Manuscript accepted on :05-Sep-2020
Published online on: --
Plagiarism Check: Yes
Reviewed by: Ahmed Salah
Nafe M Al‐Tawarah1, Haitham Qaralleh1, Ali M Khlaifat2*, Mohammad nebih nofal3, Khaled M. Khleifat4, Muhamad O. Al-limoun4, Moath Alqaraleh1 and Mohammad Ahmed Al Shhab5
1Department of Medical Analysis, Mutah University, Mutah, Karak, 61710, Jordan.
2Department of Nursing, Faculty of Prince Aysha for Applied Health and Nursing, Al-Hussein Bin Talal University, Ma'an, Jordan.
3General surgery department, Faculty of medicine, Mutah University, Mutah, Karak, 61710, Jordan.
4Department of Biology, Mutah University, Mutah, Karak, 61710, Jordan.
5Department of pharmacology, School of medicine, The university of Jordan.
Corresponding Author E-mail: alkh_kha@hotmail.com.
DOI : https://dx.doi.org/10.13005/bpj/1985
Abstract
Many medicinal herbs are widely used in traditional folk medicine in most countries of the world, including Jordan, to treat many human diseases including microbial infections, diabetes and cancer. This study was conducted to evaluate the anti-cancer and antibacterial effects of essential oil (EO) of Varthemia Iphionoides, as well as the synergistic effect of EO and biosynthesiszed silver nanoparticles (AgNPs) against many cancer lines including human breast cancer, leukemia, pancreatic cancer, and prostate cancer. The chemical composition of V. iphionoides essential oil was analyzed using GC/MS. Twenty-two compounds were identified representing 96.1% of the V. iphionoides EO. The anticancer effect of EO from V. Iphionoides, as well as its synergistic effect with AgNPs, were evaluated using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) for all mentioned cell lines, in which human periodontal ligament fibroblasts (PDL) was used for verification of selectivity. It is noted that the oil has anti-proliferative activity against many types of cancer cells. The study revealed that the combination of essential oil and AgNPs shows a synergistic, antiproliferative effect on all cancer cell lines at a low concentration whereas when they are used individually, the effect is only shown when using a high concentration. EO of Varthemia Iphionoides has clearly demonstrated its effectiveness on pancreatic cancer cells compared to doxorubicin and with a high selectivity rate on synergy with AgNPs. Regarding antibacterial assays using the well diffusion methods, EO of Verthemia Iphionides was active against all five-tested bacteria, with inhibition zone ranged between 15 mm and 26.5 mm. The results of MIC for tested bacteria were between 50 and 370 µg/mL. The best synergistic capacity resulted from combination of AgNPs and EO of Verthemia Iphionides was against S. aureus, Therefore, synergistic studies can be novel strategic plan by furnishing an interesting stage in the future for this kind of research.
Keywords
Anticancer; Antibacterial; AgNPs; Varthemia Iphionoides
Download this article as:| Copy the following to cite this article: Al‐Tawarah N. M, Qaralleh H, Khlaifat A. M, Nofal M. N, Khleifat K. M, Al-limoun M. O, Alqaraleh M, Shhab M. A. A. Anticancer and Antibacterial Properties of Verthemia Iphionides Essential Oil /Silver Nanoparticles. Biomed Pharmacol J 2020;13(3). |
| Copy the following to cite this URL: Al‐Tawarah N. M, Qaralleh H, Khlaifat A. M, Nofal M. N, Khleifat K. M, Al-limoun M. O, Alqaraleh M, Shhab M. A. A. Anticancer and Antibacterial Properties of Verthemia Iphionides Essential Oil /Silver Nanoparticles. Biomed Pharmacol J 2020;13(3). Available from: https://bit.ly/2SonwIu |
Introduction
Verthemia species belong to the family Compositae (Asteraceae), in which the most widely distributed species in Jordan and Middle east is Verthemia iphionoides (also known as Chiliadenus iphinoides).1 In fact, V. iphionoides, a 30-80 cm long bushy-perennial, is the most important medical herb used in Jordan.2 In traditional herbal medicine, it plays a major role in the management of various diseases; relieving pain, healing wounds and in the treatment of eye complaints and urine retention.3 V. iphionoides has also been shown to exhibit some medicinal properties including antimicrobial,4 antiplatelet,5 and cytotoxic6 activities . Furthermore, it has been used for the treatment of many diseases, including cancer.7
Recently, the application of nanotechnology has grown rapidly especially in the green synthesis of nanomaterials that have a wide range of medical applications. The green synthesis method of producing nanomaterials has rapidly grown in the nanotechnology field; including uses in drug delivery,8 ointments, nanomedicine,9 chemical sensing,10 data storage,11 cell biology,12 agriculture, cosmetics13 and textiles,14 the food industry and photocatalytic organic dye–degradation activity,15 antioxidants16 and antimicrobial agents17,18. Since there is no effective treatment with a high level of selectivity for most cancer types, searching for bioactive chemicals in a traditional medicinal plant is a novel way to prevent patient suffering from different kinds of cancer. So, the aims of this study are to investigate the possible antiproliferative efficacy of the V. iphionoides essential oil and silver nanoparticles (AgNPs), which was synthesised using the culture of the supernatant of the fungal strain Tritirachium oryzae W5H, separately and in combination against different cancer cell lines in vitro.
