Manuscript accepted on :25-Mar-2020
Published online on: 03-04-2020
Plagiarism Check: Yes
Divakar M S1 , Raju Krishna Chalannavar2
, Raju Krishna Chalannavar2 * Shivakumar Hugar3
* Shivakumar Hugar3 and Karunakar Hegde4
and Karunakar Hegde4
1Biotechnology Unit, Department of Biosciences, Mangalore University, Mangalagangothri, Karnataka, India-574 199
2Department of Applied Botany, Mangalore University, Mangalgangothri, Karnataka, India-574 199
3Department of Pharmacology, BLDEA’s SSM College of Pharmacy and Research Center, Vijayapur, Karnataka, India-586103
4Department of Pharmacology, Srinivas College of Pharmacy, Valachill, Post- Farangepete, Mangalore, Karnataka, India-574143
Corresponding Author E-mail :drrajkc@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1911
Abstract
This study was designed to evaluate the oral acute toxicity of the ethyl acetate extract of Syzygium kanarense leaves. Medicinal plants play an important role in ethnomedicine for the treatment of multiple diseases. Syzygium is a common fruit tree with different species under Myrtaceae family; Syzygium cumini and Syzygium calophyllifolium are known medicinal plant for the treatment of diabetes mellitus and various diseases. Syzygium kanarense one of the unexplored plants tested for toxicity for the purpose of therapeutic value. The ethyl acetate extract of Syzygium kanarense (Talbot) Raizada (Family: Myrtaceae) leaves was investigated for the study of cytotoxicity and oral acute toxicity in female Wistar albino rats according to OECD guideline 425. The preliminary phytochemical screening was carried out and confirmed the cytotoxicity by MTT assay using NIH/3T3 mouse embryonic fibroblast cell line with 5 concentrations (100 to 500µg/ml) of the extract. For oral acute toxicity study female rats were divided into two equal groups. One group served as vehicle control (VC) while the other group received an orally single dose at the 2g/kg body weight (B.W) of Syzygium kanarense leaves extract from ethyl acetate (SKLEE). Then both groups were observed carefully for 14 days. After blood samples were collected carefully by cardiac puncture, under anesthesia and were subjected to haematological and biochemical analyses. The vital organs of rats were preserved in 10% formalin for histopathological examination. The extract contains phenolics, terpenoids, tannins, saponins steroids, volatile oil, glycosides, carbohydrates, and proteins. The result of cytotoxicity on NIH/3T3 cell line with IC50=425.07µg/ml and the percentage of viable cells was calculated. In the acute toxicity study found no mortality, no significant changes in B.W, blood parameters and also with the study of histopathology. The data revealed that LD50 of the leaves extract was more than 2g/kg B.W. The study concluded that the extract presence of phytochemicals is useful for traditional medicine for various diseases because they were non-toxic when administered orally at the dose of 2g/kg B.W to rats. To the best of our knowledge, this is the first report of the extract from Syzygium kanarense leaves.
Keywords
Raizada Leaves; Syzygium kanarense; Wistar Rats
Download this article as:| Copy the following to cite this article: Divakar M S, Chalannavar R. K, Hugar S, Hegde K. Toxicological Evaluation of Ethyl Acetate Extract of Syzygium Kanarense (Talbot) Raizada Leaves in Wistar Rats. Biomed Pharmacol J 2020;13(1). |
| Copy the following to cite this URL: Divakar M S, Chalannavar R. K, Hugar S, Hegde K. Toxicological Evaluation of Ethyl Acetate Extract of Syzygium Kanarense (Talbot) Raizada Leaves in Wistar Rats. Biomed Pharmacol J 2020;13(1). Available from: https://bit.ly/2R7yrGC |
Introduction
Medicinal plants have been used as therapeutic agents that take a holistic approach to treat various diseases. The immense variety of plants have played indispensable roles in the life of human beings and animals by the source of foods (fresh fruits, vegetables, grain, seeds and nuts), medicine (health care), aesthetic values and ecological monitors. Plant-derived products are present in 14 of the 15 therapeutic categories of pharmaceutical preparation that are currently recommended by medical practitioners and they are an important part of the health care the system in the Western world1 because drugs obtained from plants are frequently considered as less toxic and free from side effects than synthetic ones2. The usage of natural products have considerably improved and this increases order with the fact that the population believes that natural products do not present any toxic effects3, 4. Based on the World Health Organization (WHO), 75-80% of the world’s populations primarily depend on medicine obtained from plant materials and products5. Many benefits of using plant products for health-related issues are plenty because of the rich source plant-based like fibre, vitamin, carbohydrates, protein, calcium, magnesium, zinc, omega-3 and omega-6 fatty acids are amazing health benefits like keep body full energy to carry out day to day activities and against many diseases. Vital organs are most important in the body to play role in the regulation of physiological processes, so the organ damage is recovered, the risk of death can be reduced to a significant extent and frequency of a health problem can be minimized effectively from the plant products. Many unexplored plants are present in Western Ghats of India.
