Manuscript accepted on :31-01-2020
Published online on: 24-02-2020
Plagiarism Check: Yes
Reviewed by: Saiful Irwan Zubairi
Second Review by: S Parasuraman
Final Approval by: Dr. Beatrice O. Ondondo
Maysaa Kadhim Al-Malkey1*![]() , Aidaa Hussain Ibrahim2
, Aidaa Hussain Ibrahim2![]() , Munira CH Ismeel3
, Munira CH Ismeel3![]() , Fadhaa O Sameer1
, Fadhaa O Sameer1 ![]() , Istabraq A. Beram1 and Iftikhar AH Jasim1
, Istabraq A. Beram1 and Iftikhar AH Jasim1
1Tropical Biological Research Unit, College of Science, University of Baghdad, Baghdad-Iraq
2Department of Biotechnology, College of Science, University of Baghdad, Baghdad-Iraq
3Department of Microbiology, College of Science, Al-Karhk University of Science, Baghdad-Iraq
Corresponding Author E-mail : maysakadhim@uobaghdad.edu.iq
DOI : https://dx.doi.org/10.13005/bpj/1877
Abstract
Antibiotic resistance increment is a major problem for the human society nowadays which encourages the efforts to look for new therapeutic alternatives from natural defenses. Synergistic antibacterial activity of epidermin and staphylolysin LasA A against Staphylococcus aureus (Staph aureus), Escherichia coli (E. coli) and Pseudomonas aeruginosa (Ps. aeruginosa) was evaluated. The antibacterial activities of epidermin from Staphylococcus epidermidis (Staph epidermidis) and Staphylolysin (LasA) from Ps. aeruginosa using the agar well diffusion assay were evaluated, and then using the micro dilution method to evaluate the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC). The checkerboard method and fractional inhibitory concentration (FIC) were used to evaluate the combination between epidermin and LasA toward targeted clinical isolates of Staph aureus, E. coli and Ps. aeruginosa. The results revealed a synergistic effect between epidermin and LasA on all clinical isolates growth. The highest MIC and MBC of epidermin were 36.04 μL⁄mL and 51.73 μL/mL against Staph aureus; meanwhile, the highest MIC and MBC of LasA were 44.38 μL/mL and 50 μL⁄mL against Staph aureus. The FICindex revealed synergistic interactions in combination of epidermin and LasA which recorded 0.286 for Staph aureus while for E. coli was 0.327 and for Ps. aeruginosa was 0.390 respectively showing a synergism effect. This study finds that combination of epidermin with LasA had inhibitory activity on the targeted clinical isolate growth, which can be useful for designing and developing alternative therapeutic strategies against pathogens causing wound and burn infections.
Keywords
Antibacterial Activity; Epidermin; LasA; Pathogenic Bacteria; Synergism
Download this article as:| Copy the following to cite this article: Al-Malkey M. K, Ibrahim A. H, Ismeel M. CH, Sameer F. O, Beram I. A, Jasim I. AH. Synergistic Antibacterial Activity of Epidermin and Staphylolysin LasA against Pathogenic Bacteria. Biomed Pharmacol J 2020;13(1). |
| Copy the following to cite this URL: Al-Malkey M. K, Ibrahim A. H, Ismeel M. CH, Sameer F. O, Beram I. A, Jasim I. AH. Synergistic Antibacterial Activity of Epidermin and Staphylolysin LasA against Pathogenic Bacteria. Biomed Pharmacol J 2020;13(1). Available from: https://bit.ly/2SQDPPw |
Introduction
Infectious diseases mortality is estimating about 50,000 people every day worldwide [1]. The increment of antibiotic resistance cases has motivated scientists to explore alternative therapeutic strategies [2]. The multidrug resistant (MDR) emergence of bacterial strains leading to treatment failure of infections is becoming escalating problem. In developed countries, the reemergence of tuberculosis and pneumonia that were almost diminish and occurrence of MDR in Gram-positive and Gram-negative such as Staphylococcus, Bacillus, Escherichia coli, Pseudomonas aeruginosa, Shigella spp. and Salmonella as well as and other bacteria from all over the world was concurrently reported due to the misuse of antimicrobials [3]. The compounds extracted from the natural source are highly used since the past times for different diseases treatment and as remedy for live improvement [4].