Moreover, to study the broad-spectrum antibacterial activity of AgNPs used singly and in combination with EOs from the leaves of V. iphionoides plants. The effects of the V. iphionoides EO after synergizing with AgNPs were investigated against five bacterial strains using well diffusion and microdilution methods. This is the first report using EO of V. iphionoides combined with biosynthesized AgNPs for studying the antibacterial capacity and anticancer effect of different cell lines.
Materials and Methods
Plant material
In 2018, the aerial parts of V. iphionoides were collected in June and July from Al Karak province, in the south of Jordan. The plant was identified as previously reported in Khlaifat et al.,19
Preparation of the essential oil
A sample of 50g of the dried aerial part of the plant was hydro-distilled using a simple Clevenger apparatus for three hours. This procedure was repeated more than 20 times. The oil was extracted from the aqueous phase using diethyl ether. The diethyl ether was evaporated, and the oil was dried over anhydrous sodium sulfate. Finally, the extracted oil was stored at 4oC until further analysis.
Preparation and characterization of AgNPs
AgNPs were prepared in our laboratory as recently reported by Al-limoun et al.,20
Gas chromatography/Mass spectrometry (GC/MS) analysis
In this study, a Varian chrompack CP-3800 GC/MS-200 equipped with a split-splitless injector was used. The extracted components were separated on a DB-5 GC column and the mass detector was set to scan ions between 40-400 m/z using full scan mode and electron impact (EI, 70 eV). The temperature of the injector was set at 250°C with a split ratio of 1:10. The detector and transfer-line temperatures were 160°C and 230°C respectively. For separation of the different oil components, a linear temperature program was used. The heating rate was programmed at 3°C/min starting from 60°C (initial temperature) to 250°C (final temperature) and held at 250°C for 5 min with a total run time of 68 minutes 25 seconds. The identification of compounds was made by comparing the retention time with reference samples, based on their linear indices relative to a series of n-alkanes (C8-C20) in the same chromatographic conditions and mass spectra, by matching the essential oil constituents with NIST library and published reports. Whenever possible, the co-chromatography of certain standard compounds was performed under similar chromatographic condition.
Bacterial strains
For antibacterial activity five bacteria were employed involving three Gram-negative bacteria: E. aerogenes ATCC 13048, E. coli ATCC 11293 and the clinical isolate Extended Spectrum Beta-Lactamase producing P. aeruginosa (ESBL) and two Gram-positive bacteria: the clinical isolate Beta-Lactamase producing S. aureus (BL) and S. epidermidis ATCC 12228. The clinical isolates were obtained from the Department of Biology, Faculty of Science, Mutah University, Mutah, Jordan.
Preparation of bacterial suspension
Bacterial broth cultures were made following the M07-A9 procedure of the Clinical Laboratory Standards Institute (CLSI, 2012). One bacterial colony was cultivated in sterile 5 ml nutrient broth (NB) at 37°C and incubated overnight. The resultant growth cultures were adjusted to 0.5 McFarland Standard using sterile NB broth. The adjustment of bacterial suspensions to the density of the 0.5 McFarland standard was done spectrophotometrically at (A 620 nm) to obtain a final absorbtion of 0.1.
Antimicrobial activity
The antibacterial activities of EO and AgNPs alone or in combination were evaluated using well diffusion method and microdilution method. In well diffusion method, EO, AgNPs and combination of AgNPs:EO were evaluated at concentrations equal to 10 mg, 172.6 mg and 86.28 µg : 5 mg per well, respectively. In microdillution method, three fold dilution were prepared from a stock solution of 100 mg , 1.726 mg and combination of 86.28 µg and 5 mg per mL for EO, AgNPs and AgNPs:EO, respectively.