The rare plant Syzygium (S.) kanarense described by Talbot and reported in 1897 from the green forests of the Gerusoppa ghat in North Kanara district of Karnataka, India. In 1986 Ahmedullah and Nayar reported this species to be endemic in Shimoga and North Kanara districts of Karnataka, India. In 1996 S. kanarense was considered as critically endangered species and last seen by Talbot discounting the collection of R. Sundararaghavan. After a long gap of 47 years, this species rediscovered from the evergreen forests and it belongs to Myrtaceae family6. Total of 52 constituents was reported from the essential oil of S. kanarense7and no data available in leaves extract of S. kanarense7. The phytochemical of alkaloids affect the different metabolic system in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes, inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the nervous system. Several alkaloids may affect multiple functions8. To study the toxicity of plant extracts and compounds, the cytotoxicity test is one of the biological evaluations and screening tests that use tissue cells in vitro to observe toxicity by cell growth or not9. The aim of the present study is to accessed deals the analysis of phytochemicals, cytotoxicity, oral acute toxicity, hematological and biochemical parameters, histopathological in vital organs from SKLEE.
Materials and Methods
Chemicals
Biochemical kits such as urea, creatinine, alkaline phosphatase, S.G.O.T (A.S.T), S.G.P.T (A.L.T), total protein kits were purchased from Gappe Hills, Pattimattom (PO), Dist. Ernakulam, Kerala – 683 562, India.
Collection of Plant Material
The fresh leaves of S. kanarense were collected from Pilikula Nisargadhama, Mudushedde (N 12.9299, E 74.8958), Mangalore, Dakshina Kannada district, Karnataka state, India (voucher specimen No. PND-800, flower: 2385 and plant fruiting: 2520).
Preparation of Crude Extract
About 2 kg quantity of leaf material was thoroughly washed with clean water and spread on clean paper. Removed foreign particles with the help of cutter and then kept at room temperature for air drying for 20 days. The dried leaves were finely powdered mechanically and 150gm powder extracted first with hexane (500 ml) to remove overall lipid content, then the residue was extracted with ethyl acetate (500ml) using soxhlet apparatus and then filtered by Whatman no.1 filter paper. The excess solvent was removed by a rotary evaporator at 50°C. Then dried crude extract 18.6 gm (12.4% yield) was stored in the refrigerator (below 5°C) prior to use.
Phytochemicals Screening
The phytochemicals screening of SKLEE was carried out to reveal the presence or absence of chemical constituents using standard procedures10,11,12,13,14.
Alkaloids Test
Mayer’s Test
About 2 ml of plant extract, 2 drops of Mayer’s reagent were added along the sides of the test tube. The appearance of white creamy precipitate indicated the presence of alkaloids11.
Wagner’s Test
A few drops of Wagner’s reagent were added to the plant extract along the sides of the test tube. A reddish-brown coloured precipitate indicated the presence of alkaloids11.
Hagner’s Test
The filtrate was treated with a few drops of Hagner’s reagent. The Formation of yellow precipitate indicated the presence of alkaloids14.
Test for Phenolic Compounds
Ferric Chloride Test
The extract (50 mg) is dissolved in 5 ml of distilled water. To this, a few drops of neutral 5% ferric chloride solution was added. Dark green colour indicates the presence of phenolic compound11.
Gelatin Test
The extract (50 mg) is dissolved in 5 ml of distilled water and 2 ml of 1% solution of Gelatin containing 10% NaCl is added to it. White precipitate indicates the presence of phenolic compounds11.
Lead Acetate Test
The extract (50 mg) is dissolved in of 5ml distilled water and to this 3 ml of 10% lead acetate solution was added. A bulky white precipitate indicates the presence of phenolic compounds11.
Test for Flavonoids
Alkaline Reagent Test
Extract was treated with a few drops of sodium hydroxide solution. Formation of intense yellow colour, which becomes colourless on the addition of dilute acid, indicates the presence of flavonoids14.
Lead Acetate Test
Extract was treated with a few drops of lead acetate solution. Formation of a yellow colour precipitate indicates the presence of flavonoids13.
Test for Terpenoids
2mg crude extract was shaken with chloroform (2ml) followed by the addition of concentrated H2SO4 (2 ml) along the side of the test tube, the reddish-brown colouration of the interface indicates the presence of terpenoid12.
Test for Tannins
The 50 mg extract was stirred with distilled water (10 ml) and then filtered. A few drops of 5% ferric chloride were then added. Black or blue-green colouration or precipitate was taken as a positive result for the presence of tannins12.
Test for Saponins
The extract (50 mg) is diluted with distilled water and made up to 20 ml. The suspension is shaken in a graduated cylinder for 15 minutes. A 2 cm layer of foam indicates the presence of saponins14.
Test for Steroids
About 100 mg of dried extract of S. kanarense was dissolved in 2ml of chloroform. H2SO4 was carefully added to form a lower layer. The reddish-brown colour at the interface was indicative of the presence of the steroidal ring14.
Test for Glycosides
The extract (50 mg) was hydrolysed with concentrated hydrochloric acid for 2 hours on a water bath, filtered and the hydrolysate was subjected to the following tests11.