One strategy to overcome of the new emerging antimicrobial resistance is to use bacteriocins as therapeutic possibilities in clinical settings, bacteriocin by definition “are ribosomally-synthesized antimicrobial peptides produced by bacteria and can exhibit narrow spectra of activity” meanwhile others may display a broader spectra of activity [5]. Bacteriocins show strong activity against their target strains, often within the nanomolar range, making them in some cases more effective than their counterparts with antibiotics; thus, bacteriocins have potential to be used in clinical settings [6].
Epidermin is “a tetra cyclic peptide produced and secreted by Staphylococcus epidermidis”. It is lantibiotic family member which is a group of plasmid-encoded, ribosomal synthesized and post transitionally modified antimicrobial peptides [7]. Epidermin is bactericidal to Gram-positive bacteria, the bacteriocin inhibited the synthesis of DNA, RNA, protein and polysaccharides simultaneously, leading to insufficient energy to carry biosynthetic processes, and eventually the energy-transuding cytoplasm membrane may be the primary biochemical target and seems to affect the membrane permeable barrier by forming water-filled membrane channels or pores, probably by a barrel-stave mechanism [8].
Pathogenic bacteria possess an extensive arsenal of virulence factors that allow them to survive in the host and cause disease. Among those are secretion of extracellular proteases which facilitate bacterial colonization by inducing damage to host tissue and actively subverting immune responses [9]. LasA protease enzyme: “Is also designated as staphylolysin”. Its elastolytic and staphylolytic endopeptidase secreted by Ps. aeruginosa. LasA is synthesized as propoenzyme that mediates proteolysis to omit a 22 kDa” amino-terminal peptide; it is protease and one of the M23 family of β-lytic Zinc metalloendopeptidase [10]. The potential of LasA as antistaphylolytic therapy has been confirmed in vivo in Staphylococcal experimental model of keratitis [11].
In a few studies, bacteriocins combinations with other antimicrobials have been conducted to overcome the development of antimicrobial resistance and/or increase antimicrobial potency. It is possible that the use of antimicrobials that function synergistically with bacteriocins will increase the killing effects of each other, thus increasing the likelihood of production of resistance to either the bacteriocin or the antimicrobial stressor. Such bacteriocin-antimicrobial combinations could have great value, in terms of reducing the likelihood of resistance development because of the involvement of two different of antimicrobial action mechanisms [12]. Therefore, this study aimed to evaluate the antibacterial ability of combined lantibiotic with bacteriocin (epidermin and LasA) against some pathogens causing wound and burn infection, including Staph aureus, E. coli and Ps aeruginosa.
Patients and Methods
Bacterial Isolation, Identification and Antimicrobial Resistance
Seventy swab samples were collected from patients with wound and burn infection admitted at Al-Kadhimia Hospital and Central Childhood Hospital, Baghdad-Iraq from the period May 2014 till November 2015. Approval was obtained from our scientific review board and Ministry of Health-Iraq. Standard methods for bacterial isolation were used for bacterial isolates (Staph epidermidis, Staph aureus, E. coli, and Ps aeruginosa). They were cultured on primary and selective media (Nutrient agar, Blood agar, Mannitol salt agar and McConkey agar, HiMedia, India), then bacterial identification was performed using biochemical tests, and then antimicrobial sensitivity test was done for the targeted isolate [13].
A Mueller Hinton agar (MHA) with disk diffusion method using commercially antibiotics was applied. Antibiotics were from (HiMedia Laboratories Ltd, India). In this study Antibiotic sensitivity profiles determination of the reference bacteria, the following antibiotics (concentration µg/disc) were used: amoxicillin (10), cephotaxime (30), chloroamphinecol (10), ciprofloxacin (10), erythromycin (15), gentamicin (10), tetracycline (10) and vancomycin (30) (Bauer et al., 1996). Diameters of inhibitory zone were compared with the standards following the Clinical and Laboratory Standards Institute instructions’ (CLSI, 2019) [14].