Well diffusion method
Hundred µl of bacteria suspension adjusted to an equivalent of 0.5 McFarland standards (approximately 108 CFU/mL) was spread on Muller Hinton agar then 100 µl from the prepared stock solutions were loaded into the well that previously was prepared. The prepared plates were incubated at 37oC for 24h and the inhibition zones were measured as mm diameter.
Microdilution methods
A three-fold dilution series was performed using Muller Hinton broth from the prepared stock solutions (100 mg per well, 1.726 mg per well and 86.28 µg: 5 mg per well for EO, AgNPs and AgNPs:EO (in combination), respectively) for each sample tested using 96-well plate. Then 10 µL of the bacterial suspension equivalent to 0.5 McFarland standards (approximately 108 CFU/mL for bacteria) was added to each well. In addition to DMSO as negative control, three different antibiotics including chloramphenicol (Cm), kanamycin (Km) and ampicillin (Amp) were used as control. The inoculated plates were incubated at 37 C for 24h. The growth of the microorganisms was monitored by the subculture of each well content on nutrient agar. The MIC values were defined as the lowest concentrations of the plant essential oil found to inhibit the growth of microorganisms.21
Cancer cell lines culture
Human breast cancer cell lines; namely MDA-MB-231 (mammary gland/breast; derived from metastatic site: pleural effusion. ATCC HTB-26, chronic myelogenous leukemia; namely K-562 (ATCC HTB-243), pancreatic cancer cell line Panc1 (ATCC CRL1469), human prostate cancer cell lines; namely PC3 (ATCC CRL-1435) and human periodontal ligament fibroblasts (PDL). All these cells were cultured in DMEM containing 10% FBS, HEPES Buffer (10 mM), L- glutamine (100 μg/ml), gentamicin (50 µg/mL), penicillin (100 µg/ml), and streptomycin (100 mg/ml).
Cell harvesting and counting
All cells were propagated in a humidified 5% CO2 incubator at 37◦C. Firstly, all the cells were washed in 75 cm2 flasks with 3–5 ml of phosphate buffer saline (PBS),then 1–2 ml of trypsin was added to each flask until the cells detached. An equal amount of fresh media was added for each cell line, then gentle pipetting was performed to disturb any clumps and ensure a uniform single cell suspension. The frequency and ratio of the cell propagation was distinct for each cell line. The cells were propagated every 2-3 days, after reaching the desired number of cells. Cells were counted by mixing 100µl of 4% trypan blue dye and 25 µl of the harvested cells, then transferring the cell suspension to the edge of a hemacytometer counting chamber.
Cytotoxicity assay
The essential oil and AgNPs were assayed for cell toxicity. Cytotoxicity measurements were based on the viability of the cells present in the culture. Cells were seeded into 96-well plates at a density of 1×104 cells per well and incubated for 24 h at 37 C in DMEM, then incubated with DMEM containing different concentrations of oil and AgNPs for 48 hours. MTT assay was then performed as follows: the medium was removed and cells in each well were incubated with 20 μl of MTT solution (5 mg/ml) for 4 hours at 37°C. MTT solution was then discarded and 200 μl of dimethyl sulfoxide (DMSO) were added to dissolve insoluble formazan crystals. Optical density was measured at 570 nm and 630 nm. Data were obtained from triplicate wells. Human periodontal ligament fibroblasts (PDL) are a primary cell culture used for verification of selective cytotoxicity with the least antiproliferative IC50 value obtained. As a robust and classical antineoplastic reference agent, doxorubicin was used for comparison purposes. All the assays were performed in triplicate and the calculated IC50 antiproliferative activities were reported as the mean values ± SD (n=3).
The combined Effects of V. iphionides essential oil and AgNPs
To assess the synergistic effects of the essential oil in combination with AgNPs, cells were seeded into 96-well plates at a density of 1×104 cells per well and incubated for 24 hours at 37 oC in DMEM. After incubation, all cell lines were treated with a combination of oil and AgNPs at IC25, I
C50, and IC75 values. After 48hours the MTT solution (20 𝜇l) was added to each well and incubated for 4 hours. The MTT-formazan crystals formed were dissolved in 100 𝜇l of DMSO and the absorbance was measured at 570 nm and 630 nm. Data were obtained from triplicate wells. The combination index (CI) was calculated by CompuSyn software (Paramus, NJ, USA) to analyse the synergistic inhibitory effect.
