Manuscript accepted on :
Published online on: --
Plagiarism Check: Yes
Reviewed by: Dr. Asim Faraz
Dahlan-Daud C. K1, Zain Z. N2, Tham C. L.1, Yong Y. K.3 and Hakim M. N1,4
1Department of Biomedical Sciences, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Malaysia.
2Faculty of Medicine and Health Sciences, Universiti Sains Islam Malaysia, Kuala Lumpur, Malaysia.
3Department of Human Anatomy, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Malaysia.
4Laboratory of UPM-MAKNA Cancer Research (CANRES), Institute of Bioscience, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia.
Corresponding Author E-mail: nazrulh@upm.edu.my
DOI : https://dx.doi.org/10.13005/bpj/1974
Abstract
The 6-Mercaptopurine (6-MP) is a purine sulphur derivative used in the treatment of children with leukaemia as an antitumor medication. Nonetheless, the anti-proliferation and anti-rheumatoid arthritic activities of the drug have not been previously described. Therefore, the current study objective was to determine the cytotoxicity effects of 6-MP, 6-mercaptopurine riboside (6-MPR), 6-thioguanine (6-TG) and 6-thioxanthine (6-TX) on murine macrophages (RAW 264.7) and rabbit synoviocytes (HIG-82) cell lines. An inflammatory stage of RAW 264.7 and HIG-82 cell lines stimulated with Escherichia coli lipopolysaccharide (LPS) and phorbol-12-myristate acetate (PMA), respectively were treated with the drugs at a serials concentration 3.125 – 100 µM. Subsequently, the MTT assay was used to determine the viability of cells. The inhibitory effects were measured based on nitric accumulation in the conditioned media. The results showed that all drugs tested did not show any cytotoxic effect on both cell lines at low and medium concentrations, which the HIG-82 cell viability was more than 80%. However, the drugs display anti-proliferation property on RAW 264.7 cells compared to control. Reduction on cell proliferation was found on all tested drugs. Among its, the diclofenac significantly reduced the proliferation on HIG-82 cell compared to other drug compounds. Inhibitory effects of compound on nitric oxide production PMA-stimulated HIG-82 had only a small inductive effect, but excellent inhibition was observed on RAW 264.7 cell. Meanwhile, 100 μM 6-thioguanine and diclofenac had cytotoxic effect to RAW 264.7 and HIG-82 cell respectively. All thiopurines enhanced the proliferation of HIG-82 and RAW 264.7 cells with no cytotoxic effects. This finding opens new avenues for treating RA and the anti-rheumatic activities during synovial inflammation stage of RA. The inhibitory effects of 6-MP and 6-MPR to inflammatory cells marker such as synovial fibroblast and macrophages by proliferating healthy synoviocytes more potenly than 6-TG and 6-TX. The current study has demonstrated the potential use of 6-MP and its associated thio compounds in the in-vitro approach of HIG-82 cell culture in curing rheumatoid arthritis disease.
Keywords
Cytotoxicity; HIG-82; Nitric oxide; RAW 264.7
Download this article as:| Copy the following to cite this article: Dahlan-Daud C. K, Zain Z. N, Tham C. L, Yong Y. K, Hakim M. N. Effects of 4 Thiopurine Compounds on Nitric Oxide Production and Cell Viability of HIG-82 Synoviocytes and RAW 264.7 Macrophages. Biomed Pharmacol J 2020;13(3). |
| Copy the following to cite this URL: Dahlan-Daud C. K, Zain Z. N, Tham C. L, Yong Y. K, Hakim M. N. Effects of 4 Thiopurine Compounds on Nitric Oxide Production and Cell Viability of HIG-82 Synoviocytes and RAW 264.7 Macrophages. Biomed Pharmacol J 2020;13(3). Available from: https://bit.ly/3d09l6a |
Introduction
Drugs for the treatment of inflammatory arthritis particularly related to rheumatoid arthritis (RA) have been not comprehensively explored. Worldwide, RA is a serious health problem that affects up to 1.0% of adults in developed countries (Parada-Turska et. al., 2006; Wang et. al., 2006). In the USA alone, the total annual cost of arthritis to society is compensated for by more than 20 million RA patients with severe developmental disabilities on a daily basis and estimated at $100 billion (Scott et. al., 2010). In Malaysia, 5 in 1000 adults are reported to be the RA patient by a year (Shahrir et. al., 2008). As consequent, RA has a significant impact on physical, emotional, psychological and social activities of patients in the daily basis; they restricted in most functioning on works as well reducing life expectancy, premature mortality further causing the massive economic burden to the society (Too et. al., 2012).
To date, patients suffering from arthritis such as Crohn’s, ulcerative colitis, and chronic arthritis have been prescribed with a long-single potent drug that has experienced serious side effects. As an example, the potent drugs like disease-modifying anti-rheumatic drugs (DMARD’s) (e.g: azathioprine, methotrexate and tofacitinib), non-steroidal anti-inflammatory drugs (NSAID’s) (e.g; diclofenac) and glucocorticoids (Nielsen et. al., 2001). DMARD drugs are often used with a combination with NSAIDs or glucocorticoids greatly improved the quality of life for many patients after 4-6 months to be fully effective. Unfortunately, DMARDs target the immune system of the patients, which weaken the immune system’s ability to fight infections. Subsequently, the chemotherapy to the cells affected disease become resistance toward the drugs. This is a major factor in the failure of many forms of chemotherapy, especially to cancer patients. These problems of course reduce the drug’s efficacy (Shanker et. al., 2010).
Hyper-proliferation of synovial fluid (SF) and synoviocytes in the synovial membrane lead to the development of pannus which subsequently cause the invasion to the joint and bone that causes damage to the affected area (Smolen et. al., 2014; 2007; Firestein, 1996) and SF play a vital role in facilitating inflammatory action, cartilage degradation and joint destruction in RA (Mor et. al., 2005). A study using SCID mouse revealed that intrinsic factor with the immune system support contributes to join damage and degradation. Thus, it indicates that the invading products, if synoviocytes may be isolated from inflammatory cells and serve as a key target for the progression of arthritis (Muller-Ladner et. al., 2007). In this research, the HIG-82 synoviocyte cell line, which retains both the morphology and differentiation markers of synovial fibroblast, was used as a RA cell model to evaluate the therapeutic action of thiopurine compounds. Phorbol myristate acetate (PMA) is a well-known stimulator to activate necrosis factor-kappa B (NF-κB) in the cell was applied to resemble the inflammatory and arthritic condition in activated synoviocytes (Kim and Ro, 2005; Smith et. al., 1998; Baeuerle and Henkel, 1994; Hasan et. al., 2012). NF-κB regulates the expression of target genes that encode inflammatory cytokines such as interleukin (IL)-1, IL-6, IL-12, tumour necrosis factor-alpha (TNF-α), interferons (IFNs), chemokines and enzymes that cause inflammatory response such as COX-2 and inducible nitric oxide synthase (iNOS) (Piccinini and Midwood, 2010).
Nitric oxide (NO) is a short-lived, reactive species molecule synthesized by NO synthases (NOS) catalyzed by L-arginine (Nathan, 1991). Many biological responses involved by NO molecule such as inflammation, cell-mediated immune response, relaxation of the blood vessels and neurotransmission (Miyasaka, 1997). There are at least two NOS isoforms; Ca2 + Calmodulin-dependent constitutive NOS (cNOS), present primarily in endothelial cells and brain, and Ca2 + Calmodulin-independent inducible NOS (iNOS), residing predominantly in macrophages and smooth muscle cells (Nagy et. al., 2007). Nitric oxide (NO) is generally produced in large quantities by activated macrophages as one of the host defence during inflammation. NO can catalyse by distinct three isoforms of nitric oxide synthase. However, the enzyme which plays the most critical role in inflammation is iNOS. After activated by endotoxin or pro-inflammatory cytokines such as IL-1, IFN-gamma (IFN-γ), TNF-α (Miyasaka, 1997), murine macrophage produces large amount of NO (Laroux et. al., 2000). Purinethol or 6-mercaptopurine (6-MP) (Figure 1) is a derivative of purine sulphur approved by the Food and Drug Administration ( FDA) as an antitumor drug in 1953 (Pilar et. al., 1996). 6-MP was among the first effective drugs for childhood leukaemia and subsequently refined as an effective anti-cancer and immunosuppressant medication in the previously poor prognosis of the disease (Zaza et. al., 2005; Bell et. al., 2004; Elion, 1989).
