glory Science Co. for HSP 70 8. The Raylor Centre, James Street, Bioquote Ltd, York, YO10 3DW, United Kingdom
MicroRNA was extracted from serum by miRNA assay Plasma/Serum Kit (Qiagen, Hilden, Germany). Preparations of serum RNA were quantified using NanoDrop 1000 (Wilmingtion, Delaware, Nanodrop, USA). Measurement of serum microRNA levels was performed by microRNA-specific stem-loop primers (part of the TaqMan microRNA Assay Kit; Applied Biosystems) and TaqMan microRNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA). Real-time Quantitative PCR was done using the Quantistudio 12Kflex Real-Time PCR System (Applied Biosystems). The results were analyzed using the RQ manager software (Applied Biosystems).
The formula 2−⊿Ct was used to estimate serum levels of miRNA, where ⊿Ct =mean (Ct of internal references) − Ct of target miRNA. The relative expression levels of miR-26a and miR-27a were calculated and normalized to miR-16 (Applied Biosystems, Foster City, CA) 17 using the comparative ⊿Ct method and the equation 2−ΔΔCT, as described previously 18.
Quantification of Homocystien (Hcy)
It is estimated in serum by tandem mass spectrometry;ClinMass® LC-MS/MS Application MS2000, Gmbh Dessauerstraße 3 · D-80992 münchen.
Statistical analysis of the results was performed using statistical package for social science and Microsoft Excel 2010 (SPSS version 24.0) for windows (SPSS IBM., Chicago, IL). Simple t- test and Pearson correlation were done according to Hirsh and Riegl 19. All reported P- values were two-tailed, value <0.05 was considered significant.
Results
Demographic, laboratory, and clinical data of the studied groups are illustrated in table 1.
Table [2] illustrates the t-test results of each of α FP, Hyc, HSP 70 and MiRNAs (RQ26a& RQ27a). α FP result showed insignificant difference between control & HCV group (P≤0.05) but the difference between HCV infection-associated HCC and control, HCV infection-associated HCC and HCV were highly significant (P=0.00).
The same table showed that Serum Hyc level was insignificantly different in HCV group with controls (P≤0.05), where the difference was highly significant between HCV infection-associated HCC and control (p=0.001).
In addition, there were significant difference between HCV and HCV infection-associated HCC groups was found (p= 0.000). Concerning HSP70 Results, statistical analysis denoted a high significant difference among the HCV and control groups.
Comparison of the mean values molecular parameters RQ26a and RQ27a between studied groups showed significant differences between the three group exceptfor the HCV infection-associated HCCand control groups in RQ26a with down-regulation in HCV infection-associated HCC group less than HCV.
Correlation of the parameter showed that α-FP was correlated positively with ALT and bilirubin and was negatively correlated with Hcy and albumin.