Statistical analysis
The results were presented as means± standard deviation (SD) of three independent experiments. Statistical differences between a and different treatment groups were determined using GraphPad Prism ANOVA followed by Dunnett’s post hoc test. For all statistical analysis, a p-value of less than 0.05 was considered statistically significant. P values of less than 0.001 were considered of statistically highly significant.
Results
The chemical composition of V. iphionoides essential oil
The chemical composition of V. iphionoides essential oil was analyzed using GC/MS (Table 1). Twenty-two compounds were identified representing 96.1% of the V. iphionoides essential oil. The major classes of compounds were characterized as oxygenated monoterpene (50%) and Monoterpene hydrocarbons (41%). The result also showed that Eucalyptol (53.65) is the most dominant compound followed by Bornyl acetate (11.26) and Borenol (8.31%).
Table 1: Chemical composition of V. iphionoides essential oil using GC/MS
| no | RT | Compound | KI exp | Ki let | Conc (%) |
| 1. | 5.450 | Santolina triene | 913.57 | 908 | 0.76 |
| 2. | 6.116 | Alpha thujene | 933.4 | 930 | 1.02 |
| 3. | 7.505 | Sabinene | 977.0 | 969 | 0.69 |
| 4. | 7.684 | Beta pinene | 982.9 | 974 | 0.79 |
| 5. | 8.318 | Yomogi alcohol | 1001.6 | 999 | 0.71 |
| 6. | 8.976 | Alpha terpinene | 1020.0 | 1014 | 0.72 |
| 7. | 9.286 | Para cymene | 1027.9 | 1024 | 2.84 |
| 8. | 9.434 | Limonene | 1032.2 | 1029 | 1.30 |
| 9. | 9.608 | Eucalyptol | 1036.5 | 1031 | 53.65 |
| 10. | 10.519 | Gamma terpinene | 1061.2 | 1054 | 1.88 |
| 11. | 11.173 | Cis-sabinenehydrate | 1078.0 | 1065 | 1.71 |
| 12. | 11.521 | Artemisia alcohol | 1087 | 1080 | 1.23 |
| 13. | 12.480 | 1-terpineol | 1112.7 | 1133 | 1.28 |
| 14. | 14.435 | Nerol oxide | 1157.0 | 1173 | 0.58 |
| 15. | 14.872 | Cis-chrysanthenol | 1167.6 | 1160 | 0.79 |
| 16. | 15.145 | Lavandulol | 1174.9 | 1161 | 0.77 |
| 17. | 15.529 | Borenol | 1183.2 | 1165 | 8.31 |
| 18. | 15.767 | Terpinen-4-ol | 1188.9 | 1177 | 1.10 |
| 19. | 16.491 | Alpha terpineol | 1206.1 | 1189 | 1.98 |
| 20. | 16.660 | Ethyl octanoate | 1210.2 | 1196 | 1.27 |
| 21. | 20.148 | Bornyl acetate | 1290.0 | 1284 | 11.26 |
| 22. | 23.357 | Neryl acetate | 1365.2 | 1359 | 1.45 |
| Total | 96.10 | ||||
| Monoterpene hydrocarbons (9) | 10.71 | ||||
| oxygenated monoterpene (11) | 82.67 | ||||
| oxygenated sesquiterpene (1) | 1.45 | ||||
| Aliphatic ester (1) | 1.27 |
The modulation of the proliferation of different cancer cell lines and fibroblasts by V. iphionides EO and AgNPs
The antiproliferative efficacies of doxorubicin on breast, pancreatic and prostate cancer and leukaemia cell lines are further illustrated in table 2. Nevertheless, doxorubicin lacked selective cytotoxicity in fibroblasts. Table 2 further displays the antiproliferative efficacies of V. iphionides essential oil and AgNPs against the same cancer cell lines. Moreover, the V. iphionides essential oil has an equipotent effect to doxorubicin against all cancer cell lines, except pancreatic cancer, where he essential oil showed promising antiproliferative effects compared to doxorubicin (table 2). The AgNPs also showed cytotoxity effects against all the cancer cell lines. Surprisingly, the AgNPs were not as potent as doxorubicin or V. iphionides essential oil. In cancer cell lines such as PANC1, K562 and PC3, the V. iphionides essential oil and the doxorubicin showed significantly antiproliferative effects compared to the AgNPs. Nevertheless, both V. iphionides and AgNPs had selective efficacies in the PC3 cell line (see figure 1).