Thiopurine compounds are purine anti-metabolic, which interferes with a biochemical process involving endogenous purines, which are vital components of DNA, RNA and certain co-enzymes (Coulthard, 2012; Cara et. al., 2004; Coulthard et. al., 2002; Lennard, 1992). The thiopurines were initially tested for leukaemia treatment and as an immunosuppressant for organ transplantation. The compounds of thiopurine have a fairly small therapeutic index and are capable of life-threatening toxicity (Sahasranaman et. al., 2008). Thiopurines can have a cytotoxic effect in a myriad of areas. Previous reported uses the thiopurines for inflammatory bowel disease and ulcerative colitis, eventually by controlled clinical trials (Konidari and El-Matary, 2014; Frei et. al., 2013; Mahadevan et. al., 2000; Ricketts, 1998). 6-MP and its derivatives block the bio-activation of NF-κB and its related cytokines (Chang et. al., 2012). They prevent bio-activation by inhibiting de novo purine synthesis and integrate specific cells, including neutrophils, macrophage, lymphocyte, and endothelial cells, into nucleic acids (Ordentlich et. al., 2003; Hussein-Al-Ali et. al., 2012). Possessions are dose-related, low doses of drugs are anti-inflammatory, but higher doses are immunosuppressive and cytotoxic (Polifka and Friedman, 2002). Therefore, effects of thiopurine compounds namely, 6-mercaptopurine (6-MP), 6-MP riboside (6-MPR), 6-thioguanine (6-TG) and 6-thioxanthene (6-TX) on cell viability and inhibitory effects on nitric oxide production were examined on PMA-activated HIG-82 synoviocytes fibroblast and LPS-induced murine macrophage, RAW 264.7 cell lines. The therapeutic effects of thiopurines compounds, particularly on the cell viability with less cytotoxicity is crucial. Therefore, the objectives of the current study: firstly, to examine the effects of immunosuppressive thiopurine anti-metabolites compounds on cell viability of phorbol myristate acetate (PMA)-activated HIG-82 synoviocytes fibroblast and Escherichia coli lipopolysaccharide (LPS)-induced RAW 264.7 murine macrophage cell lines. Secondly, to examine inhibitory effects of immunosuppressive thiopurine anti-metabolites on inducible nitric oxide production of phorbol myristate acetate (PMA)-activated HIG-82 synoviocytes fibroblast and Escherichia coli lipopolysaccharide (LPS)-induced RAW 264.7 murine macrophage cell lines and thirdly, to investigate the cytotoxicity effects of immunosuppressive thiopurine anti-metabolic compounds due to nitric oxide production in responding to compound dosage. We hypothesized that these thiopurine compounds will be potentially useful for the treatment of RA.
Materials and Methods
Thiopurine compounds
All reagents and chemical used were purchased from commercial manufactures. Antibiotics (glutamine-penicillin-streptomycin 100x), fetal bovine serum (FBS) and trypsin (0.25% – EDTA in HBSS w/o calcium w/o magnesium w/ phenol red) from Biowest (France), nutrient mixture Ham’s F-12 from Sigma Chemicals (St. Louis, USA), dimethyl sulfoxide (DMSO) and phosphate buffer saline (PBS) from Amresco (Solon, USA), phorbol-12-myristate 13-acetate (PMA) and thiopurine compounds (6-mercaptopurine and 6-mercaptopurine riboside) from Acros Organic (New Jersey, USA), 6-thioguanine and 6-Hydroxy-1,6-mercaptopurine (6-thioxanthene) from Sigma-Aldrich (St. Louis, USA), diclofenac sodium from Sigma-Aldrich (St. Louis, USA), MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] from PhytoTechnology Laboratories (KS, USA); Griess reagent from Merck-Chemical (Germany); sodium nitrate (NaNo2) from Sigma Chemicals (St. Louis, USA).
The 6-MP, 6-MPR, 6-TG and 6-TX and diclofenac (Figure 1) were dissolved in 100% dimethyl sulfoxide (DMSO) at stock 0.05 M stock solution. Further, from the stock, a serial dilution with Dulbecco’s Modified Eagle’ medium (DMEM) (Sigma-Aldrich, St Louis, USA) to give a final concentration 100 µM with 0.2% DMSO is used for Murine macrophage RAW 264.7 cell line. Meanwhile, a serial dilution with nutrient mixture Ham’s F12 with L-glutamine (Sigma-Aldrich, St Louis, USA) is used for Rabbit synoviocytes HIG-82 cell line. Further diluted with diluents to 6 different serial concentrations between 100 µM and 3.125 µM. The final concentration of DMSO as a vehicle remained constant at 0.15%.
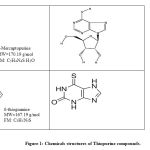 |
Figure 1: Chemicals structures of Thiopurine compounds. |
Macrophage abelsen murine leukaemia virus-transformed (RAW 264.7 cell line)
Mouse macrophage RAW 264.7 cell line, obtained from ATCC (American Type Culture Collection, Manassas, VA, USA) was stored at 37 °C in a humidified atmosphere of 95% O2 and 5% CO2. The culture medium constituted of Nutrient Mixture DMEM (Sigma Chemicals, St. Louis, MO, USA) supplemented by 10% foetal bovine serum ( FBS; Biowest, South America), 100 U/ ml penicillin (Biowest) and 100 μg/ml streptomycin (Biowest). The cells were sub-cultured every 2–3 days at 1:5 split ratios after 85 -100% confluency.
Rabbit synoviocytes fibroblast (HIG-82 cell line)
HIG-82 synoviocyte cell line (ATCC CRL-1832) was obtained from ATCC was kept at 37 °C in a humidified 95% O2 and 5% CO2. The culture medium consisted of Nutrient Mixture F-12 Ham (Sigma Chemicals, St. Louis, MO, USA) supplemented with 10% of foetal bovine serum (FBS; Biowest, South America), 100 U/ml penicillin (Biowest) and 100 μg/ml streptomycin (Biowest). Culture medium was changed every 3-4 days. After entering the confluence, which took 2–3 weeks, cells were subcultured serially using a solution of 0.25% Trypsin-EDTA with a subculture ratio of 1:2 to 1:4 and subjected to experiments below passage 10.
Induction and stimulation of inflammation phase
Methods previously described by Parada-Turska et. al. (2008) and Jeoung et. al. (2013) were employed with slight modifications. The cells growing at exponential phase with 80–90% confluence were detached from the plastic surface by gently trypsinized with Trypsin-EDTA (0.25%) solution. After the cells had completely detached from the surface of the flask, then the completed media was added to stop the trypsin activity. The cell suspension was relocated into a centrifuge tube of 15 mL and centrifuged at 4 ° C at 190 x g for 10 minutes. After removal of the supernatant, the cell pellets were re-suspended with 1 mL of completed growth medium; RAW 264.7 cell in DMEM with 10% fetal bovine serum and HIG-82 cell line in Ham’s F12 containing 10% fetal bovine serum and the concentration of cells were adjusted to 1 x 106 cells/mL and 1 x 104 cells/mL for both cells respectively by adding the calculated volume of completed growth medium above. Basically, 10 uL of suspension cells were mixed at ratio 1:1 with 0.4% trypan blue solution and the number of cells was calculated using a haemocytometer. Dead cells absorbed the blue colour of trypan blue due to the membrane permeability, while the viable cells remained unstained. The viability of cells for the assay had to be at least 95%. The cell viability and the volume of the completed growth medium needed were calculated.
For cell stimulation and treatment, 50 µL of the diluted cell suspension (1 x 106 cells/mL) were dispensed into a tissue culture grade 96–well flat bottom plate (Becton Dickinson, NJ, USA) except for blank. The plate was incubated for 3 hours for RAW 264.7 cell line and 24 hours for HIG-82 cell line at 37 °C, 5% CO2 to allow the cells to attach to the surface. While waiting for the cell attachment, the tested compound stock was serially diluted to a decreasing concentration with completed growth medium. For preliminary screening, six concentration of tested compound was prepared (100, 50, 25, 12.5, 6.25 and 3.125 µM). After incubation hours, the media was gently removed to discard unattached cells and the attached cells were then induced with 50 mL of 10 µg/mL (final concentration) of Escherichia coli lipopolysaccharide (LPS) from serotype 055:B5 (Sigma, USA) and 10 nM (final concentration) phorbol myristate acetate (PMA) for the cells respectively. Then, 50 µL from each of serially diluted samples were transferred into each well of the prepared tissue culture plate except the control groups. The five controls were set up and the control group were prepared in the last row of the tissue culture plate. The plate was then incubated for another 24 hours overnight at 37 °C, 5% CO2. After incubation, the culture cells were prepared for MTT cytotoxicity assay.