Extraction of Crude Epidermin
During the log phase, crud bacteriocin produced by Staph epidermidis was extracted using anaerobically incubated tryptone soya broth for 24 hours at 37◦C. After incubation, the cultures were centrifuged (6000 rpm at 4ºC for 10 min) to obtain culture-free supernatant which was filtered using 0.22 µm pore sterilized filter (Puradisc 25mm, India). To eliminate possible inhibition effects of organic acids, the pH was adjusted to pH 7 with 1 M NaOH. Protein content was determined using colorimetric at maximum absorption at 600 nm, using brilliant blue G-250 and Bradford method by bovine serum albumin [15]. Partial purification of bacteriocin was performed by ion exchange chromatography assay; final concentration was 160 μg/ml.
LasA Protease Production
The crude LasA protease was extracted from Ps. aeruginosa isolates cultivated on skimmed milk agar (MRS) 1% (HiMedia, India) according to Diggle et al., (2002) [16]. Ammonium sulfate participation was used according to Nadeem and Mukhtar, (2013) for partial purification of LasA according to previous literature [17]. Estimation of protein concentration by Bradford method was done using the standard curve of bovine serum albumin (BSA) at concentrations (1.6, 1.4, 1.2, 1, 0.8, 0.6 and 0.4) mg/ml [18]. The extracted LasA that produced by precipitation with ammonium sulfate were loaded inside dialysis bag at molecular weight cut-off 10000 kDa [19]. After the gel filtration chromatography was done followed (Mohsen et al., 2013) the protein concentration was 40 µg/ml [20]. LasA protease activity detection was done by as follows: 100 µl of partial purified LasA was added to 500 µl of bacterial suspension then incubated at 37°C for 30 min, after that the reading of absorbance at the wave length 600 nm. To determine the proteolytic potency of the isolates, agar well diffusion assay was performed.
Antimicrobial Activity of Bacteriocin by Agar Well Diffusion Assay
To determine the activity of the supernatant, agar well-diffusion method was performed in triplicate. Bacterial suspension inoculated with 10 µl of (1 × 108 cfu/ml), by swabbing over the entire surface of the plates. Wells nearly 6 mm in diameter and about 2.5 mm in depth were made on the surface of solid cultured medium plates using a sterile pasture pipette. Later, 10 µl of bacteriocin was inoculated into wells then 24 hr of incubation at 37°C was done, zone of inhibition for each plate was examined, the control for each zone was prepared using un-inoculated sterile BHI broth without extract served as negative control while standard antibiotics, 10 µl of gentamicin (10 µg/mL) or ciprofloxacin (10 µg/mL) were used as reference positive controls. The plates were incubated at 37°C for 24 hrs, and then zones of inhibition measured manually. Zones of inhibition ≥ 8 mm in diameter were considered as positive.