Borntrager’s Test
To 2 ml of filtered hydrolysate, 3 ml of chloroform was added and shaken, the chloroform layer was separated and 10% ammonia solution was added to it. A pink colour indicates the presence of glycosides.
Legal Test
About 50 mg of extract was dissolved in pyridine sodium nitroprusside solution was added and made alkaline using 10% NaOH. Presence of glycoside is indicated by pink colour.
Test for Volatile Oil
For volatile oil estimation 50 mg of powdered material (crude drug) is taken and subjected to hydrodistillation. The distillate is collected in a graduated tube of the assembly, wherein the aqueous portion automatically separated out from the volatile oil11.
Test for Carbohydrates
Molish’s Test
To 2 ml of extract, two drops of alcoholic solution of α- naphthol was added. The mixture was shaken well and a few drops of concentrated sulphuric acid were added slowly along the sides of the test tube. A violet ring indicates the presence of carbohydrates11.
Benedict’s Test
To 0.5 ml of filtrate, 0.5 ml of Benedict’s reagent was added. The mixture was heated in a boiling water bath for 2 minutes. A characteristic coloured precipitate indicates the presence of sugar11.
Test for Proteins
The extract (100 mg) was dissolved in 10 ml of distilled water and filtered through Whatman No. 1 filter paper and the filtrate was subjected to test for proteins11.
Millon’s Test
To 2 ml of filtrate few drops of Millon‟s reagent was added. A white precipitate indicates the presence of proteins.
Biuret Test
Extract (2 ml) was filtrate is treated with 1 drop of 2% copper sulphate solution. To this 1 ml of ethanol (95%) was added, followed by addition of potassium hydroxide pellets. Pink colour ethanolic layer indicates the presence of protein.
Cytotoxicity Assay
Cell culture from the normal mouse embryonic fibroblast cells (NIH/3T3) was used for cytotoxicity screening of the SKLEE by MTT assay. NIH/3T3 the cell line was obtained from National Centre for Cell Sciences (NCCS), Pune, India.
MTT Assay
To evaluate the cell viability MTT assay is a standard method for cytotoxicity15, 16. The cell line of NIH/3T3 was seeded in 96-well plates at 200μl density of suspension contained approximately 10,000 cells. Following 24-hrs incubation and attachment, the cell line was treated with different concentrations of extract from 100µg/ml to 500µg/ml of extract. The 96 well plates were incubated at 37ºC with 5% CO2 for 24 hrs. Then for the further incubation period, the media (3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide or MTT) was aspirated with carefully without disturbing the crystals of MTT at the wells bottom and 100µl of solubilisation solution of DMSO was added and then well plate was shaken gently in a shaker to solubilise the formazan. The microplate reader was used for the absorbance at the wavelength of 570nm was noted using Spectrophotometer17. The following formula was used to calculate the % viability.

Approval from Animal Ethics Committee
The experiments were conducted in accordance with the guidelines cleared by the Animal Ethics Committee in Shri Sanganabasava Mahaswamiji College of Pharmacy and Research Centre, Vijayapur, Karnataka State, India (Registration number: BLDE/BPC/2019-2020/6).
Evaluation of Acute Toxicity Assay
Accordance to OECD guidelines-425 (up and down procedure) dosing at 2g/kg, B.W experiment was performed on non-pregnant female Wistar albino rats weighing 150-250gm were selected18. They were procured from Mohan Bhat, Laboratory Suppliers, B.E.M. School, Cross Road, Mangalore. The animals were kept under standard laboratory conditions maintained (12hr dark: 12hr light) at room temperature of 22-25ºC. The rats were freely supplied with pellet diet and continuous supply of drinking water. Before dosing, animals were kept under the standard conditions for 5 days. The extract was homogeneously mixed with 1% Carboxymethyl cellulose (CMC) gel and administrated orally using gavage needle at a single dose of 2g/kg B.W of rats (n=5), for VC group CMC gel administered the same volume as that of the treated group received. The acute toxicity was monitored with the extract treated rats and observed closely during the first 30 minutes to 4 hours. The behaviour of the animal was observed systematically for survival or death. The observation was made twice a day for up to 14 days. The clinical observations were made daily for the study of physical condition, food, water intake, skin, fur, eyes, mucous membrane, salivation, sleep, coma, diarrhoea, convulsion and any changes in the level of activity in both group of rats. At the end study of oral acute toxicity, the rats were weighed and blood samples were collected by puncture of cardiac under anesthesia using ketamine (50 mg/kg) and xylazine (5 mg/kg) intraperitoneal injection respectively then vital organs were excised and preserved in 10% formalin for the study of histopathological study in both groups.
Collection of Blood Samples
5 ml of blood samples was collected by puncturing the cardiac with syringe needles into labelled heparinised (ethylene diamine tetra acetate [EDTA]) tube for haematological evaluation and blood in the non-heparinised tube (without EDTA) allowed to coagulate, then centrifuged and the sera were separated for the study of biochemical analyses.
Hematological Analysis
The blood indices like haemoglobin (Hb), red blood cells (RBC), white blood cells (WBC), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), neutrophils, lymphocytes, monocytes, eosinophils, packed cell volume (PCV) were evaluated by automated hematology analyzer (Yumizen H500 – Horiba).