3-(4,5-dimethylthiazole-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) cell viability assay
The cell viability was determined by assaying the reduction of MTT reagents to formazan salts. MTT is a water-soluble tetrazolium salt which yellow in colour. Metabolically active cells are able to convert MTT molecule into a water-insoluble dark blue formazan salt by reductive cleavage of the tetrazolium ring (Pozzolini et. al., 2003). After treatment, the supernatant of the 96-wells plate containing cells was replaced with the fresh media and 20 µL of MTT reagents (5 g/mL) were added into each well. After 4 hours, the spent media was removed completely and the formazan salts were dissolved with 100 µL 100% DMSO. The absorbance was then measured at 570 nm using an ELISA microplate reader (Infinite M200 Tecan Microplate Reader; Tecan Inc. Durham, North Carolina, USA). The percentage of cell viability was calculated.
Griess assay
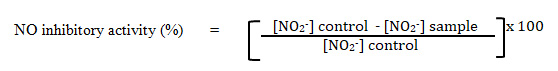
The production of nitric oxide (NO) was determined by measuring the increase of nitrite in the supernatant of the samples. The inhibitory activity of compounds on NO production assayed using Griess reaction (Ahmad et. al., 2006). Briefly, 50 µL of supernatant from each well transferred into corresponding well of a clean non-sterile 96-well plate. The exact volume 50 µL (1:1) of Griess reagent [1% sulpanilamide, 0.1% N-(1-naphthyl)-ethylene diamine dihydrochloride in 2.5% phosphoric acid] and mix well with the cell culture supernatant. The absorbance was read at 550 nm with a microplate reader (Tecan Infinite 200 PRO, Switzerland). The amount of nitrite in a sample was calculated from a sodium nitrite standard curve freshly prepared in deionised water (0-100 µM). Then, the percentage of nitrite inhibition of each sample was calculated using the formula below:
Statistical analysis
Data is expressed as mean ± SEM of duplicates using GraphPad Prism 5 software, using different experiments of triplicate sets of each template. Using variance analysis, statistical significance was established at p ˂0.05. Significant treatment means were further subjected to Bonferroni post-tests.
 |
Figure 2: Summary of methods |
Results
Results in Table 1 and 2 showed a dose-dependent suppression effects to dose increment compounds on HIG-82 synovicytes and murine macrophage RAW 264.7 cell lines due to cytotoxicity effects. Interestingly, the inhibitory effect of four thiopurine compounds on NO production were only demonstrated in LPS-induced RAW 264.7 cell line but not on PMA-induced HIG-82 cell line based on NO production. This is due to very less or untraceable concentration of NO was produced by the PMA-activated HIG-82 cell line. Thus, it cannot be compared with the control group due to there are no any significant different between treated group and control. In general, all compounds were not showed the high degree of cytotoxicity effects (cell viability < 70%) at all concentration tested for both type of cells. L-NAME, a standard NO inhibitor was used as a positive drug control; which significantly inhibited NO production at the concentration of 250 µM. The efficacy of the active compounds on cell viability and nitrite oxide production of HIG-82 synoviocytes and murine macrophage RAW 264.7 cell lines were shown in the Tables respectively below.
The effect of thiopurine compounds on cell viability
Table 1 indicates the percentage viability of HIG-82 synoviocyte and RAW 264.7 cells used in this analysis undergoing viability MTT assay for 24-hour incubation with various 6-MP doses and derivatives. HIG-82 synociocyte cell displayed a dose-dependent reduction in cell viability in all thiopurine compounds examined after 24 hours incubation. Eventually, 6-MP and 6-MP riboside compounds were demonstrated a good and moderate potentially candidate of rejuvenalization cell respectively compared to others. Furthermore, 6-MP compound was more potent compared with others on HIG-82 synoviocyte cell where cell viability calculated at 90.62, 90.45 and 88.53% at the same concentration (25 µM, 50 µM and 100 µM doses) doses respectively. In the other hand, diclofenac, a control drug, demonstrated highly reduction in cell viability compared to thiopurine compounds. The result shown there are significant different in cell viability of diclofenac treatment on PMA stimulated HIG-82. The reduction effect of diclofenac in cell viability at 25 µM (cell viability = 83.58±1.52), 50 µM (cell viability = 89.42±1.47), 100 µM (cell viability = 69.02±19.59) compared to control group; PMA stimulation HIG-82 without treatment.
However, on the right-most column, demonstrated the percentage of viability of murine macrophage RAW 264.7 cells treated with Thiopurine compounds after 24 hrs incubation. The findings showed that for RAW 264.7 cells similar pattern to HIG-82 synoviocyte cells, both compounds have a dose dependent reduction in cell viability. Treatment with thiopurine compounds were caused dose dependent effects where increasing the concentration decreasing the cell viability except 6-Thioxanthine (6-TX) with low dose dependent similar reaction in HIG-82 synoviocytes cell. Similar to the HIG-82 synoviocyte cell, the effect of 6-MP in RAW 264.7 murine macrophages was more potent than the 6-MPR or RAW 264.7 macrophages less sensitive than the HIG-82 synoviocyte cell. The viability of RAW 264.7 cell murine macrophage was 145.70 and 140.96% at the two highest 6-MPR concentrations. The most potency Thiopurine compound in this dosages on the RAW 264.7 murine macrophage cell was 6-Thioguanine. The two highest concentrations of 6-Thioguanine were demonstrated a moderate toxicity effect where the cell viability obtained are 73.03 and 69.22%, respectively.
Interestingly, 6-MP and diclofenac possess a similar effect on RAW 264.7 macrophages cell same trend effects to HIG-82 synoviocyte cell line. However, from the both findings, HIG-82 synoviocyte cell was seem more sensitive and less viability to thiopurine compounds when compared to RAW 264.7 cell within the dosage range of the compounds but the HIG-82 cell was more consistent pattern effects to thiopurine compounds. The result findings from cell viability MTT assay showed that anti-metabolic thiopurine compounds namely 6-MP, 6-MPR, 6-TG and 6-TX were not cytotoxicity to HIG-82 synoviocyte cell and RAW 264.7 murine macrophage cell line of the selected dosages range. This is because the percentage of cell viability for all four compounds were showed more than 70% viability cell at all concentrations tested.
Table 1: The effects of Thiopurine compounds on cell viability of HIG-82 synoviocytes and RAW 264.7 cell lines.
| Compound | Concentration (µM) | Cell Viability (%) | |
| HIG-82 | RAW 264.7 | ||
| None | Normal | 127.87±29.34 | 154.65±52.72 |
| 6-Mercaptopurine
|
3.125 |
92.79±7.02* |
159.85±65.82 |
| 6.25 | 99.20±6.77 | 158.25±61.40 | |
| 12.5 | 94.41±9.24* | 149.42±55.27 | |
| 25 | 90.62±10.00* | 127.39±46.38 | |
| 50 | 90.45±10.57* | 105.73±37.15 | |
| 100 | 88.53±7.13* | 93.90±34.16
|
|
| 6-Thioguanine
|
3.125 |
103.25 ± 6.75 |
83.66±3.02* |
| 6.25 | 103.84 ± 5.08 | 85.77±4.11* | |
| 12.5 | 108.07 ± 0.75 | 82.93±4.06* | |
| 25 | 107.10 ± 6.70 | 79.23±3.59* | |
| 50 | 102.99 ± 5.07 | 73.03±1.31* | |
| 100 | 96.60 ± 5.09 | 69.22±5.21*
|
|
| 6-Mercaptopurine riboside
|
3.125 |
100.99±17.29 |
177.49±69.86 |
| 6.25 | 97.59±15.28* | 169.07±63.34 | |
| 12.5 | 98.76±9.52* | 167.52±58.32 | |
| 25 | 94.12±6.14* | 146.61±65.93 | |
| 50 | 91.19±3.73* | 145.70±67.37 | |
| 100 | 92.31±10.60* | 140.96±63.67
|
|
| 6-Thioxanthine
|
3.125 |
108.57 ± 19.89 |
98.99±7.08 |
| 6.25 | 104.01 ± 3.50 | 98.63±9.68 | |
| 12.5 | 106.98 ± 4.76 | 98.61±14.07 | |
| 25 | 103.03 ± 2.88 | 99.37±13.60 | |
| 50 | 103.89 ± 9.54 | 95.28±21.42 | |
| 100 | 103.70 ± 7.25 | 93.78±17.37
|
|
| Diclofenac
|
3.125 |
92.91±1.51 |
167.21±61.01 |
| 6.25 | 90.31±5.84 | 151.54±55.58 | |
| 12.5 | 86.58±2.03 | 147.98±72.35 | |
| 25 | 83.58±1.52* | 136.74±67.60 | |
| 50 | 89.42±1.47 | 101.38±26.44 | |
| 100 | 69.02±19.59* | 91.84±35.22
|
|
Effects of thiopurine compounds on nitrite oxide (NO) production
Table 2 demonstrated the inhibitory effects of thiopurine compounds on nitric oxide upon PMA-activated HIG-82 synoviocytes cell line. Among the thiopurine compound tested, there are no inhibitory action of NO showed on PMA-activated HIG-82 cells. This is due to the PMA probably does not cause the production of NO oxide. Thus, the inhibitory assessment of thiopurine compound cannot be determined. This same goes to control drug, diclofenac and the iNOS inhibitor L-NAME action in suppressing NO cannot be determined. The dosages selected were same with dosages range with cytotoxicity effects. The evaluation of NO inhibitory effects of thiopurine compounds and its derivatives is based on the dose response activities effect at six different concentrations (3.125–100 µM; 2-folds). Among four thiopurine compounds and diclofenac were tested, the inhibitory effects of NO were not determined. This is probably due to very less or undetectable concentration of NO was produced by the PMA-stimulated HIG-82 cell line. Positive control (L-NAME) also was demonstrated same respond like the compounds due to mechanism of PMA to the type of cell.
