Manuscript accepted on :09-06-2020
Published online on: 28-06-2020
Plagiarism Check: Yes
Reviewed by: Marcos Palone
Second Review by: M. Suresh
Final Approval by: Dr Ayush Dogra
Karishma Ghosh, Shubham Tyagi and Amit Gupta*
Department of Biotechnology, Graphic Era deemed to be University, Dehradun, India
Corresponding Author E-mail : dr.amitgupta.bt@geu.ac.in
DOI : https://dx.doi.org/10.13005/bpj/1957
Abstract
Introduction and objective- Flavonoids are considered as one of the most diverse groups of phytonutrients and are reported in all kinds of fruits and vegetables. In phytonutrients, more than 6,000 types of flavonoid components have been reported. In view of this, our main objective is to extract the flavonoids from leaves of these medicinal plants i.e. Mangifera indica and Syzygium cumini, determine their properties analytically (HPLC, FTIR) and also examine their antioxidant (DPPH assay) and antimicrobial potential against gram positive and gram negative bacteria. Materials and methods- In this study, qualitative analyses of these two medicinal plants were performed as per standard procedure. In addition, extraction of flavonoids from leaves was performed using methanol and ethyl acetate as a solvent system. Studies were also conducted for total flavonoid content, antioxidant activity and antimicrobial activity using aluminium chloride colorimetric assay, DPPH (1, 1-diphenyl-2-picrylhydrazyl) free radical scavenging assay and agar well diffusion assay respectively. Results- The results showed that the phenolic content of a medicinal plant often correlates with its antioxidant activity and this was confirmed through UV-Visible spectrophotometry and FTIR which were used in order to quantify the presence of functional groups. In contrast, these flavonoids showed antimicrobial and anti-inflammatory activity at higher doses as compared to control. Conclusion- Overall, flavonoid from these two medicinal plants especially leaves shows antioxidant activity, antimicrobial and anti-inflammatory activity at higher doses. In short, these flavonoids should provide beneficial effects related to human health care.
Keywords
Antimicrobial; Antioxidant; Anti-Inflammatory; Mangifera Indica; Syzygium Cumini
Download this article as:| Copy the following to cite this article: Ghosh K, Tyagi Shubham, Gupta A. Analytical and Immunopharmacological Studies of Flavonoids: Mangifera Indica and Syzygium Cumini. Biomed Pharmacol J 2020;13(2). |
| Copy the following to cite this URL: Ghosh K, Tyagi Shubham, Gupta A. Analytical and Immunopharmacological Studies of Flavonoids: Mangifera Indica and Syzygium Cumini. Biomed Pharmacol J 2020;13(2). Available from: https://bit.ly/385l8h5 |
Introduction
Since time immemorial medicinal plants have been used for treatment of various diseases and are still used in modern medicines, nutraceutical and pharmaceutical industry.1, 2 Presence of secondary metabolites (such as flavonoids, alkaloids, tannins, terpenoids etc.) reported in medicinal plant products and some of the attributes of these medicinal plant products are known for their health benefits.3, 4 These broad spectrums of health benefits include anti-hypertension, anti-inflammatory, antioxidant, antimicrobial, anticarcinogenic, hepatoprotective activities and so on.1-4 The presence of various primary and secondary metabolites produced in plants and their potential use in the healthcare industry have been studied intensively.5, 6 Recently there has been an upsurge in the study of therapeutic benefits of medicinal plants with phenolic compounds. Such metabolites of medicinal plants with phenyl structure show active UV absorption and therefore easy to detect and isolate.6 One of the secondary metabolites i.e. flavonoids is one of the most diverse groups of naturally occurring phytonutrients with over 6,000 types containing varied polyphenolic compounds has been reported till date. They are found in the nucleus of mesophyll cells of all plant plants, especially in photosynthetic plants. Studies have shown apple and onion to have abundant content of flavonoid which correlates with their high potential for scavenging of free radical, ROS and chelating of metal ions.7-10
Syzygium cumini and Mangifera indica are traditional Indian fruits belonging to the family Anacardiaceae 11, 12 and Myrtaceae13-15 respectively. Both species are geographically populated in Southeast Asia along with Germany and Brazil. For many centuries various parts of these trees have been associated with medicinal properties11-15 Syzygium cumini also known as the Indian blackberry or Black plums11, 12 known to have high concentration of natural phenols, flavonols and anthocyanin, especially when extracted from freeze-dried samples. Furthermore these compounds have played a major role in the use of Syzygium cumini for treatment of type 2 diabetes. Furthermore both have been traditionally used as antibacterial, antifungal, antiulcerogenic, gastroprotective and so on. However there is a need to further investigate the pharmacological activity of these phytochemical compounds against various diseases. In order to achieve this objective pertaining to investigate its phytochemical compound especially flavonoids from these medicinal plants and determined its antimicrobial and antioxidant potential.