Biochemical Analysis
The blood sample was collected from animals to clean dry tubes and centrifuged at the speed of 3000 rpm at 25ºC for 10 minutes. To obtain serum was stored at ˗20ºC. Serum analyzed for the determination of serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), alkaline phosphatase (ALP), total bilirubin, total protein (TP), albumin, globulins, urea, creatinine, total cholesterol (TC), triglycerides (TG), high density lipoprotein (HDL), low density lipoprotein (LDL) and very low density lipoprotein (VLDL) was performed by using automated biochemistry analyzer (Mindray BS-200).
Histopathology Study
The vital organs were isolated from animal (liver, kidney and heart) and examined macroscopically. The morphology of internal organs was observed for the signs of toxicity. These organs were excised and fixed in 10% formalin bottles, then processed for histopathological study. The vital organs were embedded in paraffin wax; serial section (3-µm thickness) obtained by cutting the embedded tissue with a microtome and stained with haematoxylin and eosin (H & E). The slides were observed under Nikon phase contrast microscope (Y-TV55 ECLIPSE) and captured the magnified (X20 and X40) images of tissue structures for further study.
Statistical Analysis
Animal study of all data was expressed as mean ± SEM and one way analysis of variance in ANOVA followed by Dunnett T3 test for multiple comparisons. It was used to analyze the significance for the effect of SKLEE group with compared to a vehicle control group with the help of SPSS software, version 20; values are considered statistically significant when P < 0.05. All data points show the mean of the standard error of means (SEM).
Results
Phytochemical Screening
SKLEE appeared in the green colour and good smell. The phytochemical studied of extract contains phenolics, terpenoids, tannins, saponins, steroids, volatile oil, glycosides, carbohydrates and proteins (Table 1).
Table 1: Phytochemicals in SKLEE were presence of phenolics, terpenoids, tannins, saponins steroids, volatile oil, glycosides, carbohydrates and proteins
| Phytochemical tests | Extract (SKLEE) |
| Alkaloids | -ve |
| Phenolic compounds | +ve |
| Flavonoids | -ve |
| Terpenoids | +ve |
| Tannins | +ve |
| Saponins | +ve |
| Steroids | +ve |
| Volatile oil | +ve |
| Glycosides | +ve |
| Carbohydrates | +ve |
| Protein | +ve |
(+) = Present) (˗) = Absent
Cytotoxicity Study of SKLEE on NIH/3T3 Cell Line
To determine the cytotoxic effect of SKLEE by MTT assay on mouse fibroblast cell line (NIH/3T3) the viable cells were assessed in different concentration. The cell viability assay was used for cytotoxic effects on SKLEE and it was expressed as IC50 value (Table 2). Dose-dependent cell viability reduction after 24 hr was observed in extract treated on NIH/3T3 cell line. For cell growth, dose dependent exposure to SKLEE showed the viability of cell more than 50% decrease at the dosage of 500 µg/ml. Graphs were plotted using % of cell viability Y-axis and concentration of SKLEE X-axis (Figure 1). From the plotted graph the SKLEE concentration required for 50% of cell viability (IC50) was calculated as 425.07 µg/ml.
Table2: Study on the Cytotoxicity of SKLEE by using NIH/3T3 cell line.
| Concentration (µg/ml) | Mean ± standard error | % Cell Viability |
| 100 | 1.428 ± 0.015 | 94.04 ± 1.02 |
| 200 | 1.080 ± 0.092 | 71.09 ± 6.05 |
| 300 | 0.859 ± 0.057 | 56.55 ± 3.75 |
| 400 | 0.808 ± 0.040 | 53.19 ± 2.63 |
| 500 | 0.702 ± 0.063 | 46.21 ± 4.14 |
| Untreated | 1.519 ± 0.003 | 100 ± 0.19 |
| IC50 = 425.07 µg/ml R2 = 0.894 | ||
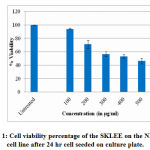 |
Figure 1: Cell viability percentage of the SKLEE on the NIH/3T3 cell line after 24 hr cell seeded on culture plate. |
Oral acute Toxicity Study
Behavioral and Body Weight
Acute toxicity studied in female Wistar rats treated orally with a single dose of 2 g/kg B.W. The B.W of SKLEE treated group compared to the vehicle control group showed no significant changes. The B.W tested in both VC and treated group with SKLEE (2 g/kg) were increased progressively and results were expressed in Table 4 and Figure 2. The behaviour of VC and treated rats after dosing effects were noted. After 24hr no mortality and no signs were observed in treated rats were noted carefully after dosage. No observable signs of toxicity were detected during the experimental period up to 14 days and all behavioural observation results of rats are illustrated in Table 3.