The antiproliferative activity of the combination of V. iphionides essential oil and AgNPs is shown in figure 2. It was clear that the synergistic effect only appeared when the V. iphionides essential oil and AgNPs were incubated with different cancer cell lines at low concentration. However, at high concentrations the combination between V. iphionides essential oil and AgNPs showed an antagonistic effect.
Table 2: IC50 values (µg/ml) of in vitro antiproliferative activity of oil and Nano on different cancer cell lines.
| Treatment (µg/ml) | Cell lines | ||||
| fibroblast | k562 | MDA-MB-231 | PANC1 | PC3 | |
| oil | 0.3±0 | 0.4±0 | 0.3±0 | 0.4±0 | 0.14±0 |
| Nano | 15.2±1.4 | 20.2±2.5 | 15.8±0.3 | 17.4±5.3 | 10.7±0.3 |
| Doxorubicin | 0.03±0.01 | 0.4±0.04 | 0.09±0.01 | 0.2±1.7 | 0.1±0.02 |
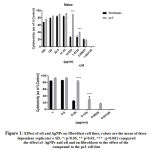 |
Figure 1: Effect of oil and AgNPs on fibroblast cell lines, values are the mean of three dependent replicates ± SD. *: p<0.05, ** p<0.01, *** : p<0.001 compared the effect of AgNPs and oil and on fibroblasts to the effect of the compound to the pc3 cell line |
 |
Figure 2: Effect of the combination between V. iphionides oil and AgNPs on PANC1, K562, PC3, MDA-MB-231 and fibroblast cell lines, values are the mean of three dependent replicates ± SD. |
Antibacterial activity using well diffusion method
The increase in the resistance to different antibiotics can be controlled using new methodologies, such as synergy with either AgNPs or the EOs of plants. The synergistic effect of AgNPs and EO was evaluated using well diffusion method (Table 3). The results showed that there were notable increasing in antibacterial activity of AgNPs:EO except against E. coli. When AgNPs and EO were examined separately, the inhibition zones exhibited by AgNPs against E. aerogenes, P. aeruginosa and S. epidermidis and S. aureus were 20, 22, 20 and 16 mm, respectively. EO of V. iphionoides against same bacteria exhibited 19.2, 15.6, 26.5 and 19 mm, respectively. After combining of AgNPs and EO in a proportion of 1:1, the inhibition zone resulted is 21, 23, 22 and 22 mm, respectively showing presence of synergistic effect among them.
Table 3: Antibacterial activity of V. iphionoides EO, AgNPs and EO:AgNPs (1:1) on the tested bacterial strains using well diffusion method.
| Bacterial strains | AgNPs | EO | AgNPs:EO (1:1) |
| E. aerogenes | 20±1 | 19.2±1.0 | 21±0.57 |
| P. aeruginosa | 22±0.57 | 15.6±0.80 | 23±1 |
| E. coli | 18±0 | 26.5±1.40 | 18±0.50 |
| S. epidermidis | 20±0.57 | 24±1.08 | 22±0.50 |
| S. aureus | 16±0.57 | 19±0.88 | 22±0.60 |
Minimal Inhibitory Concentration and Growth Curves
Table 4 shows MIC values of AgNPs and EO of V. iphionoides against E. aerogenes, P. aeruginosa, E. coli, S. epidermidis and S. aureus. MICs of AgNPs against these bacterial strains in order were 19.15, 6.38, 19.15, 6.38, 6.38 µg/mL, respectively as demonstrated by broth dilution method. MICs of EO against the same bacteria were 50, 370, 50, 123 and 50 µg/mL, respectively.