Material and Methods
Chemical and Reagents
All the chemical and reagent used were of analytical grade available in the laboratory of the Department of Biotechnology, Graphic Era deemed to be University and obtained from reliable firms and institutes.
Procurement of Plant Materials
The leaves of Mangifera indica and Syzygium cumini medicinal plants were collected from the campus of Graphic Era deemed to be University, Dehradun, India (Latitude N–30.2687; Longitude E–77.9947) in the month of January, 2020. These medicinal plants were authenticated from FRI, Dehradun.
Extraction
Take 10 g of plant tissue (leaves of M. indica and S. cumini) and macerated them separately in liquid nitrogen (-196 °C) to prepare fine powder. The powder was then immersed in 80% methanol and boiled at 100°C for 2 hours. The extract was collected as a filtrate using whatman filter and further extracted using 20 ml ethyl acetate along with 40 ml distilled water. The extract was shaken regularly for 5 minutes and then incubated for 24 hours at room temperature. After incubation two layers were observed, upper layer (i.e., ethyl acetate) and lower layer (i.e., flavonoid). It was further heated to evaporate the ethyl acetate layer and concentrate the plant extract (flavonoid) that settled at the bottom.8, 9
Phytochemical Screening
2.4.1 Test for flavonoid: Small quantities of sample (1ml) of Mangifera indica and Syzygium cumini were taken in two separate test tubes. To these tubes a few drops of diluted NaOH were added. The appearance of intense yellow colour indicates the presence of flavonoids which disappears when dilute HCl was added.
2.4.2 Test for alkaloid: Stock solution (1 ml) and diluted HCl (6 ml) were added to a test tube and then boiled gently. The solution was cooled and filtrate was collected then 2 ml of Wagner’s reagent was added. The presence of reddish brown precipitate indicates the presence of alkaloids.
2.4.3 Test for terpenoid: The stock solution of 1ml of each solvent were taken in two separate test tubes and labelled. 0.5 ml chloroform along with a few drops of concentrated sulphuric acid was added to each test tube. Presence of reddish brown precipitate indicates the presence of terpenoids.
HPLC and FTIR Method for Estimation of Flavonoid
The HPLC system consisted of a pump coupled to a variable UV absorbance detector (Model.1220 infinity LC, Aglient technologies, Mumbai) operating at 285 nm. The chromatogram was recorded using an electronic integrator (Integrated diode array detector with spectra analysis). Chromatographic separation was carried out using a C18 reversed-phase (RP) analytical HPLC column (Agilent ZORBAX Eclipse plus C18 4.6 × 150 mm, 1.8 μm (PN 959994-902), 245 bar). The mobile phase consisted of methanol–0.5% phosphoric acid in water (50:50, v/v). The flow rate was held constant at 0.9 ml/min.
The FT-IR spectra of flavonoid extracted from leaves of these two medicinal plants were recorded in Perkin Elmer Spectrum Version 10.03.09 using KBr pellet method.
