Manuscript accepted on :9-04-2020
Published online on: --
Plagiarism Check: Yes
Reviewed by: Haider Mousa
Second Review by: Javed Mottaghipisheh
Final Approval by: Mohd Abdul Daiem
Mary Shobha Rani Inala1 , Gayathree Karthikkeyan2
, Gayathree Karthikkeyan2 , Sneha M Pinto2
, Sneha M Pinto2 and Kiranmayee Pamidimukkala1*
and Kiranmayee Pamidimukkala1*
1Department of Cell Biology and Molecular Genetics, Sri Devaraj Urs Academy of Higher Education and Research, Tamaka, Kolar, Karnataka, India, 563103
2Centre for Systems Biology and Molecular Medicine, Yenepoya Research Centre, Yenepoya (Deemed to be University), Mangalore, India, 575018
Corresponding Author E-mail : kiranmayee@sduu.ac.in
DOI : https://dx.doi.org/10.13005/bpj/1951
Abstract
Screening of antibacterial compounds helps to combat the resistance exhibited by bacteria to antibiotics. The aim of this study is to isolate ‘3, 3', 4', 5, 7-pentahydroxyflavone’ (quercetin) a well- known flavonoid from edible greens of Karnataka with a focus on its biological activity. We employed column chromatography for successful extraction of compound, identified it by High Performance liquid chromatography (HPLC), UV-Visible Spectroscopy and Infra-red Spectroscopy. For knocking antibacterial action, we used five methods, the first method is screening of antibacterial activity by Agar-well diffusion method, and the second is determining anti-bacterial motility, third is biofilm formation control, fourth is determination of release of absorbed materials and fifth is DNA fragmentation assay. The results of all the methods employed were complementary with minimal concentration of compound i.e. 10 µg/ml on all the tested bacteria. Induction of DNA fragmentation with 10 µg/ml of the compound is an important observation of the presented study on all the pathogens. Hence, identification of new unexploited sources of flavonoids from edible greens helps in combating emerging drug resistance. To conclude, the exploitation of ample sources of ‘3, 3', 4', 5, 7-pentahydroxyflavone’ from commonly preferred food constituent’s increases its consumption rate thereby preventing the risk of infections.
Keywords
Anti-Bacterial; Chromatography; Identification; Phytochemicals; Quercetin; Spectroscopy
Download this article as:| Copy the following to cite this article: Inala M. S. R, Karthikkeyan G, Pinto S. M, Pamidimukkala K. Biological Activity of ‘3, 3', 4', 5, 7-Pentahydroxyflavone Isolated from Green Leafy Vegetables of Karnataka on three Bacterial Strains. Biomed Pharmacol J 2020;13(2). |
| Copy the following to cite this URL: Inala M. S. R, Karthikkeyan G, Pinto S. M, Pamidimukkala K. Biological Activity of ‘3, 3', 4', 5, 7-Pentahydroxyflavone Isolated from Green Leafy Vegetables of Karnataka on three Bacterial Strains. Biomed Pharmacol J 2020;13(2). Available from: https://bit.ly/3dmkoFy |
Introduction
India is a richest source of biodiversity with 2500 plant species, out of which 100 species are recognised as traditional healers. World Health Organisation (WHO) estimates that 80% of the world’s population rely on plants for their primary health care needs 1. Hence, knowledge of chemical composition of plants is necessary for understanding the possible mechanism of action, led to focus on principle components of plants in contributing medicinal properties. Phenolics, the broad class of secondary metabolites are ubiquitous in plants with flavonols, flavanones and flavonol subgroups. These subgroups are continuing to receive ample attention due to their vast biological potential. Four kinds of flavonoids are broadly investigated for clinical use that include polyphenols derived from tea, 3, 3′, 4′, 5, 7-pentahydroxyflavone (quercetin) and its molecular cousins. Due to massive attention on flavonoids by many investigators, today, 3, 3′, 4′, 5, 7-pentahydroxyflavone, a flavonol abundant in foods has become the most studied flavonoid 2. The common name of well-known bioflavonoid, 3, 3′, 4′, 5, 7-pentahydroxyflavone is quercetin. The presence of these polyphenolic bioflavonoids is responsible for therapeutic potency of plant that contributes antioxidant action by scavenging mechanisms3. This has drawn our attention in identifying natural antioxidants with antimicrobial potencies from locally available greens. Raphanus sativus L. (radish) and Anethum graveolens L. (dill weed) are widely grown and used vegetable of South East Karnataka. The underground tuber of radish is exhaustively used and the leaves are very well neglected. Hence, in the present study, leaves of radish and dill weed have chosen for flavonoid and compared its biological activity.
R. sativus L. is an annual root vegetable of Kolar belongs to Brassicaceae family with an annual average yield of 42,276 tonnes. It has traditional importance in countries like China, Japan, Korea and Southeast Asia due to its adaptive ability, high yield and more nutritional value4. Root is consumed as vegetable; in some places, leaves are used in salads and garnishes. The seed, root and leaf of R. sativus are claimed to have several medicinal properties 5. Raphani semen (dried ripe seed) is used in Chinese traditional medicine to promote digestion, treat dyspeptic retention, constipation, diarrhoea, breathless condition, dysentery and cough with phlegm 6-7. Even though the medicinal use of Raphani semen has historical importance more than a thousand years, there is no literature available on the flavonoid content of leaves and identification of effective flavonol, 3,3′,4′,5,7-pentahydroxyflavone for its pharmacological use.