Table 4: Minimum Inhibitory concentration (MIC) of AgNPs, V. iphionoides, EO and combination of AgNPs:EO
| Bacterial strain | AgNPs
µg/mL |
EO
µg/mL |
AgNPs:EO (1:1)
µg/mL:µg/mL |
Cm | Km | Amp |
| E. aerogenes | 19.15 | 50 | 10.63:61 | 0.75 | 0.20 | 2.30 |
| P. aeruginosa | 6.38 | 370 | 3.20:61 | 1.10 | 0.30 | 2.5 |
| E. coli | 19.15 | 50 | 6.4:184 | 0.80 | 0.15 | 0.20 |
| S. epidermidis | 6.38 | 123 | 3.20:61 | 0.16 | 0.28 | 0.32 |
| S. aureus | 6.38 | 50 | 3.20:61 | 0.15 | 0.32 | 0.25 |
Discussion
For 20 years, the extracts and phytoconstituents of V. Iphionoides have been known to possess many biological effects including; antibacterial,4 anti-diabetic,22 antiplatelet5 and anticancer activities against many cancer cell lines.23,24 In this study, we analyzed the chemical composition of V. iphionoides essential oil using GC/MS, and it was clear that oxygenated monoterpene was the major compound class identified in V. iphionoides essential oil and Eucalyptol (53.65), with Bornyl acetate (11.26) and Borenol (8.31%) the most dominant compounds identified. It has been reported that V. iphionoides essential oil collected from Jordan Valley, was rich in oxygenated monoterpene compounds and Borneol (49.3%), with bornyl formate (3.6%) and bornyl acetate (2.9%) the most dominant compounds (Avato et al., 2004). Al-Douri and co-authors25 qualitatively identified the presence of 1,8-cineole (8.4%), and camphor (3.7%) in V. iphionoides oil using Thin Layer Chromatography (TLC) and Gas Chromatography (GC) analysis.
In addition, in this study, we also reported the cytotoxity effect of the essential oil extracted from V. iphionides and the cytotoxity effect of AgNPs, (which were synthesized from fungal strain Tritirachium oryzae W5H) against breast, pancreatic and prostate cancer and leukaemia cell lines. 75 cm2 his suggests that Eucalyptol, Bornyl acetate and Borenol play a major role in this effect and we also documented that Eucalyptus has antiproliferative activity against leukemia by inducing apoptosis,26 as well as against colorectal cancer by inhibition of PI3K/Akt pathway and inducing caspase-3 cleavage leading finally to apoptosis.27 On the other hand, it has been reported that AgNPs exert antiproliferative activity against breast cancer,28 lung cancer,29 colon cancer30 and cervical cancer,28 through the inactivation of P13K/Akt signalling pathways.27 In our study we revealed the co-incubation effect between V. iphionides essential oil and AgNPs against the same cell lines, and our result showed the synergistic effect occured when V. iphionides essential oil and AgNPs were co-incubated at low concentrations. However, the cytotoxicity shows antagonistic effect at high concentrations. Consequently, we suggested that high concentrations of V. iphionides essential oil and AgNPs causes hyperactivation in the mTOR pathway leading to activation of P13K/Akt signalling pathways, which enhance proliferation. In contrast, at low concentration of V. iphionides essential oil and AgNPs, the activation of the mTOR pathway is reduced significantly, leading to the inactivation of the P13K/Akt signalling pathways, which increases the apoptosis and reduces the cells proliferation.
Antibacterial activity was estimated using a well diffusion assay (table 1). In general, EO and AgNPs revealed different effects against bacteria. EO and AgNPs exhibited reasonable antibacterial activities against the investigated bacteria, both G positive and negative bacteria. However, AgNPs exhibited stronger antibacterial activity than EO for V. iphionoides (excluding E. coli). AgNO3 showed a lower inhibition region (data not shown) compared to a similar concentration of AgNPs, which indicates that antibacterial activity is due to AgNPs no other thing. AgNPs are known to increase the surface area leading to greater surface contact with bacteria and thus a better bactericidal effect against bacteria under test.31 Previous studies, appeared that AgNPs had antibacterial activity and inhibitory zone extended between 16-22 mm against E. aerogenes, P. aeruginosa, E. coli, S. epidermidis and S. aureus at 100 µl (172.56 µg AgNPs per well) of AgNPs 18. The most susceptible strain to EO of V. iphionoides was E. coli (26.5 mm). P. aeruginosa (ESBL) showed lowest sensitivity (16.5 mm) to the EO. It might be reflect the effect of AgNPs through perforating and lysing the bacterial cell wall followed by generation of free radicals31 and disintegration of DNA.32