Table 3: Observation for the SKLEE at 2g/kg B.W of an animal
| Observation | 30 Min. | 4 Hrs. | 24 Hrs. | 48 Hrs. | 1 Week | 2 Week | ||||||
| C | E | C | E | C | E | C | E | C | E | C | E | |
| Skin and Fur | N | N | N | N | N | N | N | N | N | N | N | N |
| Eyes | N | N | N | N | N | N | N | N | N | N | N | N |
| Mucous Membrane | N | N | N | N | N | N | N | N | N | N | N | N |
| Salivation | N | N | N | N | N | N | N | N | N | N | N | N |
| Lethargy | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
| Sleep | N | N | N | N | N | N | N | N | N | N | N | N |
| Coma | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
| Convulsion | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
| Tremors | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
| Diarrhea | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
| Morbidity | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
| Mortality | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
C= Control, E= Extract, N= Normal
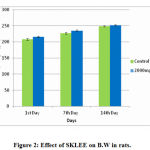 |
Figure 2: Effect of SKLEE on B.W in rats. |
Table 4: Effects of extract on B.W of rats in acute toxicity study
| Groups | Body weight (gm) | ||
| 1st Day | 7th Day | 14th Day | |
| Vehicle Control Group (1% CMC gel) | 208.00±2.54 | 227.00±2.54 | 249.00±1.87 |
| Extract (SKLEE) Group (2g/kg B.W) | 216.00±1.87 | 235.00±2.23 | 252.00±2.00 |
n=5, the values are expreseed as mean ± SEM, *P < 0.05 (Anova/Dunnett T3 test) when compared to VC and SKLEE treated group.
Organs Weight of the Rats in an Oral Acute Toxicity Study
Isolated organs were found to be free of any lesions and no significant changes occurred in the respective organs weights of the rats shown in Table 5. Organ weight is an important indication of the pathological and physiological status of animals19.
Table 5: Effects of extract on organ weight of rats in acute toxicity study
| Groups | Organs Weight (Gram) | ||
| Kidney | Heart | Liver | |
| Vehicle Control Group (1% CMC gel) | 2.07 ± 0.07 | 0.93 ±0.04 | 9.34 ± 0.13 |
| Extract (SKLEE) Group (2g/kg B.W) | 1.75 ± 0.15 | 0.97 ± 0.05 | 9.43 ± 0.25 |
n=5, the values are expreseed as mean ± SEM, *P < 0.05 (Anova/Dunnett T3 test) when compared to VC group from SKLEE treated group.
Effects of Extract on Haematological Parameters in the Acute Toxicity Study
The haematological parameters were tested in both groups by estimating the blood count is shown in Table 6. There was no significant difference in the parameters of Hb, total RBC, total WBC, MCHC, MCV, MCH, Neutrophils, Lymphocytes, Monocytes, Eosinophils and PCV count between the groups.
Table 6: Effects of extract and vehicle control treated groups on haematological parameters.
| Parameters | Vehicle Control Group (1% CMC gel) | Extract (SKLEE) Group (2g/kg B.W) |
| Hb (g/dl) | 12.62 ± 0.12 | 12.64 ± 0.12 |
| Total RBC (×106/µl) | 6.79 ± 0.16 | 6.64 ± 0.17 |
| Total W.B.C (×103/µl) | 8.16± 0.45 | 8.80 ± 0.63 |
| MCHC (g/dl) | 29.08 ± 0.18 | 29.32 ± 0.30 |
| MCV (fl) | 63.54 ± 1.65 | 64.82 ± 1.35 |
| MCH (Pg) | 18.24 ± 0.34 | 18.96 ± 0.10 |
| Neutrophils (%) | 30.40 ± 1.36 | 32.80 ± 1.77 |
| Lymphocytes (%) | 67.60 ± 1.91 | 64.20 ± 2.90 |
| Monocytes (%) | 1.40 ± 0.24 | 1.40 ± 0.24 |
| Eosinophils(%) | 1.60 ± 0.24 | 1.60 ± 0.24 |
| PCV (%) | 43.36 ± 0.48 | 42.84 ± 0.55 |
n=5, the values are expreseed as mean ± SEM, *P < 0.05 (Anova/Dunnett T3 test) when compared to VC group.
Effects of Extract on Biochemical Parameters in Acute Toxicity Study
The results in the extract treated group showed that the renal function test of urea and creatinine were not shown any significant differences (Table 7). In the liver function test parameters AST (SGOT), ALT (SGPT), globulins, albumin, total Proteins, total Bilirubin and alkaline Phosphatase showed no significant among the groups (Table 8) and Total Cholesterol, triglycerides, H D L, L D L and V L D L among the groups showed no significant variations of the report showed (Table 9). Totally the biochemical parameters showed that when the extract treated group at 2 g/kg was compared with vehicle control group report was not significant differences.
Table 7: Effects of the extract and vehicle control treated groups for renal function test of rats.
| Parameters | Vehicle Control Group (1% CMC gel) | Extract (SKLEE) Group (2g/kg B.W) |
| Urea (mg/dl) | 17.8 ± 1.01 | 18.20 ± 1.20 |
| Creatinine (mg/dl) | 0.72 ± 0.037 | 0.76 ± 0.05 |
n=5, the values are expreseed as mean ± SEM, *P < 0.05 (Anova /Dunnett T3 test) when compared to VC group from SKLEE treated groups.