HSP 70 results showed association with alb.(P= 0.04)., while a direct correlation was found between RQ 26a and RQ 27a (P = 0.00),as showed in table(3)
Table 1: Age and liver functions parameters in the studied groups
| Descriptive
Parameters |
Control
|
HCV | HCC |
| Age | 51.86±7.9o | 57.50±4.76 a | 56.00±2.73 |
| ALT (U/L) | 31.22±5.30 | 68.68±16.10 | 1.2E3±579.54 ab |
| AST (U/L) | 28.88±4.83 | 67.95±15.32 | 2.07E3±953.22 ab |
| Albumin (mg/dl)
|
4.38±0. .40 | 3.33±0.46 | 3.12±0.42 a |
| Bilirubin
(mg/dl) |
0. 57±0.1 3 | 1.18±_0. .23 a | 1.56±0. .33 ab |
Table 2: Alfa-fetoprotein (α FP), Homocysteine, HSP 70, RQ26a and RQ27a in the studied groups
| Parameters |
Control
|
HCV | HCC |
| Alfafetoprotein (pg/ml) | 3.55±3.07 | 7.80±0.613 a | 9.56E2±384.70 ab |
| HSP70 (pg/ml) | 73.26±10.37 | 60.00±10.614 a | 67.33±7.500 |
| RQ_26a
x 102 |
0.69±0.73 | 85.00±30.27 a | 30.66±12.42 ab |
| RQ_27a | 1.40±2.45 | 16.40±6. 39 a | 4.42±1.92b |
| Homocysteine(nmol/L) | 13.05±3.87 | 13.03±0..79 | 16.98±1.38 a |
 |
Table 3: Correlation between the studied parameters
|
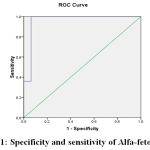 |
Figure 1: specificity and sensitivity of Alfa-fetoprotein |
Area Under the Curve
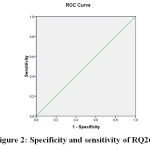 |
Figure 2: specificity and sensitivity of RQ26 |
Area Under the Curve
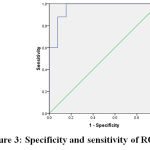 |
Figure 3: specificity and sensitivity of RQ27 |
Area Under the Curve
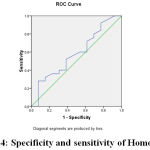 |
Figure 4: specificity and sensitivity of Homocystein |
Area Under the Curve
Discussion
Hepatocellular carcinoma (HCC) is found to be the greatest considerably diagnosed cancers internationally, and it is prevalent in Africa and Asia 20. Patients with history of chronic hepatitis and cirrhosis mostly develop HCC as a result of continuous regeneration and inflammation of hepatocytes. HCC is considered the second cause of cancer-related fatality across the world 21. Our study aim was to detect specific and sensitive markers to help early prediction of such deviation to afford proper treatment.
Our study results showed that the liver functions of both patient groups were significantly deteriorated as compared with controls. Obviously due to HCV infection, inflammation, fibrosis, and cirrhosis which lead to liver failure fulminate in HCC. These results were in agreement with Fathy and coworker 20 who found that there was a highly significant difference in all measured liver function tests as well as α-FP between healthy control group and HCC group 20.
The present study also revealed that αFP results indicated that the difference between HCC and HCV was significantly high. These results showed that αFP could be considered as signal for carcinoma. αFP is currently widely used to distinguish between HCC and benign liver lesions.Diagnosis of HCC after HCV chronic hepatitis without confirmatory pathology can be obtained by measuring serum αFP level in combination with techniques of imaging, that include ultrasonography, computerized tomography and magnetic resonance imaging 21,22. A study by Filmuset al.23showed limited sensitivity of αFP (41-65%|0 23.
On the other hand, serum Hcy results of this study were highly significant in HCC group when compared to controls, while the difference was highly significant between HCC and HCV. These results denoted that only affected hepatocytes do express Hcy, and its expression is significantly high in hepatic diseases like HCC. The same conclusion was reached by Malaguarneraetal.24 considered serum Hcy as a valuable biomarker for patients with HCC 24.
Mustafaetal.25 concluded that Hyc level is increased significantly in patients with HCv when compared with healthy controls, decreased following treatment. The present study showed that there is a positive correlation between Hyc and HCC, a result which help in establishing diagnosis of HCC 25.
Studies showed that the Hcy levels were different in early and advanced cancer grades. Nevertheless, we hypothesize that early stage cells might not produce Hcy, as it enhance the process of proliferation of tumour cells. Researchers have found that elevated homocysteine levels lead to enhanced proliferation of cells in Caco-2 cell lines. This increased proliferation was found to be reversed by the culture medium with folate supplementation or by supplementation with its metabolites at a later step, such as 5-MTHF. Nevertheless, advanced-grade tumour cells might produce Hcy because elevated Hcy plasma levels might be also cytotoxic to the malignant cells. Thus, it could be of great importance for proliferating cells to keep an optimized Hcy level. However, this hypothesis needs additional experimental validation. 7.