The concentration used for screening NO production of RAW 264.7 murine macrophage cell was covered a wide range (3.125–100 µM) to determine the effective working concentration for each compounds. Table 2 showed the effects of 6-MP, which dose dependent effects decreasing the level of nitrite concentration. The decreasing level of NO production pattern also demonstrated by other thiopurine compounds. Among four thiopurine compounds used, 6-mercaptopurine showed most potent NO inhibitory effect on LPS-induced RAW 264.7 murine macrophage cells with 11.61±10.85% NO inhibition activity at 100 µM compared to the rest compounds. The potency thiopurine compounds as NO inhibitor from strongest to weakest compounds as following in order; 6-mercaptopurine > 6-mercaptopurine riboside > 6-thioxanthine > 6- thioguanine. Interestingly, the inhibitory effects of NO production of all thiopurine compounds were showed at very strong activity of immunosuppressive when compared to L-NAME, a standard iNOS inhibitor as a positive drug control, which significantly inhibited NO production at the concentration of 250 µM. The efficacy of the thiopurine immunosuppressive compounds on nitrite production of RAW 264.7 murine macrophage cell line is shown in Table 2. Table 3 and Table 4 demonstrated the percentage of cell viability reduction and inhibitory effects on NO production at 100 µM respectively compared to Normal after 24 hrs incubation.
![]()
Table 2: The effect of thiopurine compounds on NO inhibition of HIG-82 cell lines and RAW 264.7 cell lines.
| Compound | Concentration (µM) | NO inhibitory
Activity (%) |
|
| HIG-82 | RAW 264.7 | ||
| 6-Mercaptopurine
|
3.125 |
1.24 ± 0.42 |
59.67±24.66 |
| 6.25 | 0.81 ± 0.44 | 55.90±25.37 | |
| 12.5 | 1.04 ± 0.78 | 48.10±23.76 | |
| 25 | 1.01 ± 0.88 | 34.41±20.79* | |
| 50 | 0.94 ± 0.71 | 33.41±20,29 | |
| 100 | 1.24 ± 0.66 | 11.61±10.85**
|
|
| 6-Thioguanine
|
3.125 |
1.41 ± 0.68 |
75.68±17.71# |
| 6.25 | 1.02 ± 0.66 | 60.65±13.90 | |
| 12.5 | 0.83 ± 0.43 | 34.84±27.02* | |
| 25 | 0.80 ± 0.57 | 37.35±15.09* | |
| 50 | 1.35 ± 0.23 | 30.92±22.21* | |
| 100 | 0.93 ± 0.53 | 34.70±13.10*
|
|
| 6-Mercaptopurine riboside
|
3.125 |
1.01 ± 0.53 |
70.66±18.08 |
| 6.25 | 0.43 ± 0.43 | 80.66±9.59# | |
| 12.5 | 0.59 ± 0.44 | 72.07±17.05 | |
| 25 | 1.07 ± 0.54 | 56.58±15.57 | |
| 50 | 0.86 ± 0.44 | 49.26±28.30 | |
| 100 | 1.37 ± 0.65 | 22.61±10.12**
|
|
| 6-Thioxanthine
|
3.125 |
1.44 ± 0.78 |
69.45±15.28 |
| 6.25 | 0.66 ± 0.55 | 55.46±11.29 | |
| 12.5 | 1.02 ± 0.91 | 59.40±35.03 | |
| 25 | 0.90 ± 0.59 | 39.78±25.09 | |
| 50 | 1.14 ± 0.41 | 43.33±29.63 | |
| 100 | 2.10 ± 0.64 | 29.71±18.76*
|
|
| Diclofenac
|
3.125 |
0.99 ± 0.58 |
NA |
| 6.25 | 0.75 ± 0.40 | NA | |
| 12.5 | 0.74 ± 0.41 | NA | |
| 25 | 1.05 ± 0.46 | NA | |
| 50 | 0.95 ± 0.42 | NA | |
| 100 | 1.44 ± 0.47 | NA
|
|
| L-NAME | 250 | 1.37 ± 0.38 | 33.99±12.82* |
| Normal-Induced | 1.21 ± 0.48 | 79.69± 6.88# | |
Table 3: Percentage of cell viability reduction at 100 µM compare to the normal after 24 hours incubation.
| Cell line
Compound |
Percentage (%) | |
| HIG-82 | RAW 264.7 | |
|
6-MP |
30.77 ± 4.119* |
39.28 ± 17.08 |
| 6-MPR | 27.81 ± 6.120 | 8.85 ± 31.84 |
| 6-Thioguanine | 24.45 ± 5.094 | 55.24 ±3.005 |
| 6-Thioxanthine | 18.90 ± 7.258 | 39.36 ±10.03 |
| Diclofenac | 46.02 ± 11.31* | 40.61 ± 17.61 |
Table 4: Percentage of inhibitory effects on NO production at 100 µM compare to the normal-induced after 24 hours incubation.
| Cell line
Compound |
Percentage (%) | |
| HIG-82 | RAW 264.7 | |
|
6-MP |
2.48 ± 0.6562 |
85.43 ±6.267* |
| 6-MPR | 13.22 ± 0.6544 | 71.63 ±5.842* |
| 6-Thioguanine | 23.14 ±0.5288 | 56.46 ±7.563* |
| 6-Thioxanthine | 73.55 ±0.6416 | 62.72 ±10.83* |
| L-NAME | 13.22 ±0.3769 | 57.35 ±8.678* |
Discussions
Results of the present study demonstrated the cytotoxicity effects and NO production by HIG-82 synociocyte and RAW 264.7 cell lines after treated with four immunosuppressive thiopurine anti-metabolic compounds, namely the 6-mercaptopurine (6-MP), 6-mercaptopurine riboside (6-MPR), 6-thioguanine (6-TG) and 6-thioxanthine (6-TX). As mentioned these compounds exhibits dose-dependent cytotoxicity effects on HIG-82 synoviocytes and murine macrophage RAW 264.7 cell lines after incubation 24 hours. In general, the cytotoxicity effect on PMA-activated HIG-82 and LPS-induced RAW 264.7 cell lines after treated with thiopurine compounds were demonstrated a dose-dependent manner with higher proliferation activity on HIG-82 synoviocytes cells compared to murine macrophage RAW 264.7 cells approximately 70% (30.77% reduction) and 60% (39.28% reduction) proliferation activity after 24 hrs incubation (Table 3). Furthermore, all thiopurine compounds except 6-MP riboside were showed more toxicity effects on LPS-induced RAW 264.7 murine macrophage cell line especially after treated with 6-thioguanine. In other side, only 6-MP riboside was demonstrated less toxicity to RAW 264.7 murine macrophage cell, thus promoting more proliferation activity (Mawatari et. al., 2001). According to our finding in this study, 6-thioguanine was demonstrated a very potent thiopurine as well at 100 µM on RAW 264.7 murine macrophage cell (Table 3) was believed involved in HGPRT up-regulation in inflammation mechanisms. Endless, the toxic accumulation of 6-TG in cells has activated an apoptosis or cell death programme which therefore provokes tumour regenericity in tissues and organs (Yatscoff and Aspeslet, 1998).