Table 8: Effects of extract and vehicle control treated groups for liver function test in female rats.
| Parameters | Vehicle Control Group (1% CMC gel) | Extract (SKLEE) Group (2g/kg B.W) |
| S.G.O.T (A.S.T) (U/L) | 29.42 ± 1.93 | 26.66 ± 2.83 |
| S.G.P.T (A.L.T) (U/L) | 26.80 ± 1.46 | 26.60 ± 1.56 |
| Alkaline Phosphatase (IU/L) | 43.43 ± 1.68 | 42.92 ± 1.58 |
| Total Bilirubin (mg/dl) | 0.23 ± 0.01 | 0.22 ±0.02 |
| Total Protein (g/dl) | 7.3 ± 0.11 | 7.1 ± 0.19 |
| Albumin (g/dl) | 3.4 ± 0.10 | 3.4 ± 0.08 |
| Globulins (g/dl) | 3.8 ± 0.16 | 3.9 ± 0.16 |
n=5, the values are expressed as mean ± SEM, *P < 0.05 (Anova /Dunnett T3 test) when compared to VC group from SKLEE treated groups.
Table 9: Effects of extract on the serum lipid profile of vehicle control treated and SKLEE treated groups.
| Parameters | Vehicle Control Group
(1% CMC gel) |
Extract (SKLEE) Group
(2g/kg B.W) |
| Total Cholesterol (mg/dl ) | 118.00 ± 5.94 | 127.00 ± 4.03 |
| Triglycerides (mg/dl) | 99.60 ± 5.00 | 107.40 ± 3.05 |
| H.D.L (Cholesterol) (mg/dl) | 38.40 ± 1.93 | 41.00 ± 1.30 |
| L.D.L (Cholesterol) (mg/dl) | 59.68 ± 3.00 | 64.52 ± 2.14 |
| V.L.D.L (Cholesterol) (mg/dl) | 19.92 ± 1.00 | 21.48 ± 0.61 |
n=5, the values are expreseed as mean ± SEM, *P < 0.05 (Anova /Dunnett T3 test) when compared to VC group.
Histopathology Study of Oral Acute Toxicity
The liver, kidney and heart the tissue in SKLEE (2 g/kg B.W) treated rat revealed characteristic of normal structure and no morphological changes detected at the cellular level compared with VC treated group.
In histopathological study of liver sections was observed in treated SKLEE rat after 14 days (Figure 3, C and D) at the dose of 2 g/kg B.W The SKLEE treated rat organs of liver section comparison with the VC treated rat liver section (Figure 3, A and B) showed central vein normally, nucleus (N), kupffer (K) cells and contained RBC (Red Blood Cells). In kidney histopathological study of VC rat (Figure 7, A and B) and SKLEE treated (2 g/kg B.W) rat (Figure 4, C and D) were showing the same structure in both of the glomerulus (G) and Tubules (T) (Figure 4, A and B). In heart tissue sections were showing normal structure in both of VC and SKLEE treated groups (Figure 5, A, B, C and D). Liver, kidney and heart histopathological study confirmed a normal structure in SKLEE treated group.
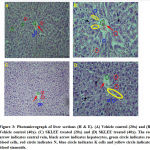 |
Figure 3: Photomicrograph of liver sections (H & E). |
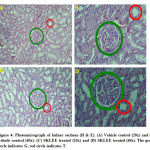 |
Figure 4: Photomicrograph of kidney sections (H & E). |
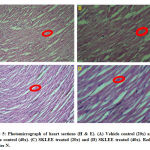 |
Figure 5: Photomicrograph of heart sections (H & E). |
Discussion
Before the study of pharmacological and development of the pharmaceutical product from the medicinal plant, the acute toxicity studies provide valuable information for medicines20,21. Medicinal plants presumed to be safer side without any compromising side effects on health22,23 but in many studies reported the harmful effects of improper use of medicinal plants24. Therefore, the evaluation of toxicological effects from the extract of any medicinal plant intended to be used in the animal model is a crucial part of its assessment for potential toxic effects. S. kanarense is a medicinal plant and more than 50 compounds have been identified in aerial parts7. Medicinal properties of SKLEE are due to the presence of phytoconstituents. The presences of phytochemicals in SKLEE are phenolics, terpenoids, tannins, saponins steroids, volatile oil, glycosides, carbohydrates, and proteins (Table 1). These constituents may be responding to hyperglycemia. No data available on the studies of toxicity effect in S. kanarense leaves extracts. Many studies exist on the oral acute toxicity of plant extracts using rat models22,25. The determine cytotoxicity activity by MTT assay on mouse embryonic fibroblast cell line (NIH/3T3) was used for cytotoxic effects of the extract were expressed as IC50 values (Table 2). The MTT assay indicated that SKLEE on NIH/3T3 cell line showed toxicity more than 50% cytotoxic effects in the concentration of 500µg/ml.