In vitro Antibacterial Activity of Epidermin and LasA:
Determination of Minimum Inhibitory Concentration (MIC)
The broth microdilution technique was adopted using 96-well microtiter plates with a volume of 300 µl were used with tetrazolium salt (2,3,5-triphenyltetrazolium chloride, Sigma, India) as an indicator to determine the MIC (50 µL of 0.5% solution) according to the procedures by the (CLSI, 2019) [14]. Different concentrations of bacteriocin and LasA were prepared by serial dilutions of 100 μL with 2 fold dilutions in the range of (0.12-250) µL/mL prepared by incorporation of the bacteriocin (epidermin) and LasA into Muller Hinton Broth (MHB) (HiMedia, India). Then 100 µL (a bacterial inoculum), corresponding to 5 ×105 CFU/mL was added to 100 µL of serial fold dilutions of the bacteriocin or LasA in the wells of microtiter plates with a final volume 200 µL for each well was performed. Two wells containing microbial media broth served as positive control and negative control, respectively. Incubated for 24 hrs at 37○C of the parafilm sealed microtiter plates were done, and then the growth of the bacteria was observed. A color changing to pink indicates growth of bacteria. The MIC value of the bacteriocin was taken as “the lowest concentration of bacteriocin that inhibits visible growth of individual test bacteria”. MIC values were detected by ELISA reader (Bio-Rad, Germany) at 492 nm [21]. The percentage of inhibition in growth of test bacteria due to antimicrobial proteins was calculated as the following formula:
% of reduction in growth = OD Value of Control Well – OD of Test Well / OD Value of Control Well × 100
Minimum Bactericidal Concentration (MBC)
The next step was transferring 100 μL of liquid from each well without visible growth on to MHA for determination of MBC was used and incubated at 37°C for 48-72 hrs. Finally, “the lowest concentration of antimicrobial agent being able to reduced 99.9% of the bacteria was assessed as MBC”. Triplicate procedures were done.
Synergism Test of Bacteriocin Combination with LasA by Fractional Inhibitory Concentration (FIC)
A checkerboard microdilution method was chosen to assess the efficacy of possible interaction between epidermin and LasA which could be synergistic, additive, antagonist or exhibiting no interaction against the pathogens. Inoculation were prepared spectrophotometrically and further diluted to obtain final concentrations (0.5 × 106) CFU/mL. Each microdilution well included 100 μL of the diluted (two times) concertation of both antimicrobials (bacteriocin and lantibiotic) was inoculated with 100 μL of the diluted (two times) inoculum suspension with 200 μL as a final volume of each well, the trays were incubated at 37°C, and the results were read at 24 hours visually using an ELISA reader system.
The fractional inhibitory index (∑ FIC) index was calculated by the following formula:
FICindex = FICA + FICB = MICA (A in presence of B) / MIC (A alone) + MICB (B in presence of A) / MIC (B alone)
Where “MICA alone:” is the MIC value of bacteriocin “A” tested alone; “MICB alone” is the MIC value of staphylolysin “B” tested alone; “MICA combined” is the MIC value of bacteriocin “A” tested in combination with staphylolysin “B”; “MICB combined” is the MIC value of staphylolysin “B” tested in combination with bacteriocin “a”. According to this method, synergistic effect if the FIC index is of ≤ 0.5; additive effect if the FIC index of (0.5 < FIC ≤ 1.0); no interactive effect if the FIC index of (1 < FIC ≤ 4.0) and antagonism effect if the FIC index ≥ 4.0 [22].
Statistical Analysis
The data were analyzed using SPSS 18.0 program for Windows was used to analyze the results obtained by analysis of variance (ANOVA) with significance level set at P ≤ 0.05.
Results
A total of 70 skin swabs were collected from wound and burn infection, then standard laboratory identification such as cultural characteristics and biochemical tests were applied on samples. Fifty samples were identified as Staph epidermidis using mannitol salt agar (HiMedia, India) as selective media, of which 5 strains were epidermin producers. Only one isolate was used in this study. Targeted bacteria (E. coli, Ps aeruginosa and Staph aureus) were isolated from corneal scraping samples which were taken according to ophthalmologist order from patients suffering of microbial keratitis (corneal ulcer) referred to Ibn Al-Haitham Teaching Eye Hospital Laboratory in Baghdad as shown in Table (1). Antibiotic susceptibility pattern of the targeted isolates was summarized in Table (2). The antibacterial activities of epidermin from Staph epidermidis isolate were determined using the agar well diffusion assay summarized in Figure (1). The antibacterial activity quantitatively assessed on the basis of the inhibition zone. Epidermin showed antibacterial activity against staph aureus with concentration 160μg/ml after purification with gel filtration through sephadex G-75; whereas E. coli and Ps. aeruginosa isolates came with poor inhibition zones.