A. graveolens L. is an annual herb belongs to family Apiaceae. It is grown widely in Eurasia and in Karnataka and spread widely in Bijapur with a yield of 646 tonnes per year8. In Unani medicine, it is used in curing digestive problems and also used in more than 56 Ayurveda preparations like Maharasnadi kashayam, Mrithasanjeevani, Gugguluthiktaquatham and Sraswatharishtam. It was tremendously used by Egyptian doctors from over 5000 years ago9. The pharmacological importance of A. graveolens is known for its anti-microbial, anti-hyper lipidemic and anti-hyper cholesterolemic activities10. Furthermore, the fruit is used in treating gastrointestinal disorders11. Since, the plant has medicinal importance and there is a gap in literature on the 3, 3′, 4′, 5, 7-pentahydroxyflavone content of leaves of the plant, exploring its phytochemical components contributing in medicine is essential. Hence, the objectives of the study were screening of phytochemicals, identification and structural elucidation of flavonol, 3, 3′, 4′, 5, 7-pentahydroxyflavone (quercetin) from leaves of A. graveolens L. and R. sativus L. and exploring its biological potency.
Materials and Methods
Collection of Material
The seeds of R. sativus and A. graveolens were grown in pesticide free environment, leaves were collected after 2-3 days water stress shade dried, powdered and stored. The authentication of the plant samples with voucher specimen number of R. sativus (2232) and A. graveolens (1236) were provided by Dr. Madhava Shetty, Assistant Professor, Department of Botany, S.V. University, Tirupathi.
ATCC strains of Pseudomonas aeruginosa (27853), Staphylococcus aureus (25923) and Escherichia coli (25922) were obtained from HI media Laboratories, India. All others chemicals and reagents used were of analytical grade obtained from Sigma Aldrich.
Extraction and Phytochemical Analysis
Adopted protocol was same for both the plants. About 25 grams of leaf powder was taken in 100 ml of ethanol and was subjected to constant shaking at 120-130 rpm for 48 hours at room temperature. After 48 h, the extracts were filtered using Whatmann No. 1 filter paper and the solvent was evaporated until dryness. About 12g of dried extract was obtained from both the plant materials and stored at 4°C for further analysis.
Phytochemical analysis was performed for both the leaf extracts according to standard protocol and total flavonoid content was determined by Aluminium chloride method12.
Purification of 3, 3′, 4′, 5, 7-Pentahydroxyflavone
Column chromatography was performed according to the procedure of Chandrappa et al.13 The concentrated plant compound obtained from column chromatography at 1mg/ml was subjected to HPLC along with the standard quercetin (3,3′,4′,5,7-pentahydroxyflavone) to check purity of the compound.
Characterization of 3, 3′, 4′, 5, 7-Pentahydroxyflavone
Mass Spectrometry-Based Multiple Reaction Monitoring (MS-MRM)
The qualitative analysis of the metabolite, 3, 3′, 4′, 5, 7-pentahydroxyflavone, was carried out using Multiple Reaction Monitoring (MRM) assay using QTRAP 6500, AB-SCIEX mass spectrometer. The samples were diluted to 1: 5 in 0.1% formic acid in water and analysed in MS1, MS2 and MRM modes for the presence of 3,3′,4′,5,7-pentahydroxyflavone using the selected ion pair transitions14.
H 1 Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR is primarily related to the magnetic properties of certain atomic nuclei; notably the nucleus of the hydrogen atom and will demonstrate the number of atoms in neighbouring groups. Hence, in the present study, it was used as one of the tools for 3, 3′, 4′, 5, 7-pentahydroxyflavone identification 15.
UV-Vis Spectrophotometry and FTIR Measurements
Spectrophotometric analysis of the isolated 3, 3′, 4′, 5, 7-pentahydroxyflavone was performed by UV-Vis Lambda 20 Spectrophotometer between 200 and 700 nm wavelength with a band width of 2 mm. Infrared absorption (IR) spectrum of flavonol 3,3 ‘, 4’, 5 ,7-pentahydroxyflavone was recorded with Fourier Transform Infrared (FTIR) absorption spectrometer. The spectrum was recorded in the region between 4000 cm-1 and 400 cm-116.
Determination of Biological Activity
Antibacterial Activity
Antibacterial activity of 3, 3′, 4′, 5, 7-pentahydroxyflavone extracted from both the plant species was detected primarily by agar well diffusion method by using ATCC strains of Pseudomonas aeruginosa (27853), Staphylococcus aureus (25923) and Escherichia coli (25922). 3, 3′, 4′, 5, 7-pentahydroxyflavone was prepared in 10 μg/ml, 20, 30, 40 50, 100, 150, 200 and 250 μg/ml concentrations and checked for antibacterial activity. The same strains of bacteria were also tested with the high-end antibiotic discs by performing antibiotic susceptibility test on nutrient agar plates. The zone of inhibition was measured for both the experiments. All the experiments were repeated thrice and results were noted 17.
Determination of Motility Assays
Swarming, swimming and twitching motility of bacteria were detected with minimal to maximum concentration of 3, 3′, 4′, 5, 7-pentahydroxyflavone i.e.; 10 (Minimal Inhibitory Concentration) and 100 µg/ml (as less concentration as possible) concentration extracted from two plants. The appearance of spreading zones from the point of inoculation from the bottom of petri dish is noted for all the assays and results were noted 18.