Estimation of Total Flavonoid Content
10 mg of quercetin was dissolved in 10 ml methanol to prepare a stock solution of 1mg of quercetin per ml. The stock solution of 1mg/ml was then diluted to different concentrations (0.1 mg/ml, 0.01 mg/ml and 0.001mg/ml) of quercetin using methanol. Different concentrations of quercetin were used as standard to prepare a calibration curve for the estimation of total flavonoid content in Mangifera indica and Syzygium cumini. 0.5 ml of each plant extract and dilutions of standard quercetin were taken in different test tubes respectively. To each test tube 1.5 ml methanol, 0.1 ml potassium acetate, 0.1ml of 10% aluminium chloride and 2.8ml of distilled water were added. Corresponding blanks were prepared for each extract and standard quercetin dilution by replacing aluminium chloride with distilled water. The test tubes were incubated for 30 min and absorbance was measured at 415nm. This method is referred to as the Aluminium chloride colorimetric assay and is used for quantification of total flavonoid content (mg/ml) 16
Analysis of Antioxidant Activity of Flavonoid
The DPPH free radical scavenging assay was performed to calculate the percentage antioxidant activity of each plant extract. 0.5 ml of each extract was taken in test tubes separately. To these test tubes 0.3ml of DPPH (0.5mM in ethanol) and 3 ml of absolute ethanol were added. Respective blanks were prepared by replacing DPPH with ethanol. Control was prepared for the DPPH assay by adding 3.3 ml ethanol and 0.3 ml DPPH to a test tube. These respective test tubes were all incubated for 100 minutes at room temperature. After incubation absorbance was measured at 517 nm against corresponding blank.17
Antimicrobial Assay
To evaluate the potential antimicrobial activity of these two medicinal plants especially leaves, three bacterial strains were selected namely Bacillus subtilis, Pseudomonas aeruginosa and Salmonella enteritidis. The Nutrient agar medium (NAM) was used to culture microbes required for antimicrobial susceptibility test. Microbial strains were cultured on to the agar plate and the broth, followed by overnight incubation at 37̊ C for 72 hrs. The antimicrobial activity of the extracts were determined using MHA (Mueller Hinton Agar), developed in 1941 by Mueller and Hinton, which has been widely used for antimicrobial susceptibility tests because of its non- selective , non – differential properties and high starch content. MHA is a loose agar and usually appears as translucent and light amber in colour.
The antimicrobial activity of two different species was tested using the disc diffusion method. 100 µl of microbial culture of the respective strains were taken and spread with the help of an L- shaped spreader on MHA plate (20 ml/ plate). The sterile discs (6 mm in diameter) containing residues of flavonoids were independently impregnated on the agar plates which have previously been inoculated with the selected microbial strain. Erythromycin was used as a positive control for bacteria. In this study, discs without samples, were considered as negative control. The plates were then incubated at 37̊ C for 24 hours and at 30̊ C for 48 h. Antimicrobial activity was then determined by measuring the diameter of the growth- inhibition zone in millimetres.18
Anti-Inflammatory Activity
This activity is done in whole human whole blood samples and consent a letter was obtained from a healthy volunteer. In this study, human whole blood was mixed with equal volume of sterilized phosphate buffered solution. This solution was centrifuged (4000 rpm; 10 min) and the pelleted cells (containing RBCs) were collected. These pelleted cells were used for determining its anti-inflammatory activity using different concentrations of flavonoids (62.5-500 µg/ml) from Mangifera indica and Syzygium cumini. All these samples were incubated (37 °C, 30 minutes) and centrifuged at 3000 rpm for 10 min. Finally, supernatant was decanted and its haemoglobin content was estimated through spectrophotometer at 570 nm.19
Statistical Analysis
The results for these studies related to flavonoids (using variable concentration) against control and data expressed as means ± standard error (SE) of triplicate. The data were analysed using one-way analysis of variance (ANOVA; Bonferroni multiple comparison test; *P < 0.05; **P < 0.01; ***P < 0.001).
Result
Qualitative Analysis
Qualitative analysis of primary and secondary metabolites was done and these studies revealed the presence of protein, flavonoids, alkaloids, and terpenoids.
HPLC and FTIR Analysis of Flavonoids
The results of these studies related to flavonoids was obtained by performing HPLC and FT-IR for analysing the content as shown in Fig.1. These studies indicate that flavonoids showed several peaks (using HPLC) and its confirmation through presence of functional groups (using FT-IR).
Estimation of Phenolic Content and Antioxidant Activity
The total phenolic content of this medicinal plant product especially leaves, were calculated from the calibration curve using quercetin as standard. The total phenolic content was seen more in case of Syzygium cumini as compared to Mangifera indica. These phenolic compounds showed redox properties and antioxidant properties. For confirmation of its antioxidant activity, DPPH radical assay was used which is widely accepted. The results showed that DPPH scavenging activity in case of Syzygium cumini was 70.4 % at a concentration of 500 μg/ml flavonoid content, while that of Mangifera indica, was 64%.