Table 1: The prevalence of Staphylococcus epidermidis isolated from wound and burns skin swabs
| Bacterial isolates | No. of swabs | Percentage % |
| Staph epidermidis | 50 | 71% |
| Others | 20 | 29% |
| Total | 70 | 100% |
Table 2: Antibiotic Susceptibility Pattern of Target isolates as Determined by the Disc-Diffusion Techniquea
| Antibiotic | Concentration µg/disc | Targeted isolates | ||
| Staph aureus | E.coli | Ps. aeruginosa | ||
| Amoxicillin | 10 | R | R | R |
| Cephotaxime | 30 | S | S | S |
| Chloroamphinecol | 10 | S | R | R |
| Ciprofloxacin | 10 | S | S | S |
| Erythromycin | 15 | R | R | R |
| Gentamicin | 10 | S | R | R |
| Tetracycline | 10 | R | S | R |
| Vancomycin | 30 | S | S | S |
| a Abbreviation: S = sensitive; R = resistance | ||||
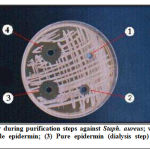 |
Figure 1: Epidermin activity during purification steps against Staph |
The results of the MIC and MBC of epidermin were determined by the microdilution method and are shown in Table (3). The MIC of epidermin against Staph aureus was 36.04 µL/ml followed by 19.95 against Ps. aeruginosa, whereas; the MIC of LasA against Staph aureus was 51.73 µL/ml, followed by 15.76 µL/ml against Ps. aeruginosa. Regarding the MBC of epidermin against Staph aureus was 44.38 µL/ml followed by 30.33 against E. coli, whereas; the MBC of LasA against Staph aureus was 50 µL/ml, followed by 27.48 µL/ml against E. coli.
Table 3: The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of bacteriocin (epidermin) and staphylolysin (LasA) against targeted pathogenic bacteria
| Bacteria | MIC µL/ml | MBC µL/ml | ||
| Epidermin | LasA | Epidermin | LasA | |
| Staph aureus | 36.04 | 51.73 | 44.38 | 50 |
| E. coli | 13.85 | 7.48 | 30.33 | 27.48 |
| Ps. aeruginosa | 19.95 | 15.76 | 11.91 | 15.82 |
The FIC value for epidermin and LasA were shown in Table (4). The FIC of synergism between epidermin and LasA against all targeted bacteria showed synergistic effect.
Table 4: The Fractional Inhibitory Concentration (FIC) of bacteriocin (epidermin) combined with staphylolysin (LasA) against targeted pathogenic bacteria
| Bacteria | MICA (Alone) | MICB (Alone) | MICA in the presence of B | MICB in the presence of A | FICindex | Activity |
| Staph aureus | 36.04 | 51.73 | 1.55 | 1.08 | 0.286 | S |
| E. coli | 13.85 | 7.48 | 2.03 | 3.76 | 0.327 | S |
| Ps. aeruginosa | 19.95 | 15.76 | 0.99 | 1.26 | 0.390 | S |
FICindex = FICA + FICB; FICA = (MICA combined/MICA alone) and FICB = (MICB combined/MICB alone).
FIC ≤ 0.5: synergistic effect (S); 0.5 < FIC ≤ 1: additive effect (AD); 1 < FIC ≤ 4: no interactive effect (I); FIC > 4: antagonistic effect (A)
(I); FIC > 4: antagonistic effect (A)
Discussion
Bacteriocins encoding genes located in the genomes of most Gram-negative pathogens, including Ps. aeruginosa, E. coli and Klebsiella pneumoniae (K pneumoniae) [23]. Taking in mind, they are highly had specific antibacterial activity that kill only bacteria closely related to the producer and are deployed during the fight for resources with competitor strains [24]. This makes them attractive as therapeutics as they offer a more targeted approach. In fact, one major problem with conventional antibiotics is the dysbiosis induced by broad-range killing of bacteria. While the narrow killing spectrum of bacteriocins means that the bacteria responsible for the infection have to be identified prior to treatment which gives the advantage of being able to specifically target one species, or even one strain of bacteria, spearing the normal healthy microflora intact [25].