Effect of 3, 3′, 4′, 5, 7-Pentahydroxyflavone on Biofilm formation
Anti-biofilm activity of 3, 3′, 4′, 5, 7-pentahydroxyflavone was performed with 10 and 100 µg/ml concentrations of plant extracts by the method described by Lee19. Average of the three biological replicates was calculated for the percentage of biofilm inhibition by using the following equation.
OD of test/ OD of control x 100
Measurement of Absorbing Materials and Proteins
Fresh bacterial culture was inoculated in nutrient broth and cells in log phase were collected by centrifugation at 5000 X g for 15 min. The collected cells were washed three times with 0.1 M PBS (pH 7.4) and re suspended in PBS. Cell suspension of 100 ml was incubated at 370C in shaking incubator for 4h in treatment with 10 and 100 µg/ml concentrations of 3,3′,4′,5,7-pentahydroxyflavone extracted [control, 1X MIC and 2X MIC]. The suspensions were removed from shaking incubator after 4h and centrifuged at 6000 X g for 5 min. The supernatants were read at 260 nm for 4 h and 24 h. Along with; the concentration of proteins in supernatant was assessed by using Lowry method. Absorbance was measured for three replicates at 280 nm20.
DNA Fragmentation Assay
DNA fragmentation assay was performed according to standard procedure 21. About 104 cells of all the bacteria were treated with 10 and 100 µg/ml concentrations of 3, 3′, 4′, 5, 7-pentahydroxyflavone extracted for 4h, centrifuged at 5000 rpm for 5 min and discarded the supernatant To the pellet, 20 μl of TES (1 M Tris, 20 mM EDTA and 1%SDS) was added to lyse the cells and 10 μl of RNase was added followed by incubation for 1h at 370C. Added 10 μl of proteinase K, incubated at 500C for 1 hr and 30 min. The resultant solution was loaded onto 1% agarose gel and documented by using UV-trans illuminator (Biorad).
Results
Phytochemical analysis of both the leaf extracts revealed the presence and absence of various phytochemical components which is shown in table 1. The flavonoid content of the leaf extract was found to vary between the two extracts tested. The exhibited concentrations after solvent extraction were 128 mg and 190 mg from A. graveolens and R. sativus respectively. The retention time of isolated 3, 3′, 4′, 5, 7-pentahydroxyflavone and the standard noted was the same as (Figure 1). The mass/charge (m/z) ratio of isolated samples was in agreement with the standard (Figure 2). The peaks of proton NMR of test samples and the standard were matching as shown in (Figure 3). The UV- visible spectrum of both the samples showed maximum absorption from 230 nm to 450 nm which is similar to standard quercetin (3, 3′, 4′, 5, 7-pentahydroxyflavone) (Figure 4).
 |
Figure 1: HPLC chromatograms of 3, 3′, 4′, 5, 7-pentahydroxyflavone |
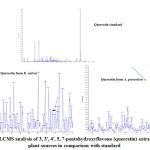 |
Figure 2: LCMS analysis of 3, 3′, 4′, 5, 7-pentahydroxyflavone |
 |
Figure 3: NMR analysis of 3, 3′, 4′, 5, 7-pentahydroxyflavone |
 |
Figure 4: UV-Vis analysis of 3, 3′, 4′, 5, 7-pentahydroxyflavone |
Table 1: Phytochemical content of R. sativus and A. graveolens leaf extracts
| Name of the Phytochemicals | R. sativus | A. graveolens |
| Phenolics | Present | Present |
| Flavonoids | Present | Present |
| Proteins | Present | Absent |
| Glycosides | Present | Present |
| Terpenoids | Absent | Present |
| Alkaloids | Present | Present |
| Cardiac glycosides | Mildly present | Present |
| Tannins | Present | Absent |
| Gums and Resins | Absent | Absent |
| Saponins | Absent | Absent |
The FTIR spectrum of quercetin extracted from A. graveolens and R. sativus were shown in fig 5 with its characteristic bands. The results of the FTIR were as follows; with respect to A. graveolens OH group stretches were found at 3409 and 3124 cm−1, OH bending of the phenol function was detected at 1382 cm−1. The C=O aryl ketonic stretch absorption was evident at 1664 cm−1. C=C aromatic ring stretches were found at 1610, 1562, 1522 cm−1. The in plane bending of C-H in aromatic hydrocarbon was detected at 1319 cm−1 and out plane bending bands were found at 941, 864, 852, 767, 724, 695, 639, 603 which were attributed to C-O stretch in the aryl ether ring, the C-O stretching in phenol and C-CO-C stretch and bending in ketone respectively. Where as in R. sativus OH group stretches were found at 3414 cm−1, OH bending of the phenol function was detected at 1383 cm−1. The C=O aryl ketonic stretch absorption was evident at 1660 cm−1. C=C aromatic ring stretches were found at 1611, 1563, 1523 cm−1. The in plane bending of C-H in aromatic hydrocarbon was detected at 1320 cm−1 and out plane bending bands were found at 942, 865, 853, 768, 725 respectively which were attributed to C-O stretch in the aryl ether ring, the C-O stretching in phenol and C-CO-C stretch and bending in ketone group. These results confirmed that the compound extracted is 3, 3′, 4′, 5, 7-pentahydroxyflavone.
 |
Figure 5: FTIR spectrum of 3, 3′, 4′, 5, 7-pentahydroxyflavone |
3, 3′, 4′, 5, 7-Pentahydroxyflavone as a Potent Bactericidal Agent
The antibacterial activity of 3, 3′, 4′, 5, 7-pentahydroxyflavone extracted from both the leaf materials were promising.