Thus, Hcy-elevating treatments should be selectively prescribed to HCC patients, and physicians should evaluate Hcy levels after surgery or chemotherapy. Recently, the impact of Hcy on the tumor cells proliferation & growth is still poorly clarified. A closer understanding of the impact of Hcy on growth and proliferation of tumour cells would result in novel, promising strategies to cure cancer. However, Hcy could be of a great value as a potential tumor biomarker for different types of malignancies 7.
HSP70 was lower in our HCV group morethan control group (p=0.003). The opposite results were reached before by Sakamoto et al.26, who stated that by gene profiling and gene expression from about 12 600 genetic analysis, HSP70 is significantly upregulated in early HCC[26]. HSP70 expression is not observed in benign nodules or other non-malignant nodular lesions, focal nodular hyperplasia and hepatocellular adenoma. Thus HSP70 might be considered as a beneficial biomarker to distinguish early HCV from precancerous nodules and to distinguish malignant and benign liver nodules.
In addition, Shin E et al.27stated that HSP70 may be used as an indicator of prognosis for HCC. Its conclusion was established in 282 out of 392 cases of HCC (71.9%), however only 14 of 115 benign liver tissues expressed HSP70 (P<0.001) 27. Whereas Tremosini et al. 28 specified that the specificity and sensitivity in the detection of HCC of HSP70 were speculated as 57.5% and 85%, respectively [28]. The same results were reached by Gehrmann M et al. 29 who concluded that serum HSP70 levels are consecutively increased in patients with HCV and HCC and thus might have a prognostic value 29.
MicroRNAs (miRNAs) are evolutionarily preserved small no coding RNAs involved in the protein translation and regulation of gene expression.
Several researchers have identified their important role in driving tissue and organ differentiation in the embryogenesis period and in the fine-tuning of fundamental biological processes, like apoptosis and proliferation30. Results of the present study showed that MiRNAs level (RQ26a & RQ27a) were significantly down regulated in both HCC and HCV groups less than controls, where the lowest level was found in HCC cases. Furthermore, our study revealed that RQ26a level was directly associated with RQ 27a in HCV-associated HCC group (P = 0.000). Zhao et al. specified that MiR-27a-3p was identified as a tumor suppressor in other tumours 31. Hayes et al stated that growing evidence detected that their deregulation has a crucial role in malignant tumour onset and progression, where they function as oncogenes or onco-suppressors32. The same conclusion was reached by Thurnherr et al. mentioned that miRNAs ( miR-122, miR-26a and miR-130a) were found to be down-regulated in HCC, also, their genetic targets that up-regulated were combined mainly with abnormal cell proliferation that includes replication of DNA, nucleotide metabolism and transcription33. On the contrary, He et al. detected that the miR-27b expression levels were increased significantly in HCC cell lines, if compared to normal human hepatic cells. Also, in HCC tissues miR-27b was mostly up regulated, when compared to normal adjacent tissues34. Moreover, increased expression levels of miR-27b were correlated significantly with tumor differentiation, vascular invasion and Metastasis stage of Tumor Node (P<0.05). MiR-27b knockdown expression inhibited HCC cell invasion and migration34. MiRNA panel in plasma had significant clinical importance for HCC early diagnosis and might be useful tool for patients to benefit from optimal therapeutic protocols35. Researchers detected a panel of miRNA (miR-26a, miR-27a, miR-801miR-122, miR-192, miR-21 & miR-223,) that was found to be of high diagnostic accuracy for distinguishing cases with HCC from the healthy population36. Also Wang et al. suggested that miR-26a loss as a tumor suppressor are specific marker of HCV infection-associated HCC while miR-122,miR-181 and miR-23a altered expression appears to be a less specific marker of HCV infection-associated HCC37.