In this study, the synergism between epidermin and staphylolysin LasA had been applied in an attempt to reach for antibacterial activity against the foremost among the Gram-negative pathogens; Ps. aeruginosa and E. coli which pose serious threats to global healthcare and patient safety; same goes for Staph aureus under the hypothesis that whether treatment with combination of bacteriocin (epidermin) and lantibiotic (staphylolysin LasA) have higher antimicrobial activity against targeted pathogens (Staph aureus, E.coli and Ps. aeruginosa), the first step in which a strains needs to fulfill is that they should be resistant or multi-resistant to antibiotics after that, the combination between epidermin and LasA was further tested to evaluate the possible synergistic effect against targeted pathogens.
A numerous studies have been conducted, involving combinations of bacteriocins with other antimicrobials, to reveal the development of antimicrobial resistance and/or increase antimicrobial potency, Turgis et al., (2016) performed a research on synergistic antimicrobial effect of combined bacteriocins (nisin, pediocin, enterocin MT104b and enterocin MT162b) against food pathogens and spoilage bacteria, his results concluded into that combination of nisin with MT104b caused a synergistic effect on the elimination of Staph aureus which agrees with this study results [22]. A study by Field et al., (2016) found that nisin was effective against Ps aeruginosa biofilms when used together with polymyxins [26]. Ps aeruginosa biofilm-forming abilities contributes to its pathogenicity and causing cystic fibrosis in lungs of patients which warrants further extensive research to target its biofilm forming and consequent pathogenic properties [27]. Biswas et al., (2017) investigate antibacterial and synergistic activity bacteriocin of lactic acid bacteria (LAB) against β-lactamase-producing nosocomial bacteria (E. coli ,Streptococcus pyogenes, Enterococcus faecalis, Klebsiella pneumoniae and Bacillus cereus), revealed that there is a bacteriocingenic activity of LAB against nosocomial pathogens which agrees with study results [28]. Another study by Zbar et al., (2018) revealed that the antibacterial activity of partially purified bacteriocin produced by Cronobacter sakazakii against E. coli, Staph aureus, K. pneumoniae, Shigella dysenteriae, Proteus vulgaris and Serratia marcescens showed that the synergistic effect of bacteriocin with both amikacin and tetracycline in different ratios [29].
A recent study by Bhola and Bhadekar (2019) revealed that the inhibitory potential was seen in a combination of the three Lactobacillus species, Lactobacillus plantarum, Lactobacillus acidophilus and Lactobacillus casei var. rhamnosus in the ratio 1:1:1 had the highest antimicrobial activity with whole broth and cell lysate of Lactobacillus consortium exhibited up to 85% inhibition of multi-drug resistant Staphylococcus aureus both standard strain isolate (Staph aureus NCIM 2127 and clinical isolates [30]. The synergism effect of mixing bacteriocins can be as powerful as combination between bacteriocin and antibiotics.
In regards to staphylolysin LasA antibacterial activity, a study by Al- saa’edi et al., (2015) investigate the experimental treatment of bacterial keratitis (in vivo) of infected rabbits eyes caused by Staph aureus and revealed that the efficacy of LasA protease was effective as Lysostaphin drug in eradicating the Staph aureus from the infected corneas comparing to Vancomycin drug that revealed late healing period (approximately after 3 days) after application of treatment [31]. A study by Jose et al., (2017) showed that Ps aeruginosa LasA protease was having lytic action on bacterial cell walls other than that of Staph aureus and its application in rapid extraction of DNA from a wide range of bacteria [32].