In case of 3, 3′, 4′, 5, 7-pentahydroxyflavone from A. graveolens zone of inhibition (ZOI) was from 10 μg/ml to 250 μg/ml concentration, maximum zone being 22 mm was noted at 250 μg/ml concentration on S. aureus, which is streamlined with Cefoxitin, Vancomycin and Ciprofloxacin. For P. aeruginosa, the maximum inhibition zone was 20 mm for 250 μg/ml concentration which is in coordination with antibiotics Piperacillin, Ceftazidime and Imipenem. Similarly, the ZOI was 18 mm for both the test compounds and the antibiotics (Ampicillin, Sulphomethexazole and Chloramphenicol) used for E. coli.
In case of R. sativus, 24 mm ZOI was exhibited by 3, 3′, 4′, 5, 7-pentahydroxyflavone for S. aureus which is in consistent with antibiotics Penicillin and P. aeruginosa exhibited 20 mm which is consistent with Piperacillin and Imipenem. In case of E.coli 3, 3′, 4′, 5, 7-pentahydroxyflavone exhibited highest (27 mm) among the antibiotics tested Tables 2 and 3.
Table 2: Antibacterial activity of 3, 3′, 4′, 5, 7-pentahydroxyflavone (quercetin) extracted from A. graveolens and R. sativus
| Concentration in
µg/ml |
P. aeruginosa
(ZOI by quercetin from both the plants)
|
S. aureus
(ZOI by quercetin from both the plants)
|
E.coli
( ZOI by quercetin from both the plants)
|
| A. graveolens R. sativus A. graveolens R. sativus A. graveolens R. sativus | |||
| 10 | 06 06 | 06 06 | 06 06 |
| 20 | 06 06 | 06 6.5 | 07 07 |
| 30 | 07 6.5 | 07 6.5 | 08 7.2 |
| 40 | 07 07 | 08 7.5 | 09 7.5 |
| 50 | 7.5 07 | 10 8.5 | 12 10 |
| 100 | 08 08 | 14 12 | 14 13 |
| 150 | 9.5 09 | 16 15 | 16 18 |
| 200 | 13 12 | 18 18 | 17 23 |
| 250 | 20 20 | 22 24 | 18 27 |
Table 3: Antibacterial activity exhibited by antibiotics on selected pathogens
| P. aeruginosa | S. aureus | E. coli | |||
| Antibiotic | ZOI (mm) | Antibiotic | ZOI (mm) | Antibiotic | ZOI (mm) |
| Piperacillin | 20 | Azithromycin | 19 | Ampicillin | 18 |
| Ceftizidime | 19 | Erythromycin | 19 | Cephelexin | 23 |
| Gentamycin | 18 | Ciprofloxacin | 22 | Amikacin | 20 |
| Tazobactum | 21 | Penicillin | 25 | Amoxycillin | 22 |
| Amikacin | 18 | Trimethoprim | 16 | Sepifime | 19 |
| Aztronam | 24 | Cefoxitin | 22 | Chloramphenicol | 18 |
| Ciprofloxacin | 23 | Tetracycline | 18 | Sulphomethoxazole | 18 |
| Imipenem | 20 | Vancomycin | 22 | Sepfuroxi | 23 |
| Levofloxacin | 18 | Gentamycin | 16 | Trimethoprim | 21 |
3, 3′, 4′, 5, 7-pentahydroxyflavone shared antibacterial activity more or less similar to the tested antibiotics and in case of E. coli 3, 3′, 4′, 5, 7-pentahydroxyflavone extracted from R. sativus exhibited potent inhibitory zone than all the high-end antibiotics tested. It is reported from the literature that the antibiotics used for broad spectrum antibacterial activity like Cefoxitin, Vancomycin, Piperacillin, Ceftazidime, Ampicillin and Imipenem inhibit cell wall synthesis and disrupt cell membrane22.
The obtained results by 3, 3′, 4′, 5, 7-pentahydroxyflavone extracted from both the plants which are on par with the results of antibiotics, might be exhibiting the similar mechanism of action which is depicted in figure 6. Hence further investigations are necessitated in order to rule out the exact mechanism of action of 3, 3′, 4′, 5, 7-pentahydroxyflavone.
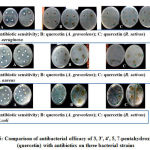 |
Figure 6: Comparison of antibacterial efficacy of 3, 3′, 4′, 5, 7-pentahydroxyflavone |
The prime requisite is to understand the action of any drug on microbial cell wall and membranes, the study is carried over for further parameter’s assessment.
Limiting Bacterial Motility by 3, 3′, 4′, 5, 7-Pentahydroxyflavone
3, 3′, 4′, 5, 7-pentahydroxyflavone extracted from both the plant species restricted twitching motility better than the other two motility patterns at 100 µg/ml concentration in all pathogenic bacteria tested. In E.coli, 3, 3′, 4′, 5, 7-pentahydroxyflavone extracted from R. sativus showed potent twitching motility restriction which is coping up with the results of antibacterial activity.
Whereas, 3, 3′, 4′, 5, 7-pentahydroxyflavone from A. graveolens presented good results than 3, 3′, 4′, 5, 7-pentahydroxyflavone from R. sativus on P.aeruginosa and S.aureus (Figure 7).