NO is an important modulator of the inflammatory cascade (Korhonen et. al., 2005). However, due to the instable and volatile properties of NO if long air exposure, it is difficult to quantify the level of NO production in biological samples. Griess assay is a commonly used, simple and rapid spectrophotometric method to detect the presence of organic nitrite compounds in biological samples, which is also one of the short stable end product(s) of NO formation (Moshage, 2009). Nitric oxide (NO) is a free radical that commonly involved in biological process (Stadler et. al., 1991). However, overproduction of NO can cause cytotoxic and cytostatic effect (Choudhari et. al., 2013; Connelly et. al., 2001; Palmer et. al., 1988). Few years back, several study in RA patients had revealed with strong evidences that overproduction of NO may be important in the pathogenesis of RA suggested a predominant M1 macrophage phenotype (McInnes and Schett, 2011) and the inflammation joints in RA are the main source of NO (Korhonen et. al., 2005; Miyasaka, 1997). In human, level of nitrite in synovial fluid was highly elevated compared to serum in RA patient thus suggesting that inflamed synovial joint and synoviocytes were contributed to RA (Moshage, 2009; Jovanovic et. al., 2002).
NO producing cells can be varied inflammatory synoviums. Several specialized cells are capable of providing NO in the inflamed synovium, including osteoblasts, osteoclasts, macrophages, fibroblasts, neutrophils, and endothelial cells. (Otero and Goldring, 2007). Articular chondrocytes synthesise NO in response to IL-1 and lipopolysaccharide (LPS) reactions (Otero and Goldring, 2007; Stadler et. al., 1991). Nevertheless, the number of chondrocytes existing in the synovium may not be sufficient to compensate for the level of NO produced in the synovium. Synovial fibroblasts develop NO in response to IL-1 and TNF-α, while its development is inhibited by the transforming growth factor-β (TGF-β) (Stichtenoth et. al., 1995). In comparison, it also notes the localization of iNOS immunoreactivity in synovial macrophages (Miyasaka, 1997). The NOS inhibitor (L-NAME) has been reported to reduce disease activity in experimental RA (McCartney-francis et. al., 1993). However, in this study, the level of nitric oxide produce cannot be determined due to no or too little of NO production being induced by the PMA-activated HIG-82 synoviocyte cell. It seems PMA suppresses its production as same reaction demonstrated by TGF-β (Stichtenoth et. al., 1995). This is because PMA was not an appropriate activator for the HIG-82 synoviocyte cell to produce NO instead of activating protein kinase C (PKC) signalling pathways for modulating diverse cellular responses such as gene transcription, cell proliferation and differentiation, induction of apoptosis, immune response, and receptor desensitisation (Chang et. al., 2005). PMA has a minimal effect on NO synthesis (Hulkower et. al., 1992; Stadler et. al., 1991; Georgescu et. al. 1988; Zakaria et. al., 2006). On the other hand, PMA is a protein kinase C (PKC) activator alone had no effects, whereas PMA with recombinant interferon (rIFN)-gamma synergistically increased NO synthesis (Yoon et. al., 1994). PMA-sensitive PKC isoforms (a and e) function in such a negative regulatory role of NOS and PMA-sensitive isoforms (a, b or e) may play a role in the inhibition of NOS induction (Paul et. al., 1997). These findings revealed that the dosages of thiopurine compounds were not toxic to synoviocytes HIG-82 cells.
In contrary, the thiopurine compounds have pronounced NO-suppressing activity on RAW 264.7 cell line. Off these, 6-MP was most potent in inhibiting the production of nitrite oxide compared to 6-MPR, 6-TG and 6-TX. Previous study suggested that thiopurines did not act as inhibitor on iNOS system, but they were significantly influenced many respective pharmacological proteins functions which responsible expressing the iNOS especially necrosis factor-κappa B (NF-κB) (Chang et. al., 2012). The central transcriptional mediator in the pathogenesis of several diseases, including stress response, cerebral ischemia and various immune reactions, which have been activated in abundance of cells and stimulated by oxidative stress-related cytokines, is considered to be a vital role of NF-κB. (Chang et. al., 2012). A common response to both cytokines and bacterial lipopolysaccharide (LPS) is an increase in apparent nitric oxide synthase (NOS) activity. This corresponds to the induction of the 130 kDa isoform of the enzyme and has been described in macrophages, smooth muscle cells, renal mesangial cells and hepatocytes (Förstermann and Sessa, 2012; Kunz et. al., 1994; Hortelano et. al., 1993; Förstermann et. al., 1991).
Our results revealed that 6-MP and 6-TG were decrease more cell viability compared to 6- MPR and 6-TX. This might be due to metabolism of 6-MP to S-methyl-thioinosine 5’- monophosphate (6-Me-Thio-IMP), which is strong inhibitor of purine de novo synthesis (Sahasranaman et. al., 2008). Purine de novo synthesis inhibition is well known to achieve the impact of immunosuppression and block the proliferation of different forms of lymphocyte lines and contribute to the cytotoxic behaviour (Erb et. al., 1998). Thus, 6-TG metabolism produces 5 “triphosphate (dGS) deoxy-6-thioguanosine. It has been shown that integrating dGS into DNA causes cell-cycle arrest and apoptosis via a mechanism involving the mismatch repair pathway (Swan et. al., 1996). It is commonly assumed that cell damage caused mainly by the incorporation of the thioguanine nucleotides (TGN) into DNA is of decisive importance for the cytotoxic effect of 6-MP as well as 6-TG. At the other hand, the antagonists of purine de novo synthesis (PDNS) in vitro are methylthioinosine monophosphate (MeTIMP) (Erb et. al., 1998) and, to a lesser degree, methylthioguanosine monophosphate (MeTGMP) (Karim et. al., 2013; Coulthard et. al., 2002; Allan and Bennett, 1971).
Previous studies have also shown that DNA has been impaired by the introduction of 6-TG into p388 murine leukemic cells and human cells, which has shown morphological alterations size enlargement and multinucleated nuclei (Almosailleakh and Schwaller, 2019; Daehn et. al., 2011). It’s terribly difficult to synthesise, and since its mode of action emerged to be close to that of 6-MP, its metabolic fate and clinical behaviour was addressed a little subsequently (Kowalska et. al., 2015; Mathews, 2012; Elion, 1989). Those were possibly the key reasons behind 6-TG that have never been used in the maintenance treatment of acute lymphoblastic leukaemia, however, unlike 6-MP, it is more active and less harmful than 6-TG that is further converted to cytotoxic TGN and can be degraded only after deamination by xanthine oxidase (Erb et. al., 1998). In other hands, 6-MPR is a derivative of 6-MP, while 6-TX is major products of metabolism of 6-MP by enzyme xanthine oxidase (Polifka and Friedman, 2002). From the results, 6-MPR decrease more cell viability compare to 6-TX. Although 6-MPR is a minor metabolite, it may be a clinical importance compare the known metabolite, 6-TX which is has less anti-neoplastic activity (Ono et. al., 1997). In contrast, 6-MPR has known has anti-neoplastic activity (Miron et. al., 2009; Solomon et. al., 1984).
Interestingly, our results showed that 6-MP and derivatives exhibits a very good therapeutic ranges in term of less cell dead and high cell lives than diclofenac, a common NSAID used for treating inflammation diseases. Diclofenac is a type NSAID that commonly used to relieve the join swelling but only have minor effect on the disease progression. Diclofenac was expected to inhibit COX synthesis of prostaglandin and help regulate symptoms but fail to prolong even the healing of RA-related joint damage (Wang et. al., 2011; Andreas et. al., 2009). Diclofenac therapy has been shown to have mild impacts on gene expression in SFs (Andreas et. al., 2009). All thiopurine compounds tested in this study were effective in promoting proliferation activity with low toxicity towards HIG-82 synoviocytes and RAW 264.7 murine macrophage cell lines after 24 hrs treatment. However, critical adverse drug reactions (ADR’s) in the human body such as fever , rash, diarrhoea, hypertension, hepatitis, pancreatitis, bacterial liver abscesses, cytomegalovirus infections, life-threatening myelosuppression, bone marrow suppression, gastrointestinal symptoms, hypersensitivity reactions, and tumorigenicity may be caused by long-term use of potent drug such as 6-MP in high dose (Gaya et. al., 1995). In addition, the introduction of 6-MP pro-drug; azathioprine may effect the second generations of animal infant in pregnancy’s mother (Ramsey-Goldman and Schilling, 1997) and birth defects after first trimester exposure to azathioprine, but the issue is still under debates (Rubinstein and Weinberg, 2012). Intracellular absorption of 6-thioguanine nucleotides in infants is also considered to be responsible for the cytotoxic effects of these drugs by blocking purine synthesis involving DNA synthesis and replication of RNA to new infant tissues and organ (Lennard, 1992).