A meta-analysis done by Petrizzo et al. microarray-based transcriptional profiles of HBV on large datasets and HCV-associated HCC showed inhibited state for manymiRs. Particularly specific inhibition of miR-146,miR-16, and the let-7 family of miRs in HCC while miR-124 ,miR-26, and miR-155 were inhibited in HBV-associated HCC.miR-29, miR-24, , miR-29andmiR-1241 were inhibited in HCV-associated HCC38.
The prognosis of HCC patients does depend only on tumor size and number but also affected by a complex interplay between different genetic, epigenetic and environmental factors39. Thus, the ability to predict patient prognosis is complex.
However, results reported by Shi and his colleagues declared that HCC patients with miR-26 low levels respond better to interferon-alpha treatment compared to patients with higher levels, suggesting that miR-26 expression can be considered as good predictor and indicator of the interferon-alpha therapy response40.
classical biomarkers to diagnose early HCC. This helps to improve HCV-associated HCC management and reach best treatment decisions for sake of the patients. Limitation of the study was the small sample size & detection of only 2 miRs. More studies on a greater number of patients are required to confirm these findings & clarify their role in disease staging and prognosis conclusion, miRNAs26a and 27a expression altered in HCV-associated HCC patients and can be used either alone or in with other biomarkers.
There is no obvious explication for why the Hcy levels differ among late and early cancer stages. Nonetheless, we contemplate that in the early stage cells might not release Hcy, as it encourages the cancer cells proliferation process. Researches have revealed that raised levels of homocysteine cause enhanced cellular proliferation in Caco-2 cell lines. This augmented proliferation could be overturned via supplementation of folate in the culture medium or via supplementation with its downstream metabolites, such as 5-MTHF. Nevertheless, cancer cells in the advanced-stage might release Hcy as a very high concentration of Hcy might also be cytotoxic to the cancer cells. Consequently, it might be significant to maintain an optimum Hcy concentration for proliferating cells. However, this conjecture demands extra experimental validation39.
Hence, Hcy-elevating drugs should be prescribed restrictively to HCV-associated HCC patients, and Hcy levels should be monitored by physicians after surgery or chemotherapy. Insight into the impacts of Hcy on the growth and proliferation of cancer cells could yield promising, novel strategies to restrain cancer. So, Hcy could be utilized as a prospect tumor biomarker for an assortment of cancers40.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
The authors are thankful to the National Research Centre for the unlimited support to carry out this work.
Conflict of Interest
This work has no fund
References
- Schütte K, Schulz C, Link A, Malfertheiner P. Current biomarkers for hepatocellular carcinoma: Surveillance, diagnosis and prediction of prognosis . World J Hepatol.; 7(2): 139–149 |(2015).
CrossRef - JiezhaoY,JuQ, LiG. Tumor markers for hepatocellular carcinoma. MolClinOncol.; 1(4): 593–598 (2013).
CrossRef - Saffroy R, Pham P, Reffas M, Takka M, Lemoine A, Debuire B. New perspectives and strategy research biomarkers for hepatocellular carcinoma. ClinChem Lab Med.;45:1169–1179 (2007).
CrossRef - Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology.; 42:1208–1236 (2005).
CrossRef - Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut.; 63:844–855 (2014).
CrossRef - Zhang D, Lou J, Zhang X, Zhang L, Wang F, Xu D, Niu N, Wang Y, Wu Y, Cui W.Hyperhomocysteinemia results from and promotes hepatocellular carcinoma via CYP450 metabolism by CYP2J2 DNA methylation.; 8(9):15377-15392 (2017).
CrossRef - HasanT, , AroraR, BansalAK, BhattacharyaR, SharmaGS, SinghLR . Disturbed homocysteine metabolism is associated with cancer Experimental & Molecular Med;51: 21(2019).
CrossRef - Luk JM, Lam CT, Siu AF, et al. Proteomic profiling of hepatocellular carcinoma in Chinese cohort reveals heat-shock proteins (Hsp27, Hsp70, GRP78) up-regulation and their associated prognostic values.Proteomics.; 6:1049–1057 (2006).