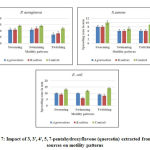 |
Figure 7: Impact of 3, 3′, 4′, 5, 7-pentahydroxyflavone (quercetin) |
Regulation of Biofilm Formation by 3, 3’, 4’, 5, 7-Pentahydroxyflavone
The formation of biofilm is an important tool for developing resistance in bacteria. Hence, control of biofilm formation is an interesting strategy. 3, 3’, 4’, 5, 7-pentahydroxyflavone extracted from both the plant species in the current study had similar action with respect to biofilm formation exhibited by S. aureus than in case of P. aeruginosa and E.coli. Both the extracted compounds exhibited 90% biofilm inhibition on S. aureus (Figure 8).
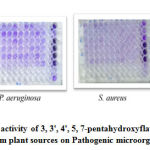 |
Figure 8: Anti-biofilm activity of 3, 3′, 4′, 5, 7-pentahydroxyflavone (quercetin) |
3, 3′, 4′, 5, 7-Pentahydroxyflavone Impact on Release of Absorbing Materials and Proteins
The 260 nm absorbing materials are used as an indicative of damage to the membrane integrity. Any drug when explored as an antibiotic shall be its maximum efficacy with minimum concentration. Keeping this in view, we used minimum inhibitory concentration (MIC-10 µg/ml) and minimum concentration with maximum efficacy (100 µg/ml). The tested bacteria lost the cell constituents at 10 µg/ml and 100 µg/ml concentrations with 3, 3′, 4′, 5, 7-pentahydroxyflavone, indicating damage to cytoplasmic membrane. As the permeability of cell membrane increases, leakage of intracellular proteins takes place in to surrounding environment thereby increasing the measurement of protein (Figure 9).
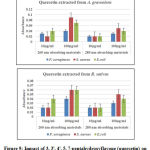 |
Figure 9: Impact of 3, 3′, 4′, 5, 7-pentahydroxyflavone (quercetin) |
3, 3′, 4′, 5, 7-Pentahydroxyflavone Induced DNA Fragmentation
The shredding of bacterial genomic DNA is one of the early events of destruction. To test the cleavage of DNA, P. aeruginosa, S. aureus and E.coli were exposed to two different concentrations of (10 and 100 µg/ml) 3, 3′, 4′, 5, 7-pentahydroxyflavone extracted from both the plants and showed marked DNA damage after 24 hr incubation. The results confirmed that the extracted 3, 3’, 4’, 5, 7-pentahydroxyflavone damaged the cell membrane and fragmented the DNA, thereby resulting in leakage of the contents and fragmentation of DNA (Figure 10).
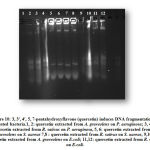 |
Figure 10: 3, 3′, 4′, 5, 7-pentahydroxyflavone (quercetin) induces |
Discussion
Bacteria have three important components like cell wall, cell membrane and cytoplasm. Special structures are also part of them like cilia, flagella and pili. Hence, in the present study mechanism of action of 3, 3′, 4′, 5, 7-pentahydroxyflavone on these structures were reported. The five methods employed for determination of antibacterial capacity of 3, 3′, 4′, 5, 7-pentahydroxyflavone components extracted from the two plants have complementary results. Flavonols like qercetin, morin, rutin, myricetin and their derivatives show potent antibacterial properties. It was also confirmed in a study performed by Mary et al. where quercetin (3, 3’, 4’, 5, 7-pentahydroxyflavone) showed significant antibacterial activity on methicillin resistant S. aureus26. Investigations made by Geoghegan, Wong and Rabie proved antibacterial activity at 0.0125 µg/ml are effective against Porphyromonas gingivalis27. It was further stated that synergistic activity was shown when S. epidermidis(resistant to Amoxicillin) was exposed to combination therapy (quercetin and amoxicillin) 28-29.
The above observations were in agreement with the results of the present study that 3, 3′, 4′, 5, 7-pentahydroxyflavone(quercetin) displays potent antibacterial activity on gram positive bacteria than on gram negative bacteria at minimal concentrations i. e 10 µg/ml. The reason behind its activity might be due to its cell membrane disruption nature as reported in the literature30-31. Understanding the variations in the cell wall structures and their modifications at the cellular level is an important approach in identifying antibacterial mechanism of any drug.
Bacterial motility plays a vital role in virulence by induction of various virulence determinants. As bacteria have outer appendages responsible for motility, it is therefore a goal to investigate the impact of 3, 3′, 4′, 5, 7-pentahydroxyflavone on motility patterns of bacteria i.e. swarming, swimming and twitching. Swarming and swimming are flagella mediated and twitching is type IV pilus mediated. Steps should be taken to prevent them, since these motilities elevate resistance to antibiotics. To our surprise, 3, 3′, 4′, 5, 7-pentahydroxyflavone tested in the present study restricted twitching motility more than the other two patterns of motilities 32. And among the three organisms, twitching motility is potent in controlling the movement of S. aureus. Though S. aureus historically been regarded as non-motile bacterium, recent findings showed that it exhibits passive movement on agar surface by spreading. These spreading movements are due to branching structures emerge from the central colony, called, dendrites. Research observations suggested that S. aureus is motile under certain circumstances33. Twitching motility is considered as one of the important factors involved in the formation of biofilm on abiotic surfaces34. Hence the control of twitching motility gains importance in the scientific research which is proven in the present study for the first time by 3, 3′, 4′, 5, 7-pentahydroxyflavone extracted from natural sources.