In regard to these side effects, this present research was performed in observations of very beneficial therapeutic doses as a novel approach to reduce the ADR caused by 6-MP inflammatory arthritis treatment. The usage of glucocorticoids with sufficient thiopurine derivative dosages is another approach to reduce ADRs. Glucocorticoids are often the most successful remission-inducing medications, however regrettably, for patients that neglect to react, and for others that experience side effects or need long-term glucocorticoid treatment, may still show severe side effects, therefore immunomodulatory or immunosuppressive medications combined with an adequate dosage are necessary treatments or substitutes (Nielsen et. al., 2001). Immunomodulatory medication such as 6-MP pro-drug, azathioprine is able to mitigate and reduce the ADR caused by the usage of 6-MP. However, the replacement of 6-MP by azathioprine still facing with immediate and long-term adverse drug reactions of 6-MP via 6-MP metabolism where involved by three routes with superior enzymes in liver and gut; (a) thiopurine-S-methyltransferase (TPMT), added the methyl group to 6-MP through methylation to form 6-methyl-MP; (b) xanthine oxidase, which catalyses 6-MP to thiourate; and (c) hypoxanthine-guanine-phosphoribosyltransferase (HGPRT), which converts 6-MP to 6-thioguanine nucleotides (Karran and Attard, 2008).
A more approach beyond the usage of glucocorticoids and pro-drug replacement is the use of a nano-encapsulated drug delivery system. Recently study was demonstrated, cell proliferation levels in the presence of anti-cancer drugs provided by gold nanoparticles conjugated have been shown to be significantly smaller than those in cells subjected to cytostatic drugs alone (Cuin et. al., 2011), indicating that the transmission of nano-participants allowed an improved sensitivity of cancer cells to drugs evaluated with ribavirin. (Tomuleasa et. al., 2012). Nano-particles of disease-modifying anti-rheumatic nano (DMARNs)-medicines medication would have increased effectiveness at a lower dosage of the product concentration in the intended tissues, resulting in a decrease of adverse drug reactions of patients (Rubinstein and Weinberg, 2012). Treatment with DMARD azathioprine/purine derivatives, gold sodium thiomalate and methotrexate successfully restored RA-related chondrocyte gene expression to ‘healthy’ levels (Andreas et. al., 2009). Anti-metabolic thiopurins are thought to be a potential pharmacological agent for the nano-encapsulated drug delivery mechanism for the treatment of multiple pathological disorders with intrinsic inflammatory pathways and components. It often inhibits systemic inflammation by inhibiting several forms of inflammatory cell activation, including antioxidants of free radical molecules in the body system, such as superoxide anion generation (Ordentlich et. al., 2003), cytokine development (Chang et. al., 2005), and molecular adhesion expression (Chang et. al., 2012). As a consequence, by retarding the trans-nuclear transcription of NF-kβ and associated cytokines, thiopurine effectively reduced the synthesis of nitric oxide synthase (Chang et. al., 2011).
Conclusion
This research demonstrated the possible use of 6-MP for the in-vitro model of HIG-82 cell culture in treating rheumatoid arthritis disease. Drug candidates are promising usefulness to be identified as a new drug compound used in the treatment of inflammatory arthritis in the future, and further studies need to be conducted with a view to reducing the adverse drug reactions in patients using a nano-encapsulated drug delivery system.
Acknowledgment
This study was supported in part by Research University Grant Scheme (RUGS) from the Universiti Putra Malaysia, grant no. 9366100.
References
- Ahmad, S., Israf, D.A, Lajiz, N.H., Shaari, K., Mohamed, H., Wahab, A.A., Ariffin, K.T., Hoo, W.Y., Aziz, N.A., Kadir, A.A., Sulaiman, M.R. and Somchit, M.N. (2006). Cardamonin, inhibits pro-inflammatory mediators in activated RAW 264.7 cells and whole blood. European Journal of Pharmacology 538:188–194.
CrossRef - Andreas, K., Häupl, T., Lübke, C., Ringe, J., Morawietz, L., Wachtel, A. and Kaps, C. (2009). Anti-rheumatic drug response signatures in human chondrocytes: potential molecular targets to stimulate cartilage regeneration. Arthritis Research and Therapy 11:R15.
CrossRef - Allan, P.W. and Bennett, L.L.Jr. (1971). 6-Methylthioguanylic acid, a metabolite of 6-thioguanine. Biochemistry Pharmacology 20(4):847–852.
CrossRef - Almosailleakh, M. and Schwaller, J. (2019). Murine models of acute myeloid leukaemia. International Journal of Molecular Sciences 20(2):453.
CrossRef - Baeuerle, P.A. and Henkel, T. (1994). Function and activation of NF-κB in the immune system. Annual Reviews of Immunology 12:141–179.
CrossRef - Bell, B.A., Brockway, G.N., Shuster, J.J., Erdmann, G., Sterikoff, S., Bostrom, B. and Camitta, B.M. (2004). A comparison of red blood cell thiopurine metabolites in children with acute lymphoblastic leukemia who received oral mercaptopurine twice daily or once daily: a Pediatric Oncology Group study (now The Children’s Oncology Group). Paediatric Blood Cancer 43(2):105–109.
CrossRef - Cara, C.J., Pena, A.S., Sans, M., Rodrigo, L., Guerrero-Esteo, M. and Hinojosa, J. (2004). Reviewing the mechanism of action of thiopurine drugs: towards a new paradigm in clinical practice. Medical Sciences Monitor 10:RA247–54.
- Chang, M., Chen, B., Yu, M., Sheu, J., Chen, T. and Lin, C. (2005). Phorbol 12-myristate 13-acetate upregulates cyclooxygenase-2 expression in human pulmonary epithelial cells via ras, raf-1, ERK, and NF-κB, but not p38 MAPK, pathways. Cellular Signalling 17:299–310.
CrossRef - Chang, C., Wu, S., Kwan, A., Lin, C. and Hwang, S. (2011). 6-mercaptopurine reverses experimental vasospasm and alleviates the production of endothelins in NO-independent mechanism-a laboratory study. Acta Neurochir 153:939–949.
CrossRef - Chang, C.Z., Wu, S.C., Lin, C.L., Hwang, S.L. and Kwan, A.L. (2012). Purine anti-metabolite attenuates nuclear factor kappa β and related pro-inflammatory cytokines in experimental vasospasm. Acta Eurochirurgica (Wien) 154:1877–1885.
CrossRef - Choudhari, S.K., Chaudhary, M., Bagde, S., Gadbail, A.R. and Joshi, V. (2013). Nitric oxide and cancer: a review. World Journal of Surgical Oncology 11(1):118.
CrossRef - Coulthard, S.A. (2012). Mechanisms of thiopurine action. Brazilian Medical Association. 58(Suppl 1).
- Coulthard, S.A., Hogarth, L.A., Little, M., Matheson, E.C., Redfern, C.P., Minto, L. and Hall A.G. (2002). The Effect of Thiopurine Methyltransferase Expression on Sensitivity to Thiopurine Drugs. Molecular Pharmacology 62(1):102–109.
CrossRef - Connelly, L., Palacios-Callender, M., Ameixa, C., Moncada, S. and Hobbs, A.J. (2001). Biphasic regulation of NF-kappa B activity underlies the pro-and anti-inflammatory actions of nitric oxide. Journal of Immunology 166(6):3873–3881.
CrossRef - Cuin, A., Massabni, A.C. and Pereira, G.A. (2011). 6-Mercaptopurine complexes with silver and gold ions: Anti-tuberculosis and anti-cancer activities. Biomedicine and Pharmacotherapy 65:334–338.
CrossRef - Daehn, I., Brem, R., Barkauskaite, E. and Karran, P. (2011). 6-thioguanine damages mitochondrial DNA and causes mitochondrial dysfunction in human cells. FEBS Letters 585(24):3941–3946.
CrossRef - Elion, G.B. (1989). The purine path to chemotherapy. Science 244 (4900):41–47.
CrossRef - Erb, N., Harms, D.O. and Janka-Schaub, G. (1998). Pharmacokinetics and metabolism of thiopurines in children with acute lymphoblastic leukemia receiving 6-thioguanine versus 6-mercaptopurine. Cancer Chemotherapy and Pharmacology 42(4):266–272.