CrossRef - Witjes CD, van Aalten SM, Steyerberg EW, Borsboom GJ, de Man RA, Verhoef C, Ijzermans JN. Recently introduced biomarkers for screening of hepatocellular carcinoma: a systematic review and meta-analysis. HepatolInt.;7:59–64 (2013).
CrossRef - Xiang ZL, Zeng ZC, Fan J, et al. Gene expression profiling of fixed tissues identified hypoxia-inducible factor-1α, VEGF, and matrix metalloproteinase-2 as biomarkers of lymph node metastasis in hepatocellular carcinoma.Clin Cancer Res.;17:5463–5472 (2011).
CrossRef - Zhang L, Wang JN, Tang JM, et al. VEGF is essential for the growth and migration of human hepatocellular carcinoma cells.MolBiol Rep.;39:5085–5093 (2012).
CrossRef - Yamamoto Y, Kosaka N, Tanaka M, et al. MicroRNA-500 as a potential diagnostic marker for hepatocellular carcinoma.Biomarkers.;14:529–538 (2009).
CrossRef - Hou J, Lin L, Zhou W, et al. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell.; 19:232– 243 (2011).
CrossRef - Tomimaru Y, Eguchi H, et al. Circulating microRNA-21 as a novel biomarker for hepatocellular carcinoma. J Hepatol.; 56:167–175 (2012).
CrossRef - Xieraili M, Yasen M, Mogushi K, et al. Villin 1 is a predictive factor for the recurrence of high serum alpha-fetoprotein-associated hepatocellular carcinoma after hepatectomy.CancerSci.; 103:1493–1501(2012).
CrossRef - TamoriA, MurakamiY, KuboS, ItamiS, Uchida-KobayashiS, MorikawaH, EnomotoM, TakemuraS, TanahashiT,TaguchiY,KawadaN. MicroRNA expression in hepatocellular carcinoma after the eradication of chronic hepatitis virus C infection using interferon therapy.Hepatology Research.; 46: E26–E35 (2016).
CrossRef - YuF, LuZ, ChenB, DongP,ZhengJ .microRNA-150: a promising novel biomarker for hepatitis B virus-related hepatocellular carcinoma .Diagnostic Pathology.; 10:129 (2015).
CrossRef - Yao J, Liang L, Huang S, Ding J, Tan N, Zhao Y, et al. MicroRNA-30d promotes tumor invasion and metastasis by targeting Galphai2 in hepatocellular carcinoma.Hepatology.;51:846–56 (2010).
CrossRef - Hirsh R, Riegl R. Studying a study and testing a test, how to read the medical literature. 2nd ed. Boston: Little, Brown and Company, (1989).
- Fathy WM, Montaser B A, El-AssalM.Angiotensin-Converting Enzyme Gene Polymorphism (insertion/deletion) and The Risk of Hepatocellular Carcinoma in Egyptian HCV and HBV Patients.Journalof American Science.;12(3) ). 116-124 (2016).
- FornerA ,Bruix J. Biomarkers for early diagnosis of hepatocellular carcinoma. Lancet Oncol.; 13:750–751 (2012).
CrossRef - NjeiB, RotmanY, DitahI, LimJK .Emerging trends in hepatocellular carcinoma incidence and mortality.Hepatology.; 61: 191–199 (2015).
CrossRef - Filmus J, Capurro M. Glypican-3 and alphafetoprotein as diagnostic tests for hepatocellular carcinoma.MolDiagn.; 8(4):207-12 (2004).
CrossRef - Malaguarnera M, CataniaVE , BorzìAM, MalaguarneraS,MadedduR, BertinoG, LatteriS. Blood homocysteine levels are increased in hepatocellular carcinoma patients with portal vein thrombosis. A single centre retrospective cohort study. International Journal of Surgery Open.;15: 60-65(2018).
CrossRef - Mustafa M, HussainS, QureshiS, MalikSA, KazmiAR,NaeemM. Study of the effect of antiviral therapy onhomocysteinemia in hepatitis C virus- infected patients. BMC Gastroenterology.;12:117 (2012).