Antimicrobial Activity
The antimicrobial activity was determined by measuring the diameter of the zone of inhibition recorded (Table 1). The results obtained in the evaluation of the antimicrobial activity of flavonoids against. Bacillus subtilis, Salmonella enteritidis and Pseudomonas aeruginosa (Fig.2) showed higher inhibition rate in case of flavonoid at higher concentration as compared to control. Overall, these flavonoids showed its antimicrobial activity against these bacterial strains.
Anti-inflammatory Activity
The results of anti-inflammatory activity related to flavonoids as shown in Fig.3. The results showed that flavonoids at higher concentration showed less haemolytic activity as compared to control. Overall, these flavonoids significant stabilization towards human RBC membranes. In this study, distilled water was used as standard and showed higher haemolytic activity.
Table 1: Antibacterial activity of the different concentrations of flavonoids from Mangifera indica and Syzygium cumini
| Test organisms | Inhibition zone (mm), Mangifera indica | Inhibition zone (mm), Syzygium cumini | ||||||||||
| Control | 5% | 10% | 25% | 50% | 100% | Control | 5% | 10% | 25% | 50% | 100% | |
| Bacillus subtilis | NA | NA | 10 | NA | NA | NA | NA | 9 | 10 | 10 | 9 | 13 |
| Salmonella enteritidis | NA | NA | NA | NA | 8 | 8 | NA | NA | NA | 10 | 10 | 12 |
| Pseudomonas aeruginosa | NA | NA | NA | 8 | 9 | 12 | NA | NA | 10 | 12 | 12 | 14 |
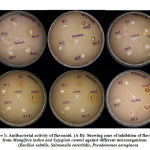 |
Figure 1: Antibacterial activity of flavonoid. (A-B) |
 |
Figure 2: HPLC and FTIR analysis of flavonoids from Syzygium cumini and Mangifera indica. |
HPLC results revealed the presence of a good percentage of flavonoid content and these were detected at a wavelength of 270 nm due to sharpness of the peaks and proper baseline and recorded its retention time (Rt min), percent area and heights. In addition, Flavonoids were treated for FTIR spectroscopy (Perkin Elmer, Spectrum Version 10.03.09 using KBr pellet method). The samples were run at infrared region between 1000 nm and 4000 nm. There was a variation in the peaks in both the flavonoid samples. The peaks showed that both the plant samples have the compounds like flavonoids
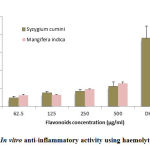 |
Figure 3: In vitro anti-inflammatory activity using haemolytic activity. |
These studies were conducted in flavonoids using different concentrations of flavonoids from Syzygium cumini and Mangifera indica in human whole blood. Values are expressed as Mean ± S.E.
Discussion
In literature, active constitutes in the form of primary and secondary metabolites which are reported in medicinal plants. Recently, medicinal plant products are commercialized and presently available in large scale pertaining to treat various human diseases or somehow partially discovered through ethnomedicinal uses. The major objective in terms of ethnobotanical or immunopharmacological based research in order to find active phytochemicals especially flavonoids on a broad scale production.19 In order to achieve this objective, we analysed the flavonoids content from the leaves of two medicinal plants by determining them analytically (using HPLC and FTIR) and also confirming their antimicrobial and anti-inflammatory activity. In literature, flavonoids are one of the groups of secondary metabolites with three ring structure (A, B and C) based on C6-C3-C6 basic conformation.19 Two benzene rings form structure A and B, linked together by a heterocyclic pyrone referred as C ring. This forms the basis of the 15 carbon skeleton structure of flavonoid. However some flavonoid may also have a C6-C1-C6 or C6-C4-C6 basic structure as observed in xanthone, homoisoflavones, rotenoids, etc. Furthermore the mode of connection between the two benzyl ring, level of oxidation of heterocyclic pyrone, degree of polymerisation and substitution of hydroxyl, methyl, methoxyl etc. on ring A and B give rise to various classes of flavonoid such as flavones, flavonols, flavonones, anthocyanidin, flavan and so on.20-22 Flavonoids are often extracted as their glycoside derivative with sugar added to 3 or 7 positions in case of O-glycoside and 6 or 8 positions in case of N-glycoside. However, aglycone derivatives of flavonoids are more common than glycoside or methyl derivatives. Since their discovery in 1936 in citrus plants, the pharmacological potential of flavonoid derived from various plants against virus, bacteria, carcinogenic hydrocarbon, free radical, cardiovascular disease etc. have been verified. Studies have presented a high possibility of determining a safe and strong flavonoid with high histamine decarboxylase inhibitory effect and that can be used as an anti-ulcer drug. Flavonoids