Determination of acute toxicity is one of the major prerequisites for the development of newly synthesized drugs. Evaluation of acute toxicity of any extract plays an important role in the designing of drugs and their toxicological effects. So, the present reports in acute toxicological study dose treatment to Wistar rats at 2 g/kg B.W concentration of SKLEE showed no mortality, clinical signs, change in the skin, eye colour change, general physique, diarrhoea, and coma were recorded (Table 3) up to 2 weeks. Throughout the 14 day periods, all animals were found to be healthy with no changes. Therefore, this SKLEE oral LD50 value should be greater than 2g/kg B.W. In rat’s exposure to potentially any toxic substances, there will be a reduction in B.W gain26. In our acute toxicity studies, all rats continued to gain weight throughout the experimental periods (Figure 2). This suggests that the administration of the SKLEE at 2g/kg B.W did not affect the B.W of the rats. The relative organ weights of the rats in the extract treated group did not show any significant changes in comparison with that of the VC treated group. It indicated that the SKLEE did not affect the appetite and no deleterious effect on the growth of animals. No significant changes were observed in rat’s physiological as metabolic activity in comparison to VC treated group. If any changes in some blood parameters indicate the toxic effects of extract in rats blood either at a physiological or pathological level. Therefore, to study focus on the liver and kidney functions test as biochemical parameters. The enzymes of alanine aminotransferase (ALT) and alkaline phosphatase (ALP) are mainly found in the liver and kidneys. An increased level of ALT, ALP and total protein in blood indicates impaired liver and kidney function27. Creatinine and urea levels rise in the blood due to poor clearance by the kidneys28. The analysis of biochemical parameters studied in particular ALT, ALP, creatinine, urea, and total protein were tested and showed there were no significant differences in the treated group rats compared to VC group rats. The report indicates in haematological, biochemical parameters and lipid profile showed good results in both groups of rats. The body has essential vital organs for proper functions that are liver and kidney. The organs functions of the liver to the metabolism of intake29 and kidney for excretion of the waste30. To assess the toxicity of any new extract, it is very essential to know the status of these vital organs, which can be checked through the liver and renal function test in female rat’s blood. The histopathological images of liver, kidney, and heart also confirmed that the SKLEE treated group at 2g/kg B.W showed no changes compared with VC treated group observed under the microscope. The obtained results showed that SKLEE is practically non-toxic after acute administration at2g/kg B.W on an animal model of Wistar rats.
Conclusion
This is the first report describing the screening of phytochemicals, cytotoxicity by MTT assay on NIH/3T3 mouse embryonic cell line, oral acute toxicity on Wistar rats and histopathological from ethyl acetate extract of S. kanarense leaves. Leaves extract of S. kanarense containing phytochemicals such as phenolics, terpenoids, tannins, saponins steroids, volatile oil, glycosides, carbohydrates, and proteins were present. Several studies from other species of the same genus confirmed the presence of these phytochemicals contributes medicinal as well as physiological properties of the plants studied in the treatment of different ailments. Therefore, extracts from one of the rare and rediscovered plant after 47 years of S.kanarense could be seen as a good source for useful drugs. Cytotoxicity by MTT assay method on mouse fibroblast NIH/3T3 cell line showed IC50 value 425.07μg/ml range. The oral acute toxicity reports confirmed the oral dose up to 2g/kg B.W and no related sign of toxicity or mortality in female Wistar rats. In B.W, hematology, biochemical serum analysis and histopathology study of vital organs did not show toxicity in SKLEE treated group comparisons with the vehicle control group. To our best of our knowledge, this is the first report from the application of S. kanarense plant extract. Further studies are under process for the isolation of bioactive compounds for anti-diabetic study on an animal model. A focus of the study in this direction certainly would bring awareness in availing of the health benefits of the natural plant.
Acknowledgment
The authors are thankful to the Department of Biosciences and Applied Botany, Mangalore University, helping to complete this research.
Conflict of Interest
The authors have declared that there is no conflict of interest.
Funding Source
Divakar M S is thankful to Mangalore University, PhD Fellowship for carrying out research.
References
- Phillipson J.D and Anderson L.A. Ethnopharmacology and western medicine. J , 1989; 25(1): 61-72.
- Valiathan M. S. Healing plants. Curr. Sci., 1998; 75(11): 1122-1127.
- Moreira T. M. S, Salgado H. R. N and Pietro R. C. L. R. O Brasil no contexto de controle de qualidade de plantas medicinais. REV BRAS FARMACOGN., 2010; 20: 435-440.
- Yuan H, Ma Q, Ye L and Piao G. The Traditional Medicine and Modern Medicine from Natural Products. , 2016; 21(5): 559.
- Singh R, Verma P and Singh G. Total phenolic, flavonoids and tannin contents in different extracts of Artemisia absinthium. J Complement Med Res., 2012; 1(2): 101.
- Shenoy H. S, Krishnakumar G and Marati R. Rediscovery of Syzygium kanarense (Talbot) Raizada (Myrtaceae) – an endemic species of the Western Ghats, India. Threat. Taxa., 2015; 7(1): 6833–6835.
- Joshi R. K, Sooryaprakash Shenoy H and Marati R. Chemical composition of the essential oil of Syzygium kanarense: An endemic and rediscovered species from the western ghats, India.Prod. Commun., 2017; 12(12): 1943–1944.
- Mith A and Boland W. Plant Defense Against Herbivores : Chemical Aspects. ANNU REV PLANT BIOL., 2012; 63: 431-450.