CrossRef - Sakamoto M. Early HCC: diagnosis and molecular markers. J Gastroenterol.; 44(19):108-11(2009).
CrossRef - Shin E, Ryu HS, Kim SH, Jung H, Jang JJ, Lee K. The clinicopathological significance of heat shock protein 70 and glutamine synthetase expression in hepatocellular carcinoma.J Hepatobiliary Pancreat Sci.; 18:544–550 (2011).
CrossRef - Tremosini S, Forner A, Boix L, et al. Prospective validation of an immunohistochemical panel (glypican 3, heat shock protein 70 and glutamine synthetase) in liver biopsies for diagnosis of very early hepatocellular carcinoma.Gut.;61:1481–1487 (2012).
CrossRef - Gehrmann M, Cervello M, Montalto G, Cappello F, Gulino A, Knape C, Specht HM, Multhoff G . Heat shock protein 70 serum levels differ significantly in patients with chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma.Front Immunol.; 1;5:307 (2014).
CrossRef - Schütte K, Schulz Ch, Link A, Malfertheiner P. Current biomarkers for hepatocellular carcinoma: Surveillance, diagnosis and prediction of prognosis.World J Hepatol.; 7(2): 139–149 (2015).
CrossRef - Zhao N, Sun H, Sun B, Zhu D, Zhao X, Wang Y, Gu Q, Dong X, Liu F, Zhang Y, Xiao L .miR-27a-3p suppresses tumor metastasis and VM by down-regulating VE-cadherin expression and inhibiting EMT: an essential role for Twist-1 in HCC. Sci Rep.; 6: 23091 (2016).
CrossRef - Hayes CN, Chayama K. MicroRNAs as Biomarkers for Liver Disease and Hepatocellular Carcinoma.Int. J. Mol. Sci.; 17: 280 (2016).
CrossRef - Thurnherr Th, Mah W, Lei Z, Jin Y, Rozen SG, Leea CG. Differentially Expressed miRNAs in Hepatocellular Carcinoma Target Genes in the Genetic Information Processing and Metabolism Pathways.Sci Rep.; 6: 20065 (2016).
CrossRef - He S, Zhang J, Lin J, Zhang C, Sun S .Expression and function of microRNA-27b in hepatocellular carcinoma.Molecular Medicine Reports.; 13 , 3: 2801-2808 (2016).
CrossRef - Giordano S, Columbano A. MicroRNAs: new tools for diagnosis, prognosis, and therapy in hepatocellular carcinoma.Hepatology.; 57(2):840-7 (2013).
CrossRef - ZhuK, DaZ,ZhoulJ.Biomarkers for hepatocellular carcinoma: progression in early diagnosis, prognosis, and personalized therapy Biomarker Research.; 1:10 (2013).
CrossRef - Wang Z, Wu N, Tesfaye A, Feinstone S, Kumar A .HCV infection-associated hepatocellular carcinoma in humanized mice. Infect Agent Cancer.; 10: 24 (2015).
CrossRef - Petrizzo A, Caruso FP, Tagliamonte M, Tornesello ML, Ceccarelli M, Costa V, Aprile M, Esposito R, Ciliberto G, Buonaguro FM, Buonaguro L. Identification and Validation of HCC-specific Gene Transcriptional Signature for Tumor Antigen Discovery. Scientific Reports. 6,; Article number: 29258 (2016).
CrossRef - Zhou J, Yu L, Gao X, Hu J, Wang J, Dai Z, Wang JF, Zhang Z, Lu S, Huang X, Wang Z, Qiu S, Wang X, Yang G, Sun H, Tang Z, Wu Y, Zhu H, Fan J. Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma.J ClinOncol.; 20;29(36):4781-8 (2011).
CrossRef - Shi J, Budhu A, Yu Z, et al, MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med.; 361:1437-1447 (2009).
CrossRef