such as rutin, quercetin and kaempferol have shown potential protective activity against gastric mucosal damage.23, 24 Similarly flavonoids with their enzyme inhibition activity have shown potential use as antiviral drug (i.e. Kaempferol, apigenin, galangin) and has been determined as strong candidate for antiviral drug against Herpes simplex virus (HSV).25 Furthermore flavonoid as an antiviral drug for influenza virus, parainfluenza virus, respiratory syncytial virus, auzesky virus etc. has also been studied.23-25 Additionally flavonoid possesses potential for prevention and treatment of oxidative stress induced cardiovascular diseases and hypertension. Among all flavonoid, quercetin is the most abundant and its prolonged administration has shown vasodilator effect resulting in reduced blood pressure and heart rate. Therefore quercetin and related bioflavonoids have been regarded to have antihypertensive effect, though active vasodilatation effect may not be the sole factor in reducing blood pressure. In view of these properties related to medicinal plant products, we focused on the total flavonoid component that is reported in these two medicinal plants and showed antioxidant activities. Further confirmation of these flavonoids is through the presence of bonds and its analysis revealed the presence of active compounds in these medicinal plants.20-22
Here anti-inflammatory activity in case of flavonoids was performed using human RBC which was selected for in vitro evaluation. This is based on the concept that erythrocyte membrane is analogous to the lysosomal membrane and its stabilization means that these flavonoids may stabilize lysosomal membranes. In short, stabilization of lysosomal membrane is crucial as well as important in case of inflammatory response because the extracellular release of lysosomal constituents of activated neutrophil containing bactericidal enzymes along with proteases, may be responsible, for causing inflammation and tissue damage. The result indicted that flavonoids at higher concentrations have significant anti-inflammatory properties.
In literature, antibiotics played a crucial role against various infectious diseases, which is mainly caused through pathogenic bacteria or fungi. For the last several years, antibiotic-resistant types of bacteria have been reported pertaining to the increase in its use.26 Drug resistance is mainly applied and executed through various multiple mechanisms. The major reasons for the emerging antibiotic resistance which includes unfit or too common use of antibiotics in fields, such as medicine, veterinary and agriculture. In this regard, a pipeline of new antimicrobial agents have been reported since the 1970s, while the number of drug-resistant bacteria has increased leading some to claim that a post-antibiotic era is imminent. Hence, there is a pressing need for exploring new types of antimicrobial drugs. In this regard, we focused on flavonoid content using methanol as a solvent system and showed antimicrobial activity against various bacterial strains. Further immunopharmacological investigations are required pertaining to show its derivatives of flavonoid that may show its antimicrobial activity.
Conclusion
From these studies, it was investigated that flavonoids from the leaves of these medicinal plants (Syzygium cumini and Mangifera indica) showed antimicrobial activity against bacterial strains. Further extraction of active molecules from these flavonoids should be helpful for elucidation of active molecules. Further immunological investigations are required as well as needed and more importantly to evaluate its various biological activities. Moreover, other parts of these plants are yet to be studied pertaining to evaluate the studied plant extracts as a potential antimicrobial agent.
Reference
- Firenzuoli, F., Gori, L., Herbal medicine today: clinical and research issues. Evid Based Complement Alternat Med. 2007, 4(1), 37-40.
- Rafieian-Kopaei, M., Medicinal plants and the human needs. J HerbMed Pharmacol. 2012, 1(1), 1-2.
- Schippmann, U.W., Leaman, D., Cunningham, A.B., A comparison of cultivation and wild collection of medicinal and aromatic plants under sustainability aspects. Frontis. 2006, 17, 75-95.
- Lindberg, M.H., Bertelsen, G., Spices as antioxidants. Trends Food Sci. Technol. 1995, 6(8), 271-7.
- Bansode, T., Gupta, A., Shinde, B., Salalkar, B.K., Partial purification and antidiabetic effect of bioactive compounds isolated from medicinal plants. Micro med. 2017, 5(1), 1-7.
- Eichholz, I., Huyskens-Keil, S., Keller, A., Ulrich, D., Kroh, L. W., Rohn, S.; UV-B-induced changes of volatile metabolites and phenolic compounds in blueberries (Vaccinium corymbosum). Food Chem. 2011, 126, 60–64.