The MICs of the NPs varied from 6.4 – 19.2 μg/mL . The MIC values for the various bacteria were as follow: 6.4 μg/mL for S. aureus, S. epidermidis and P. aeruginosa; and 19.2 μg/mL for E. coli and E. aerogenes. The results of present study showed that V. iphionoides oil exhibited marked inhibition activity against Gram positive and Gram-negative bacteria with inhibition zone ranged between 16.5 and 26.5 mm and MIC value as low as 50 µg/ml. The EO appears to be more substantial determinant for the synergetic results of antibacterial activities by AgNPs and EO of V. iphionoides, especially against S. aureus, S. epidermidis, P. aeruginosa and E. aerogenes. Although many works have been published studying AgNPs, these synergistic applications have infrequently been investigated for the dealing with the resistant bactericidal activity. However, no data have been previously reported on the potential antibacterial effects of such cooperative interaction between biosynthesized AgNPs from Tritirachium oryzae, and the EOs of V. iphionoides against the tested bacteria. It has been mentioned that the absorption of a drug is increased several times in the presence of nanoparticles, suggesting that AgNPs could be used as potential drug delivery system.19 For example, the antimicrobial effect of some antibiotics including trimethoprim, amoxicillin, vancomycin and cefotaxime were much greater than the antibacterial effect of AgNPs alone. However, combinations between these antibiotics and AgNPs led to the boosting of antibacterial activity which indicates the synergistic effect of these components with the AgNPs. Moreover, nanoparticle-EO associations could reduce the amounts of combined agents necessary, herewith lowering noxiousness and elevating antimicrobial prospect.33 In this study, the bacterial samples tested which involve those that give rise to urinary tract infection and atopic dermatitis, burn infection, besides E. coli, were mainly antibiotic-resistant.19,34–36 Thus, the raises in the scale of inhibition against these antibiotic-resistant bacteria or still antibiotic-sensitive bacteria is seen a remarkable result and an effective therapeutic master plan. However, further studies are necessary in order to determine the precise pathway when different concentrations of V. iphionides essential oil and AgNPs are co-incubated with different cancer cell lines.
References
- Al-Eisawi DM. List of Jordan vascular plants. Amman. 1982;152:79–182.
- Feinbrun-Dothan N. Flora Palaestina. Part 3, Plates Ericaceae to Compositae. In: Israel Academy of Sciences & Humanities; 1977.
- Dafni A, Yaniv Z, Palevitch D. Ethnobotanical survey of medicinal plants in northern Israel. J. Ethnopharmacol. 1984;10(3):295–310.
CrossRef - Masadeh MM, Alkofahi AS, Tumah HN, et al. Antibacterial activity of some medicinal plants grown in Jordan. Pak. J. Pharm. Sci. 2013;26(2).
- Afifi FU, Aburjai T. Antiplatelet activity of Varthemia iphionoides. Fitoterapia. 2004;75(7–8):629–633.
CrossRef - Al-Dabbas MM, Suganuma T, Kitahara K, et al. Cytotoxic, antioxidant and antibacterial activities of Varthemia iphionoides Boiss. extracts. J. Ethnopharmacol. 2006;108(2):287–293.
CrossRef - Halees RY, Talib WH, Issa RA. Varthemia iphionoides and Pelargonium graveolens Extracts as a Treatment of Breast Cancer Implanted in Diabetic Mice. Pharmacogn. Mag. 2019;15(65):698.
CrossRef - Basu S, Samanta HS, Ganguly J. Green synthesis and swelling behavior of Ag-nanocomposite semi-IPN hydrogels and their drug delivery using Dolichos biflorus Linn. Soft Mater. 2018;16(1):7–19.
CrossRef - Carabineiro SAC. Applications of gold nanoparticles in nanomedicine: recent advances in vaccines. Molecules. 2017;22(5):857.
CrossRef - Roy K. ‘Green’Synthesis of Silver Nanoparticles by using Grape (Vitis Vinifera) Fruit Extract: Characterization of the Particles & Study of Antibacterial Activity. 2012.
- Kaur R, Singh J, Tripathi SK. Incorporation of inorganic nanoparticles into an organic polymer matrix for data storage application. Curr. Appl. Phys. 2017;17(5):756–762.
CrossRef - Abdal Dayem A, Hossain MK, Lee S Bin, et al. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017;18(1):120.
CrossRef - Fukui H. Development of new cosmetics based on nanoparticles. In: Nanoparticle Technology Handbook. Elsevier; 2018:399–405.
CrossRef - Bharathi D, Josebin MD, Vasantharaj S, et al. Biosynthesis of silver nanoparticles using stem bark extracts of Diospyros montana and their antioxidant and antibacterial activities. J. Nanostructure Chem. 2018;8(1):83–92.
CrossRef - Fathima JB, Pugazhendhi A, Oves M, et al. Synthesis of eco-friendly copper nanoparticles for augmentation of catalytic degradation of organic dyes. J. Mol. Liq. 2018;260:1–8.
CrossRef - Sharma P, Bhatt D, Zaidi MGH, et al. Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl. Biochem. Biotechnol. 2012;167(8):2225–2233.