- Li W, Zhou J and Xu Y. Study of the in vitro cytotoxicity testing of medical devices. Biomed Rep., 2015; 3(5): 617-620.
- Tiwari P, Kumar B, Kaur M, Kaur G and Kaur H. Phytochemical screening and extraction: a review. Internationale pharmaceutica sciencia., 2011; 1(1): 98-106.
- Banu K. S and Cathrine L. General techniques involved in phytochemical analysis. j. adv. res. chem. sci., 2015; 2(4): 25-32.
- Edeoga H. O, Okwu D. E and Mbaebie B. O. Phytochemical constituents of some Nigerian medicinal plants. J. Biotechnol., 2005; 4(7): 685-688.
- Trease G. E, and Evans W. C. Pharmacognsy. 11th edn. Brailliar Tiridel Can. Macmillian Publishers., (1989) 0 5: 10-15.
- Yadav, R., Khare, R. K., & Singhal, A. (2017). Qualitative phytochemical screening of some selected medicinal plants of shivpuri district (mp). J. Life. Sci. Scienti. Res, 3(1), 844-847.
- Shoemaker M, Cohen I and Campbell M. Reduction of MTT by aqueous herbal extracts in the absence of cells. J Ethnopharmacol., 2004; 93(2-3): 381–384.
- Peng L, Wang B and Ren P. Reduction of MTT by flavonoids in the absence of cells, COLLOID SURFACE B., 2005; 45(2): 108–111.
- Ayaz M, Junaid M, Ullah F, Sadiq A, Subhan F, Khan M. A and Ahmad S. Molecularly characterized solvent extracts and saponins from Polygonum hydropiper L. show high anti-angiogenic, anti-tumor, brine shrimp, and fibroblast NIH/3T3 cell line cytotoxicity. Pharmacol., 2016; 7: 74
- Saleem U, Amin S, Ahmad B, Azeem H, Anwar F and Mary S. Acute oral toxicity evaluation of aqueous ethanolic extract of Saccharum munja Roxb. roots in albino mice as per OECD 425 TG. Rep., 2017; 4: 580-585.
- Raina P, Chandrasekaran C. V, Deepak M, Agarwal A and Ruchika K. G. Evaluation of subacute toxicity of methanolic/aqueous preparation of aerial parts of O. sanctum in Wistar rats: Clinical, haematological, biochemical and histopathological studies. J Ethnopharmacology., 2015; 175: 509–517.
- Palombo E A. Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. EVID-BASED COMPL ALT., 2011; 2011.
- Hasani-Ranjbar S, Nayebi N, Moradi L, Mehri A, Larijani, B, and Abdollahi M. The efficacy and safety of herbal medicines used in the treatment of hyperlipidemia; a systematic review. Pharm. Des.,2010; 16(26): 2935-2947.
- Harizal S. N, Mansor S. M, Hasnan J, Tharakan J. K. J and Abdullah J. Acute toxicity study of the standardized methanolic extract of Mitragyna speciosa Korth in rodent. J Ethnopharmacology.,2010; 131(2): 404-409.
- Hor S. Y, Ahmad M, Fars E, Lim C. P, Asmawi M. Z and Yam M. F. Acute and subchronic oral toxicity of Coriolus versicolor standardized water extract in Sprague-Dawley rats. J Ethnopharmacology., 2011; 137(3): 1067-1076.
- Kao W. F, Hung D. Z, Tsai W. J, Lin K. P and Deng J. F. Podophyllotoxin intoxication: toxic effect of Bajiaolian in herbal therapeutics. HUM EXP TOXICOL., 1992; 11(6): 480-487.
- Asare G. A, Sittie A, Bugyei K, Gyan B. A, Adjei S, Addo P and Adjei D. N. Acute toxicity studies of Croton membranaceus root extract. J Ethnopharmacology., 2011; 134(3): 938-943.
- Teo S, Stirling D, Thomas S, Hoberman A, Kiorpes A and Khetani V. A 90-day oral gavage toxicity study of d-methylphenidate and d, l-methylphenidate in Sprague–Dawley rats. , 2002; 179(3): 183-196.
- Atsamo A. D, Nguelefack T. B, Datté J. Y and Kamanyi A. (2011). Acute and subchronic oral toxicity assessment of the aqueous extract from the stem bark of Erythrina senegalensis DC (Fabaceae) in rodents. J Ethnopharmacology., 2011; 134(3): 697-702.
- Wang D, Xu K, Zhong, Y, Luo X, Xiao R, Hou Y, and Liu L. Acute and subchronic oral toxicities of Pu-erh black tea extract in Sprague–Dawley rats.J Ethnopharmacology.,2011; 134(1): 156-164
- Mitra V and Metcalf J. Metabolic functions of the liver. Anaesth Intensive Care,2012; 13(2): 54-55.
- Jaddi N. S, Alvankarian J and Abdullah S. Kidney-inspired algorithm for optimization problems. COMMUN NONLINEAR SCI., 2017; 42, 358-369.
(Visited 805 times, 1 visits today)