Furthermore, as the results of motility were encouraging, impact of 3, 3′, 4′, 5, 7-pentahydroxyflavone on bacterial biofilm were assessed. It is reported that biofilm producing organisms take a major share in causing bacterial infections; it acts as a protection barrier making the bacteria highly resistant to antibiotics35. Controlling biofilm formation is an important strategy. To the best of our knowledge, the anti-biofilm potential of 3, 3′, 4′, 5, 7-pentahydroxyflavone extracted from A. graveolens and R. sativus is being reported for the first time in the study. The prior step in the formation of biofilm relies on attachment of planktonic cells onto a substrate to form more sessile cells. The extent of inhibition of cell attachment by 3, 3′, 4′, 5, 7-pentahydroxyflavone extracted from the plants is quite encouraging in S. aureus than in P. aeruginosa and E.coli. Manner et al. demonstrated that flavonoids exhibited anti-biofilm activity against S. aureus which is in agreement with the present study36. Apart from various strategies to control biofilm, novel strategies by using plants have to be reported to gain much significance37-38. The red wine flavonoid groups, quercetin, apigenin, chrysin and kaempferol showed biofilm inhibition of S.aureus39.These are in consistent with present study results that 3, 3′, 4′, 5, 7-pentahydroxyflavone extracted from A.graveolens and R.sativus has similar biofilm inhibition on S. aureus.
Since 3, 3′, 4′, 5, 7-pentahydroxyflavone has motility and biofilm control effect, we thought of focusing on the measurement of released cell contents because of cell wall disruption. Measurement of released proteins and other materials from pathogenic bacteria treated with 3, 3′, 4′, 5, 7-pentahydroxyflavone were analysed. Bacterial cell absorbs key structural macromolecules that include proteins, nucleic acids and major amino acids (tryptophan, tyrosine and cysteine) at 260/280 nm40. It is noted that as concentrations of 3, 3′, 4′, 5, 7-pentahydroxyflavone increased, soluble proteins from the superbugs, S. aureus and E.coli increased in the supernatant, resulting in leakage of cell membrane, thereby decreasing the survival of the test organisms. Study performed by other investigators proved that leakage of cellular components at 260 nm and 280 nm were noticed when treated with spice extract essential oil against S. aureus. These observations also suggest that plant spices have cell membrane disruption activity which was in agreement with spice used in the present study A. graveolens41. Studies performed on black pepper essential oil also caused leaching of materials at 260 nm and 280 nm against S. aureus indicating the membrane permeability42. These observations prove the fact that plant components possess potent phytochemicals that aid in disruption of cell membrane.
The effective role of 3, 3′, 4′, 5, 7-pentahydroxyflavone on released materials motivated to screen for its influence on genomic DNA. When tested on bacteria, 3, 3′, 4′, 5, 7-pentahydroxyflavone induced fragmentation of DNA. It was reported by Jose and his co-investigators that E.coli upon treatment with a chemical compound, hydrogen peroxide induced DNA fragmentation43. Further Mori in his observations found that flavonoids are the strong antibacterial agents that elicit DNA damage44. Study by Ohemeg et al found that flavonoids were responsible for inhibitory activity of E.coli DNA damage and Staphylococcus epidermis which are in co-ordination with the present study results45. These observations evidenced that flavonoids act on nucleic acid system and helps in proper functions of DNA, leading to growth inhibition. To our knowledge this is the first report to identify a compound responsible for DNA fragmentation in bacterial strains extracted from plant sources where as it is well studied in cancer cells.
To conclude 3, 3′, 4′, 5, 7-pentahydroxyflavone was extracted successfully by Column chromatography from the leaf extracts. The proposed antibacterial mechanism of 3, 3′, 4′, 5, 7-pentahydroxyflavone extracted from plant source is as follows: attenuation of pathogenicity, restriction of twitching motility, reduction in biofilm formation, alteration in cytoplasmic membrane function, and induction of DNA fragmentation which are novel identifications from the selected plants.
Acknowledgements
The authors are grateful to Sri Devaraj Urs Academy of Higher Education and Research for proving all the infrastructure. The authors are thankful to Dr. Udaya Kumar and Miss Rajini from GKVK Bangalore for their support in HPLC analysis. The authors are also thankful to Dr.CV Raghuveer from Yenepoya Medical College, Mangalore for helping in Mass spectral analysis of the compound.
Conflicts of Interest
Authors declared none
References
- Azaizeh H, Fulder S, Khalil K, Said O. Ethno medicinal knowledge of local Arab practioners in the middle-east region, Fitoterapia 2003; 14: 98-108. doi: 10.1016/30367-326x (02) 00285-x
- D’Andrea G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015; 106: 256-71. doi: 10.1016/j.fitote.2015.09.018. Epub 2015 Sep 21
- Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci 2008; 4(2):89-96. PubMed PMID: 23675073; PubMed Central PMCID: PMC3614697.