Conclusions
Bacteriocins have great potential as an antimicrobial agent. In combination with each other or with other antimicrobial agents they may have empower value in decreasing use of antibiotics. The synergistic effects between bacteriocin and lantibiotic against resistant bacteria provide a new and alternative way of treatment of resistant clinical isolates. The synergistic action is of more importance in case where antibiotic(s) is no longer effective as a therapeutic agent. Combinations of bacteriocins provide an effective and economical way in combating antibiotic-resistant bacteria. The FIC indexes have indicated that they have favorable antimicrobial interactions. The combinations of bacteriocin have not been well investigated, so further studies are required to evaluate the effect of bacteriocins in combination. The combination therapies increase the treatment options and reuse of antibiotics in a case where resistance has developed.
Acknowledgments
The authors would like to thanks Tropical Biological Research Unit for supporting this work.
Conflict of interest
The authors declare no conflict of interest.
Funding Source
Self-funded by the authors.
References
- Aqil F, Zahin M, E Sayed KA, Ahmad I, Orabi KY, Arif JM. Antimicrobial, antioxidant, and antimutagenic activities of selected marine natural products and tobacco cembranoids. Drug Chem Toxicol. 2011; 34:167e179.
CrossRef - Holmes AH, Moore LS, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016; 387: 176–187. doi: 10.1016/S0140-6736(15)00473-0.
CrossRef - Panda S, Rath CC. Phytochemicals as natural antimicrobials: prospects and challenges. In: Gupta VK, ed. Bioactive phytochemicals: perspectives for modern medicine. vol. 1. New Delhi: Daya Publishing House; 2012:329–78.
- Niamah AK. Structure, mode of action and application of pediocin natural antimicrobial food preservative: A review. Basrah J Agric Sci. 2018; 31(1): 59-69. DOi:10.21276/basjas.
CrossRef - Cotter PD, Ross RP, and Hill C. Bacteriocins – a viable alternative to antibiotics? Nat. Rev. Microbiol. 2013; 11: 95–105. doi: 10.1038/nrmicro2937
CrossRef - Ming L, Zhang Q, Yang L, and Huang JA. Comparison of antibacterial effects between antimicrobial peptide and bacteriocins isolated from Lactobacillus plantarum on three common pathogenic bacteria. Int J Clin Exp Med 2015; 8: 5806–11.
- Breukin K, Wiedemann I, VanKraaij C, Kuipers O, et al. Use of the cell wall precursor lipidII by a pore-forming peptide antibiotic. Sci. 2008; 286: 2361–
CrossRef - Klaenhammer TR. Genetic of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 12, 1993, pp. 39–
CrossRef - Potempa J, Pike RN. Corruption of innate immunity by bacterial proteases. J Innate Immun. 2009; 1: 70–87. http://dx.doi.org/10.1159/000181144
CrossRef - Kessler E and Ohman DE. Staphylolysin (LasA endopeptidase), Pp. 1001-1003. In A. Barrett, N. Rawlings, and J. Woessner, The handbook of proteolytic enzymes, 2nd vol. 1. Elsevier Academic Press, Amsterdam, Netherlands. 2004
CrossRef - Barequet IS, Habot WZ, Mann O, et al. Evaluation of Pseudomonas aeruginosa staphylolysin (LasA protease) in the treatment of methicillin – resistant Staphylococcus aureus endophthalmalmitis in a rat model. Graefes Arch Clin Exp Ophthalmol. 2009; 247 (7): 7–913.
CrossRef - Mathur H, Field D, Rea MC, et al. Bacteriocin-Antimicrobial Synergy: A Medical and Food Perspective. Front. Microbiol. 2017; 8: 1205. doi: 10.3389/fmicb.2017.01205
CrossRef - Tille PM. Baily and Scott’s, Diagnostic Microbiology. 13 Elsevier Mosby. Missouri, United States of America. 2013.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 29th, CLSI supplement M100. Wayne PA: Clinical Laboratory Standards; 2019.