CrossRef - Zhang J, Si G, Zou J, et al. Antimicrobial effects of silver nanoparticles synthesized by fatsia japonica leaf extracts for preservation of citrus fruits. J. Food Sci. 2017;82(8):1861–1866.
CrossRef - Qaralleh H, Khleifat KM, Al-Limoun MO, et al. Antibacterial and synergistic effect of biosynthesized silver nanoparticles using the fungi Tritirachium oryzae W5H with essential oil of Centaurea damascena to enhance conventional antibiotics activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019;10(2):25016.
CrossRef - Khlaifat AM, Al-limoun MO, Khleifat KM, et al. Antibacterial synergy of Tritirachium oryzae-produced silver nanoparticles with different antibiotics and essential oils derived from Cupressus sempervirens and Asteriscus graveolens (Forssk). Trop. J. Pharm. Res. 2019;18(12):2605–2616.
- Al-limoun M., Qaralleh HN, Khleifat KM, et al. Culture Media Composition and Reduction Potential Optimization of Mycelia- free Filtrate for the Biosynthesis of Silver Nanoparticles Using the Fungus Tritrichum oryzae W5H”. Curr. Nanosci. 2019;15(1).
CrossRef - Massa N, Cantamessa S, Novello G, et al. Antifungal activity of essential oils against azole-resistant and azole-susceptible Candida glabrata strains vaginal isolates. Can. J. Microbiol. 2018;(ja).
CrossRef - Gorelick J, Kitron A, Pen S, et al. Anti-diabetic activity of Chiliadenus iphionoides. J. Ethnopharmacol. 2011;137(3):1245–1249.
CrossRef - Thoppil RJ, Harlev E, Mandal A, et al. Antitumor activities of extracts from selected desert plants against HepG2 human hepatocellular carcinoma cells. Pharm. Biol. 2013;51(5):668–674.
CrossRef - Yarmolinsky L, Bari G, Hamias R, et al. Preferential anti-proliferative activity of Varthemia iphionoides (Chiliadenus iphinoides). Isr. J. Plant Sci. 2015;62(4):229–233.
CrossRef - Al-Douri NA, Al-Essa LY. A survey of plants used in Iraqi traditional medicine. Jordan J Pharm Sci. 2010;3(2):100–108.
- Moteki H, Hibasami H, Yamada Y, et al. Specific induction of apoptosis by 1, 8-cineole in two human leukemia cell lines, but not a in human stomach cancer cell line. Oncol. Rep. 2002;9(4):757–760.
CrossRef - Murata S, Shiragami R, Kosugi C, et al. Antitumor effect of 1, 8-cineole against colon cancer. Oncol. Rep. 2013;30(6):2647–2652.
CrossRef - Jeyaraj M, Sathishkumar G, Sivanandhan G, et al. Biogenic silver nanoparticles for cancer treatment: an experimental report. Colloids surfaces B Biointerfaces. 2013;106:86–92.
CrossRef - Gengan RM, Anand K, Phulukdaree A, et al. A549 lung cell line activity of biosynthesized silver nanoparticles using Albizia adianthifolia leaf. Colloids Surfaces B Biointerfaces. 2013;105:87–91.
CrossRef - Sanpui P, Chattopadhyay A, Ghosh SS. Induction of apoptosis in cancer cells at low silver nanoparticle concentrations using chitosan nanocarrier. ACS Appl. Mater. Interfaces. 2011;3(2):218–228.
CrossRef - Prabhu S, Poulose EK. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. nano Lett. 2012;2(1):32.
CrossRef - Durán N, Nakazato G, Seabra AB. Antimicrobial activity of biogenic silver nanoparticles, and silver chloride nanoparticles: an overview and comments. Appl. Microbiol. Biotechnol. 2016;100(15):6555–6570.
CrossRef - Naqvi SZH, Kiran U, Ali MI, et al. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int. J. Nanomedicine. 2013;8:3187.
CrossRef - Khleifat KM, Abboud MM, Omar SS, et al. Urinary tract infection in South Jordanian population. J Med Sci. 2006;6:5–11.
CrossRef - Al-Asoufi A, Khlaifat A, Al Tarawneh A, et al. Bacterial Quality of Urinary Tract Infections in Diabetic and Non Diabetics of the Population of Ma’an Province, Jordan. Pak. J. Biol. Sci. 2017;20(4):179–188.
CrossRef - Byrd AL, Deming C, Cassidy SKB, et al. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci. Transl. Med. 2017;9(397):eaal4651.
CrossRef