CrossRef - Firestein, G.S. (1996). Invasive fibroblast-like synoviocytes in rheumatoid arthritis: passive responders or transformed aggressors? Arthritis and Rheumatism 39(11):1781-1790.
CrossRef - Förstermann, U. and Sessa, W.C. (2012). Nitric oxide synthases: regulation and function. European Heart Journal 33(7):829–837d.
CrossRef - Förstermann, U., Schmidt, H.H., Pollock, J.S., Sheng, H., Mitchell, J.A., Warner, T.D., Nakane, M. and Murad, F. (1991). Isoforms of nitric oxide synthase. Characterization and purification from different cell types. Biochemical Pharmacology 42(10):1849–1857.
CrossRef - Frei, P., Biedermann, L., Nielsen, O.H. and Rogler, G. (2013). Use of thiopurines in inflammatory bowel disease. World Journal Gastroenterology 19(7):1040–1048.
CrossRef - Gaya, S.B., Rees, A.J., Lechler, R.I., Williams, G. and Mason, P.D. (1995). Malignant disease in patients with long-term renal transplants. Transplantation 59(12):1705–1709.
CrossRef - Georgescu, H.I., Mendelow, D. and Evans, C.H. (1988). HIG-82: An established cell line from rabbit periarticular soft tissue, which retains the “activatable” phenotype. In Vitro Cellular and Developmental Biology 24(10):1015–1022.
CrossRef
- Hasan, S., Ali, H.A., Al-Qubaisi, M., Hussein, M.Z., Ismail, M., Zainal, Z., Hakim, M.N. (2012). Controlled-release formulation of antihistamine based on cetirizine zinc-layered hydroxide nanocomposites and its effect on histamine release from basophilic leukemia (RBL-2H3) cells. International Journal of Nanomedicine 7: 3351-3363.
CrossRef - Henriksson, E., Kjellen, E., Wahlberg, P., Wennerberg, J. and Kjellstrom, J.H. (2006). Differences in estimates of cisplatin-induced cell kill in vitro between colorimetric and cell count/colony assays. In Vitro Cellular & Developmental Biology Animal 42:320–323.
CrossRef - Herrlinger, K.R., Kreisel, W., Schwab, M., Schoelmerich, J., Fleig, W.E., Ruhl, A. and Stange, E.F. (2003). 6-Thioguanine – efficacy and safety in chronic active Crohn’s disease. Alimentary Pharmacology and Therapeutics 17(4):503–508.
CrossRef - Hulkower, K.I., Sagi-Eisenberg, R., Traub, L.M., Georgescu, H.I. and Evans, C.H. (1992). Synovial protein kinase C and its apparent insensitivity to interleukin-1. European Journal of Biochemistry 209(1):81–88.
CrossRef - Hussein-Al-Ali, S.H, Al-Qubaisi, M., Hussein, M.Z., Ismail, M., Zainal, Z., Hakim, M.N. (2012). In vitro inhibition of histamine release behavior of cetirizine intercalated into Zn/Al- and Mg/Al-layered double hydroxides. International Journal of Molecular Sciences 13(5):5899-5916.
CrossRef - Hortelano, S., Genaro, A.M. and Boscá, L. (1993). Phorbol esters induce nitric oxide synthase and increase arginine influx in cultured peritoneal macrophages. FEBS Letters 320(2):135–139.
CrossRef - Jeoung, B., Lee, K.D., Chang-Su, N., Young-Eok, K., Kim, B. and Kim, Y.R. (2013). Ganghwaljetongyeum an anti-arthritic remedy, attenuates synoviocyte proliferation and reduces the production of pro-inflammatory mediators in macrophages: the therapeutic effect of GHJTY on rheumatoid arthritis. Complementary and Alternative Medicine 13:47.
CrossRef - Jovanovic, D.V., Mineau, F., Notoya, K., Reboul, P., Martel-Pelletier, J. and Pelletier, J.P. (2002). Nitric oxide induced cell death in human osteoarthritic synoviocytes is mediated by tyrosine kinase activation and hydrogen peroxide and/or superoxide formation. Journal of Rheumatology 29(10):2165–2175.
CrossRef - Karran, P. and Attard, N. (2008). Thiopurines in current medical practice: molecular mechanisms and contributions to therapy-related cancer. Nature Reviews Cancer 8(1):24–36.
CrossRef - Karim, H., Ghalali, A., Lafolie, P., Vitols, S. and Fotoohi, A.K. (2013). Differential role of thiopurine methyltransferase in the cytotoxic effects of 6-mercaptopurine and 6-thioguanine on human leukemia cells. Biochemical and Biophysical Research Communications 437(2):280–286.
CrossRef - Kim, J.Y. and Ro, J.Y. (2005). Signal pathway of cytokines produced by reactive oxygen species generated from phorbol myristate acetate-stimulated HMC-1 cells. Scandinavian Journal of Immunology 62(1):25–35.
CrossRef - Konidari, A. and El Matary, W. (2014). Use of thiopurines in inflammatory bowel disease: safety issues. World Journal of Gastrointestinal Pharmacology Therapy 5:63–76.
CrossRef - Korhonen, R., Lahti, A., Kankaanranta, H. and Moilanen, E. (2005). Nitric oxide production and signaling in inflammation. Current Drug Targets, Inflammation and Allergy 4(4):471-479.
CrossRef - Kowalska, A., Latocha, M. and Pluta, K. (2015). Synthesis and anticancer activity of thiosubstituted purines. Medicinal Chemical Research 24:3107.
CrossRef - Kunz, D., Walker, G., Wiesenberg, I. and Pfeilschifter, J. (1996). Inhibition by tetranactin of interleukin 1β- and cyclic AMP-induced nitric oxide synthase expression in rat renal mesangial cells. British Journal of Pharmacology 118(7):1621–1626.
CrossRef
- Laroux, F.S., Lefer, D.J., Kawachi, S., Scalia, R., Cockrell, A.S., Gray, L., Heyde, H.V.D., Hoffman, J.M. and Grisham, M.B. (2000). Role of nitric oxide in the regulation of acute and chronic inflammation. Antioxidants & Redox Signalling 2(3):391–396.
CrossRef - Lennard, L. (1992). The clinical pharmacology of 6-mercaptopurine. European Journal Clinical Pharmacology 43(4):329–339.
CrossRef - Mahadevan, U., Tremaine, W.J., Johnson, T., Pike, M.G., Mays, D.C., Lipsky, J.J. and Sandborn, W.J. (2000). Intravenous azathioprine in severe ulcerative colitis: a pilot study. American Journal of Gastroenterology 95(12):3463–3468.
CrossRef - Mathews C.K. (2012). DNA synthesis as a therapeutic target: the first 65 years. The Federation of American Societies for Experimental Biology Journal 26(6):2231–2237.
CrossRef - Mawatari, H., Unie, K., Nisimura, S., Sakura, N. and Ueda, K. (2001). Comparative pharmacokinetics of oral 6-mercaptopurine and intravenous 6-mercaptopurine riboside in children. Pediatrics International 43:673–677.
CrossRef - McCartney-Francis, N. (1993). Suppression of arthritis by an inhibitor of nitric oxide synthase. Journal of Experimental Medicine 178(2):749–754.
CrossRef - Miron, T., Arditti, F., Konstantinovski, L., Rabinkov, A., Mirelman, D., Berrebi, A. and Wilchek, M. (2009). Novel derivatives of 6-mercaptopurine: Synthesis, characterization and antiproliferative activities of S-allylthio-mercaptopurines. European Journal of Medicinal Chemistry 44(2):541–550.
CrossRef - Miyasaka, N. (1997). Nitric oxide production in rheumatoid arthritis. Japanese Journal of RheumatoIogy 7(3):165–I72.
CrossRef - McInnes, I.B. and Schett, G. (2011). The Pathogenesis of Rheumatoid Arthritis. The New England Journal of Medicine 365(23):2205–2219.
CrossRef - Mor, A., Abramson, S.B. and Pilinger, M.H. (2005). The fibroblast-like synovial cell in rheumatoid arthritis: a key player in inflammation and joint destruction. Clinical Immunology 15(2):118–128.
CrossRef - Moshage, H. (2009). Simple and reliable measurement of nitric oxide metabolites in plasma. Clinical Chemistry 55(10):1881–1882.
CrossRef - Muller-Ladner, U., Ospelt, C., Gay, S., Distler, O. and Pap, T. (2007). Cells of the synovium in rheumatoid arthritis synovial fibroblasts. Arthritis Research and Therapy 9(6):223.