- Al-Ibadi MCh, Alwan WL and Jeber SJ. Characterization of S. epidermidis bacteriocin (epidermin). Ibn Al-Haitham. J Appl Sci. 2004; 17(3): 10–18
- Diggle SP, Winzer K, Lazdunski A, Williams P and Cámara M. Advancing the quorum in Pseudomonas aeruginosa: mva T and the regulation of N- acylhomoserine lactone production and virulence gene expression. J Bacteriol. 2002; 184 (10): 2576–
CrossRef - Nadeem U. and Muktar H. Partial purification of alkaline protease by mutant strain of Bacillus subtilis. J Pakistan Bio. 2013; 59(1): 165–
- Bradford MM. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Analyt biochem.1976; 72: 248–
CrossRef - Najeem SS and Fleah. Extractiona and Purification of Staphylolysin enzyme from Pseudomonas aeruginosa. Iraqi J Sci. 2013; 54(4): 1010–1
- Mohsen MS, Mahmoud MG, El Shebwy, K.; and Abd el Aziz MS. Purification and characterization of two thermostable protease fractions from Bacillus megaterium. J Gene Eng Biotech. 2013; 11(2): 103–
CrossRef - Naimah AK, Al-Manhel AJA, and Al-Shawi MJ. Isolation, purification and characterization of antimicrobial peptides produced from Saccharomyces boulardii. Inter J Peptide Res Theraa(2018) revealed that 2012:329ee manuscript was inPhD niversity of Baghdad. 2018; 24(3): 455–61.
- Turgis M, Khanh DV, Majid J, Behnoush M, and Monique L. Synergistic antimicrobial effect of combined bacteriocins against food pathogens and spoilage bacteria. Microb Res Inter. 2016; 4(1): 1–
- Micenková L, Štaudová B, Bosák J, et al. Bacteriocin-encoding genes and ExPEC virulence determinants are associated in human fecal Escherichia coli strains. BMC Microbiol. 2014; 14: 109. doi:10.1186/1471-2180-14-109
CrossRef - Holt KE, Thieu Nga TV, Thanh DP, et al. Tracking the establishment of local endemic populations of an emergent enteric pathogen. Proc Natl Acad Sci USA. 2013; 110: 17522–27. doi:10.1073/pnas.1308632110
CrossRef - Francino MP. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front. Microbiol. 2016; 6: 1543. doi:10.3389/fmicb.2015.01543
CrossRef - Field D, Seisling N, Cotter PD, Ross RP, and Hill C. Synergistic Nisin-Polymyxin combinations for the control of Pseudomonas biofilm formation. Front Microbiol. 2016: 7:1713. doi: 10.3389/fmicb.2016.01713
CrossRef - Reen FJ, Flynn S, Woods DF, et al. Bile signalling promotes chronic respiratory infections and antibiotic tolerance. Sci Rep. 2016; 6: 29768. doi: 10.1038/srep29768
CrossRef - Biswas K, Upadhayay S, Rapsang GF, and Joshi SR. Antibacterial and Synergistic Activity Against β-Lactamase-Producing Nosocomial Bacteria by Bacteriocin of LAB Isolated From Lesser Known Traditionally Fermented Products of India. HAYATI J Biosci. 2017; 24(2): 87–95. doi:10.1016/j.hjb.2017.08.008
CrossRef - Zbar NS, Al-Roubaiee MR, and Jasim HM. Synergistic effect of partially purified bacteriocin from Cronobacter sakazakii and antibiotics against staphylococcus aureus. World J Pharma Res. 2018; 7(9):1398 –1406.
- Bhola J and Bhadekar R. Invitro synergistic activity of lactic acid bacteria against multi-drug resistant staphylococci. BMC Complement Altern Med. 2019; 3: 1–
CrossRef - Al – saa’edi AH, Al – Abaadi MCH, Karhoot MJ and Funtil SA. Experimental Study the role of LasA Protease of Pseudomonas aeruginosa in the Treatment of Bacterial Keratitis Caused by Staphylococcus aureaus. J Fac Med. 2015;57(2):164–9.
CrossRef - Jose D, Jayesh P, Gopinath P,et al. A rapid two step bacterial DNA extraction method using LasA protease of Pseudomonas aeruginosa MCCB 123. Indian J Biotechnol. 2017;16(3):495–504.