- Gupta, A., Chavan, R., Immunosuppressive and cytotoxic potential of flavonoids from medicinal plants: Preliminary investigation for anticancer activity, Int. J. Immunotherapy Cancer res. 2017, 3(1), 7-11.
- Sharma, S.S., Gupta, A., Mane, V., Shinde, B., Immunopharmacological activity of flavonoids from Lemna minor (Duckweed) and determined its immunological activity. Current Life Sci. 2017, 3(2), 22-27.
- Gupta, A., Chaphalkar, S.R., Immunopharmacological activity of flavonoids isolated from Mesua ferrea, Ficus benghalensis and Jasminum auriculatum. Current Life Sci. 2016, 2(2), 49-54
- Gupta, A., Chaphalkar, S.R., Anti-inflammatory and immunosuppressive activities of flavonoids from medicinal plants. Herb Med Pharmacol. 2016, 5 (3), 121 -124.
- Bansode, T., Gupta, A., Salalkar, B.K., In silico and in vitro assessment on antidiabetic efficacy of secondary metabolites from Syzygium cumini (L.) Skeels. Plant Sci. Today 2016, 14(3), 1-10.
- Gupta, A., Chaphalkar, S.R., Flow cytometry based assay of formulation from Syzygium cumini in human whole blood and glycosylated red blood cells. J. Pharma res. 2014, 3 (12), 265 – 270.
- Agunbiade, S. O., Olanlokun, J. O., Studies on the nutritive value of the seed kernels of exotic and local mangoes (Mangifera indica L.). Biosci. Biotech. Res. Asia 2006, 3, 81–86.
- Ajila, C. M., Bhat, S. G. Prasada Rao, U. J. S., Valuable components of raw and ripe peels from two Indian mango varieties, Food Chem, 2007, 102, 1006–1011.
- Arogba, S.S., Physical, chemical and functional properties of Nigerian mango kernel and its processed flour. J. Sci. Food Agriculture, 1997, 73, 321–328.
- Chang, C., Yang, M., Wen, H., Chern. J., Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178-182.
- Villano, D., Fernandez-Pachon, M.S., Moya, M.L., Troncoso, A.M., Garcıa-Parrilla, M.C., Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta, 2007, 71, 230.
- Salvador, M.J., Ferreira, E.O., Pral, E.M.F., Alfieri, S.C., Albuquerque, S., Ito, I.Y. et al., Bioactivity of crude extracts and some constituents of Blutaparon portulacoides (Amaranthaceae). Phytomedicine, 2002, 9, 566-571.
- Pei, R., Liu, X., Bolling, B. Flavonoids and gut health. Curr Opin Biotechnol, 2020, 61,153-159.
- Yang Y, Zhao Y, Zuo Z, Zhang J, Shi Y, Wang Y. Investigation of a Medical Plant for Hepatic Diseases with Secoiridoids Using HPLC and FT-IR Spectroscopy for a Case of Gentiana rigescens. Molecules, 2020, 25(5), 9.
- Zahoor M, Shafiq S, Ullah H, Sadiq A, Ullah F. Isolation of quercetin and mandelic acid from Aesculus indica fruit and their biological activities. BMC Biochemistry, 2018, 19, 5.
- Dumont S, Rivoal J. Consequences of oxidative stress on plant glycolytic and respiratory metabolism. Front Plant Sci, 2019, 10, 166.
- Kumar, S., Sharma, U.K., Sharma, A.K., Pandey, A.K., Protective efficacy of Solanum xanthocarpum root extracts against free radical damage: phytochemical analysis and antioxidant effect, Cellular Bio., 2012, 58 (1), 171–178.
- Hollman, P.C.H., Absorption, bioavailability and metabolism of flavonoids, 2004, Pharmaceutical Biol, 2004, 42, 74–83.
- Cushnie, T.P.T, Lamb, A.J., Antimicrobial activity of flavonoids, Int. J Antimicrobial Agents, 2005, 26 (5), 343–356.
- Haraguchi, H., Tanimoto, K., Tamura, Y., Mizutani, K., Kinoshita, T., Mode of antibacterial action of retrochalcones from Glycyrrhiza inflata, Phytochemistry, 1998, 48 (1), 125–129.