CrossRef - Nagy, G., Clark, J.M., Buzás, E.I., Gorman, C.L. and Cope, A.P. (2007). Nitric oxide, chronic inflammation and autoimmunity. Immunology Letters 111(1):1–5.
CrossRef - Nathan, C. (1991). Nitric oxide as a secretory product of mammalian cells. The FASEB Journal 6: 3051–3064.
CrossRef - Nielsen, O.H., Vainer, B. and Rask-Madse, J. (2001). Review article: the treatment of inflammatory bowel disease with 6-mercaptopurine or azathioprine. Alimentary Pharmacology and Therapeutics 15(11):1699–1708.
CrossRef - Ono, Y., Ikeda, K., Wei, M.X., Harsh, G.R., Tamiya, T. and Chiocca, E.A. (1997). Regression of experimental brain tumors with 6-thioxanthine and Escherichia coli gpt gene therapy. Human Gene Therapy 8(17):2043–2055.
CrossRef - Ordentlich, P., Yan, Y., Zhou, S., Heyman, R.A. (2003). Identification of the anti-neoplastic agent 6-mercaptopurine as an activator of the orphan nuclear hormone receptor Nurr1*. The Journal of Biological Chemistry 278(27):24791–24799.
CrossRef - Otero, M. and Goldring, M.B. (2007). Review: cells of the synovium in rheumatoid arthritis Chondrocytes. Arthritis Research and Therapy 9(5):220.
CrossRef - Palmer, R.M., Ashton, D.S. and Moncada, S. (1988). Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature 333(6174):664–666.
CrossRef - Parada-Turska, J., Rzeski, W. and Majdan, M. (2006). Effect of glutamate receptor antagonists and antirheumatic drugs on proliferation of synoviocytes in vitro. European Journal of Pharmacology 535:95–97.
CrossRef - Parada-Turska, J., Mitura, A., Brzana, W., Jabłoński, M., Majdan, M. and Rzeski, W. (2008). Parthenolide inhibits proliferation of fibroblast-like synoviocytes in vitro. Inflammation 31(4):281–285.
CrossRef - Paul, A., Doherty, K. and Plevin, R. (1997). Differential regulation by protein kinase C isoforms of nitric oxide synthase induction in RAW 264.7 macrophages and rat aortic smooth muscle cells. British Journal of Pharmacology 120:940–946.
CrossRef - Piccinini, A.M. and Midwood, K.S. (2010). DAMPening inflammation by modulating TLR signaling. Mediators of Inflammation 672395.
CrossRef - Pilar, B.P., Boerbooms, A.M. and Putte, L.B.A (1996). Effects of anti-rheumatic agents on cytokines. Seminars in Arthritis and Rheumatism 125:234–253.
CrossRef - Polifka, J.E. and Friedman, J.M. (2002). Teratogen update: azathioprine and 6-mercaptopurine. Teratology 65(5):240–261.
CrossRef - Pozzolini, M., Scarf, S., Benatti, U. and Giovine, M. (2003). Interference in MTT cell viability assay in activated macrophage cell line. Analytical Biochemistry 313(2):338–341.
CrossRef - Ramsey-Goldman, R. and Schilling, E. (1997). Immunosuppressive drug use during pregnancy. Rheumatic Disease Clinics of North America 23(1):149–167.
CrossRef - Ricketts, R.R. (1998). The long-term outcome of ulcerative colitis treated with 6-mercaptopurine. Journal of Pediatric Surgery 33(1):145.
CrossRef - Rubinstein, I. and Weinberg, G.L. (2012). Nanomedicines for chronic non-infectious arthritis: The clinician’s perspective. Maturitas 73(1):68–73.
CrossRef - Sahasranaman, S., Howard, D. and Roy, S. (2008). Clinical pharmacology and pharmacogenetics of thiopurines. European Journal of Clinical Pharmacology 64:753–767.
CrossRef - Scott, D., Wolfe, F. and Huizinga, T.W.J. (2010). Rheumatoid arthritis. Lancet 376:1094–1108.
CrossRef - Shahrir, M., Shahdan, M., Shahid, M., Sulaiman, W., Mokhtar, A.M., Othman, M. and Yusof, A. (2008). Multicentre survey of rheumatoid arthritis patients from Ministry of Health Rheumatology Centers in Malaysia. International Journal of Rheumatic Diseases 11(3):287–292.
crossRef - Shanker, M., Willcutts, D., Roth, J.A. and Ramesh, R. (2010). Drug resistance in lung cancer. Lung Cancer Targets and Therapy 1:23–36.
crossRef - Smith, M.E., van der Maesen, K., Somera, F.P. and Sobel, R.A. (1998). Effects of phorbol myristate acetate (PMA) on functions of macrophages and microglia in-vitro. Neurochemical Research 23(3):427–434.
crossRef - Smolen, J.S. and Redlich, K. (2014). Chapter 36 – rheumatoid arthritis. In N. R. Rose, & I. R. Mackay (Eds.). The autoimmune diseases (fifth edition) (pp. 511-523). Boston: Academic Press.
crossRef - Stichtenoth, D.O., Fauler, J., Zeidler, H. and Frolich, J.C. (1995). Urinary nitrate excretion is increased in patients with rheumatoid arthritis and reduced by prednisolone. Annals of the Rheumatic Diseases 54:820–824.
crossRef - Stadler, J., Stefanovic-Racic, M., Billiar, T.R., Curran, R.D., McIntyre, L.A., Georgescu, H.I., Simmons, R.L. and Evans, C.H. (1991). Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. The Journal of Immunology 147:3915–3920.
- Swan, P.F., Waters, T.R., Moulton, D.C., Xu, Y.Z., Zheng, Q., Edwards, M. and Mace, R. (1996). Role of post-replicative DNA mismatch repair in the cytotoxic action of thioguanine. Science 273(5278):1109–1111.
crossRef - Tomuleasa, C., Soritau, O., Orza, A., Dudea, M., Petrushev, B., Mosteanu, O., Susman, S., Florea, A., Pall, E., Aldea, M., Kacso, G., Cristea, V., Berindan-Neagoe, I. and Irimie, A. (2012). Gold nanoparticles conjugated with cisplatin/doxorubicin/capecitabine lower the chemo-resistance of hepatocellular carcinoma-derived cancer cells. Journal Gastrointestin Liver Disease 21(2):187–196.
- Too, C.L., Murad, S., Dhaliwal, J.S., Larsson, P., Jiang, X., Ding, B., Alfredsson, L., Klareskog, L. and Padyukov, L. (2012). Polymorphisms in peptidylarginine deiminase associate with rheumatoid arthritis in diverse Asian populations: evidence from MyEIRA study and meta-analysis. Arthritis Research and Therapy 14:R250.
crossRef - Tsikas, D. (2007). Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: Appraisal of the Griess reaction in the l-arginine/nitric oxide area of research. Journal of Chromatography B 851(1-2):51–70.
crossRef - Wang, Y.F., Wei, D.M. and Lai, Z. (2006). Triptolide inhibits CC chemokines expressed in rat adjuvant-induced arthritis. International Immunopharmacology 6:1825–1832.
crossRef - Wang, L.S., Zhao, D.Q. and Di, L. (2011).The analgesic and anti-rheumatic effects of Thladiantha dubia fruit crude polysaccharide fraction in mice and rats. Journal of Ethnopharmacology 137:1381–1387.
crossRef - Yatscoff, R.W. and Aspeslet, L.J. (1998). The monitoring of immunosuppressive drugs: a pharmacodynamic approach. Therapeutic Drug Monitoring 20(5):459–463.
crossRef - Yoon, H.J., Jun, C.D., Kim, J.M., Rim, G.N., Kim, H.M. and Chung, H.T. (1994). Phorbol ester synergistically increases interferon-γ-induced nitric oxide synthesis in murine microglial cells. Neuroimmunomodulation 1(6).
crossRef - Zakaria, Z.A., Fatimah, C.A., Mat Jais, A.M., Henie, E.F.P., Sulaiman, M.R., Somchit, M.N., Thenamutha, M., Kasthuri, D. (2006). The in vitro antibacterial activity of muntingia calabura extracts. International Journal of Pharmacology 2(4): 439-442.
crossRef - Zaza, G., Cheok, M., Yang, W., Panetta, J.C., Pui, C., Relling, M.V. and Evans, W.E. (2005). Gene expression and thioguanine nucleotide disposition in acute lymphoblastic leukaemia after in vivo mercaptopurine treatment. Blood 106(5):1778–1785.
crossRef