- Zhao-liang LU, Li-wang LIU, Xiao yan LI, Yi-qin GONG, Xi-lin HOU et al. Analysis and Evaluation of Nutritional Quality in Chinese Radish (Raphanus sativus L.) Agricultural Sciences in China 2008; 7: 823-830. doi.org:10.1016/S1671-2927 (08)60119-4
- Aziz F, Hassan D. Radish Juice Promote Kidney Stone Deposition in Ethylene Glycol-induced Urolithiasis in Rats. Cihan University-Erbil Scientific Journal 2020; 4(1): 57-61. doi.org:10.24086/cuesj.v4n1y2020.pp57-61
- Tung-Ting Sham, Alisa Chui-Ying Yuen, Yam- Fung Ng, Chi-On –Chan, Daniel Kam Wah Mok and Shun Wan Chan. A Review of the Phytochemistry and Pharmacological Activities of Raphani Semen. Evidenced – Based Complementary and Alternative Medicine 2013; 1-16. doi.org:10.1155/2013/636194
- Xu Q, Bauer R, Hendry BM, Fan TP, Zhao Z, Duez P, Simmonds MS et al. The quest for modernisation of traditional Chinese medicine. BMC Complement Altern Med 2013; 13:132 doi.org: 10.1186/1472-6882-13-132. PMID: 23763836; PMCID: PMC3689083.
- Jana S, Shekhawat GS. Anethum graveolens: An Indian traditional medicinal herb and spice. Pharmacogn Rev 2010; 4(8):179-84.doi: 10.4103/0973-7847.70915). PMID: 22228959; PMCID: PMC3249919.
- Ravindran P, Balachandran I. Underutilized medicinal spices II, Spice India 2005; Vol. 17: India: Publisher V K Krishnan Nair: 32–6.
- Delaquis PJ, Stanich K, Girard B, Mazza G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int J Food Microbiol 2002; 25: 74(1-2):101-9. PubMed PMID: 11929164.
- Yazdanparast R, Alavi M. Anti-hyperlipidaemic and anti-hypercholesterolaemic effects of Anethum graveolens leaves after the removal of furocoumarins. Cytobios 2001; 105 (410):185-91. PubMed PMID: 11409638.
- Anyasor GN, Ogunwenmo KO, Oyelana OA, Akpofunure BE. Phytochemical constituents and antioxidant activities of aqueous and methanol stem extracts of Costus afer Ker Gawl. (Costaceae). Afr. J. Biotechnol 2010; 9:4880-84. doi.org: 10.5530/pj.2019.11.218.
- Chandrappa CP, Govindappa M, Anil Kumar NV, Channabasava R, Chandrasekhar N, UmaShankar. Identification and Separation of quercetin from ethanol extract of Carmona retusa by Thin Layer Chromatography and High Performance Liquid Chromatography with diode array detection. W J Pharma Pharmaceut Sci 2014; 3: 2021-2029.
- Ming-Hua Hao, Fan Zhang, Xing-Xia Liu, Fan Zhang, Li-Juan Wang et al. Qualitative and quantitative analysis of catechin and quercetin in flavonoids extracted from Rosa roxburghii Tratt. Trop. J. Pharm. Res 2018; 17(1): 71-76. doi.org: 10.4314/tjpr.v17i1.11.
- Selvaraj K, Chowdhury R, Bhattacharjee C. Isolation and structural elucidation of flavonoids from aquatic fern Azolla microphyllaand evaluation of free radical scavenging activity. Int J Pharm Sci 2013; 5:743–749.
- Lu Ross X, Power CF JR, Rasco BA. Determination of quercetin in onions (Allium cepa) using Infrared Spectroscopy. J. Agri. Food. Chem 2011; 59: 6376 – 6382. doi.org:10.1021/jf200953z. Epub 2011 May 25.
- Nakamura K, Ishiyama K, Sheng H, Ikai H, Kanno T, Niwano Y. Bactericidal activity and mechanism of photo irradiated polyphenols against Gram-positive and negative bacteria.J Agric Food Chem 2015; 63:7707-7713.doi.org: 10.1021/jf5058588
- Sameer Mohideen Gani S, Gopinath P. Detection of Swarming, Swimming and twitching motilities among clinical isolates of Pseudomonas aeruginosa; J Chem. Pharm Sci 2016; 9(4): 3248-3250. doi.org:10.18535/jmscr/v4i8.50.
- Lee LH, Cho MH, Lee J. 3-Indolylacetonitrile decreases Escherichia coli O157:H7 biofilm formation and Pseudomonas aeruginosa virulence. Environ Microbiol 2011; 13: 62–73.
- Xu JG, Hu QP, Wang XD, Luo JY, Liu Y, Tian CR. Changes in the main nutrients, phytochemistry and antioxidant activity in yellow corn grain during maturation. J Agric Food Chem 2010; 58:5751-5756. doi.org:10.1021/jf100364k
- Pattern.R, Hirzel, A. Apoptosis DNA fragmentation analysis protocol. Abcam. 2017.
- Kapoor G, Saigal S, Elongavan A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J Anaesthesiol Clin Pharmacol 2017; 33(3):300–305. (doi.org:10.4103/joacp.JOACP_349_15)
- Yoon J, Kim B, Lee WJ, Lim Y, Chong Y, Ahn J. Production of a novel quercetin glycoside through metabolic engineering of Escherichia coli. Appl Environ Microb 2012; 78:4256–4262.
- Holderbaum DF, Kon T, Guerra MP. Dynamics of total phenolic content in different apple tissues and genotypes: impacts and relevance for breeding programs. Sci Hortic 2014; 168: 58–63. doi.org: 10.1016/j.scienta.2014.01.020.
- Shashank Kumar, Abhay K Pandey. Chemistry and Biological Activities of Flavonoids: An Overview. The Sci World Journal 2013; 1-16. doi.org:10.1155/2013/162750.
- Mary Shobha Rani Inala, C D Dayanand, Nagarjuna Sivaraj, PM Beena, A V M Kutty. Antibacterial activity of Flavonoids Extracted from seeds of Pogamia pinnata Linn on Methicillin Resistant Staphylococcus aureus. British Microbiol Res J 2015; 10(1): 1-8. doi.org:10.9734/BMRJ/2015/20002.
- Geoghegan F, Wong R, Rabie A. Inhibitory effect of quercetin on periodontal pathogens in vitro. Phytother Res 2010; 24(6): 817–820. doi.org: 10.1002/ptr.3014.
- Šmejkal K, Chudík S, Kloucek P, Marek R, Cvacka J, Urbanová M, Holubová, P. Antibacterial C‐geranyl flavonoids from Paulownia tomentosafruits. J Natural Prod 2008; 71(4): 706–709. doi.org:10.1021/np070446u CCC: $40.75.
- Eumkeb G, Chukrathok S. Synergistic activity and mechanism of action of ceftazidime and apigenin combination against ceftazidime-resistant Enterobacter cloacae. Phytomedicine 2013; 15: 20(3-4): 262-9. doi.org:10.1016/j.phymed.2012.10.008. Epub 2012 Dec 3. PubMed PMID: 23218402.
- Kao TT, Tu HC, Chang WN, Chen BH, Shi YY, Chang TC, Fu TF. Grape seed extract inhibits the growth and pathogenicity of Staphylococcus aureus by interfering with dihydrofolate reductase activity and folate-mediated one-carbon metabolism. Int J Food Microbiol 2010; 30:141(1-2):17-27. doi.org:10.1016/j.ijfoodmicro.2010.04.025. Epub 2010 May 11. PubMed PMID: 20483185.
- Si W, Gong J, Tsao R, Kalab M, Yang R, Yin Y. Bioassay-guided purification and identification of antimicrobial components in Chinese green tea extract. J Chromatogr 2006; 1125 (2):204-10. doi.org: 10.1016/j.chroma.2006.05.061. PubMed PMID: 16797571
- Henrichsen J. Bacterial surface translocation: a survey and a classification. Bacteriol Rev 1972; 36(4):478-503. PubMed PMID: 4631369; PubMed Central PMCID: PMC408329.
- O’Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 1998; 30(2):295-304. PubMed PMID: 9791175.
- Pollitt E J G, Crusz AS, Diggle PS. Staphylococcus aureus forms spreading dendrites that have characteristics of active motility. Sci Rep 2015; 5: 17698 doi.org: 10.1038/srep17698
- Acker VH, Dijick PV, Coenye T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol 2014; 22: 326-333. doi.org:10.1016/j.tim.2014.02.001.
- Manner S, Skogman M, Goeres D, Vuorela P, Fallarero A. Systematic exploration of natural and synthetic flavonoids for the inhibition of Staphylococcus aureus biofilms. Int J Mol Sci 2013; 25:14(10):19434-51. doi.org: 10.3390/ijms141019434. PMID: 24071942; PMCID: PMC3821565.
- Singh S, Singh S K, Chowdhury I, Singh R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. The open Microbiol J 2017; 11: 53–62. doi.org:10.2174/1874285801711010053.
- Maria Esperanza Cortés, Jessika Consuegra Bonilla, Ruben Dario Sinisterra. Biofilm formation, control and novel strategies for eradication, Science against microbial pathogens: communicating current research and technological advances A. Méndez-Vilas (Ed.)
- Cho HS, Lee JH, Cho MH, Lee J. Red wines and flavonoids diminish Staphylococcus aureus virulence with anti-biofilm and anti-hemolytic activities. Biofouling 2015; 31(1):1-11. doi.org: 10.1080/08927014.2014.991319. PubMed PMID: 25535776.
- Bajpai VK, Sharma A, Baek KH. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 2013; 32: 582. doi.org: 10.1016/j.foodcont.2013.01.032
- Vani KP, Lakshmi OB. In vitro antibacterial efficacy of common spices and their effect on permeability of membrane. Int J Pharm Bio Sci 2014; 5(4):1203-11.
- Tang H, Chen W, Dou Z M, Chen R, Hu Y, Chen W Chen H. Antimicrobial effect of black pepper petroleum ether extract for the morphology of Listeria monocytogenes and Salmonella typhimurium. J food sci technol 2017; 54(7): 2067–2076. doi.org:10.1007/s13197-017-2644-2.
- Jones G A, McAllister T A, Muir AD, Cheng K J. Effects of Sainfoin (Onobrychis viciifolia Scop.) Condensed Tannins on Growth and Proteolysis by Four Strains of Ruminal Bacteria. Appl environment microbial 1994; 60(4):1374–1378.
- Mori A, Nishino C, Enoki N, Tawata S. Antibacterial activity and mode of action of plant flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochemistry 1987; 26 (8): 2231-4. doi.org:10.1016/S0031-9422 (00)84689-0
- Kwasi A. Ohemeng, Brent L Podlogar, Van N Nguyen, Jeffrey I. Bernstein, Heather M. Krause et al. DNA Gyrase Inhibitory and Antimicrobial Activities of Some Diphenic Acid Mono hydroxamides. J Medicinal Chem 1997;40(20):3292-3296.doi.org: 10.1021/jm9701583.







