Manuscript accepted on :17-Jan-2020
Published online on: 11-02-2020
Plagiarism Check: Yes
Reviewed by: M Mohan Varma
Second Review by: Exbrayat Jean-Marie
Final Approval by: Dr. Kishore Kumar Jella
Komal Saini, Vidushi Sheokand and Priyanka Chopra
Department of Periodontology, SGT University, Gurugram
Corresponding Author E-mail : drpriyankachopra79@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1875
Abstract
One of the important action of platelets is their role in haemostasis and healing of wound. Now they are gaining popularity in Dentistry in periodontal regeneration. Earlier fibrin glue was introduced as sealant, later the platelet-rich plasma (PRP); first generation of platelet concentrates was utilized in various fields of Dermatology from chronic ulcer management to trichology and also in aesthetics. Choukroun et al. in Francein 2000’s introduced the second generation of platelet concentrates (PRF)Platelet Rich Fibrin. PRF have comparatively several advantages over traditionally prepared PRP. In this review we are focusing on why Platelet Concentrates are so important in Healing and Regeneration and we will also discuss the journey of fibrin glue from PRP to PRF, i-PRF, t-PRF, L-PRF etc.
Keywords
Platelet Concentrates; PRP; PRF; Wound Healing
Download this article as:| Copy the following to cite this article: Saini K, Chopra P, Sheokand V. Journey of Platelet Concentrates: A Review. Biomed Pharmacol J 2020;13(1). |
| Copy the following to cite this URL: Saini K, Chopra P, Sheokand V. Journey of Platelet Concentrates: A Review. Biomed Pharmacol J 2020;13(1). Available from: https://bit.ly/37mKW6s |
Indroduction
Periodontal repair and regeneration is the ultimate goal in treatment of Periodontal defects. Various biomaterials, natural as well as synthetic have been tried since many years to accelerate wound healing of both soft and hard tissue. Naturally occurring materials also known as “autologous biomaterials” are present in the body and provides signalsfor repair, regeneration and healing. Similarly, synthetically generated alloplastic materials have also shown promising result in many fields of regenerative dentistry but the drawback is that they may exhibit foreign body reaction1. One of greatest disadvantage of synthetic materials considered for regeneration is that most of them are avascular in nature, to improve the acceptance the concentration of autologous biomaterials is increased to the wound site for enhancing healing.2 It has been noted that the bone graft material combined with bloodis known to promote neo-angiogenesis, new bone formation when compared to bone grafts alone .3Two important autologous biomaterials, platelets and fibrin are believed to play important role in promoting the healing of wound and regeneration.
Platelet concentrates had travelled a long way from introduction of platelet concentrates as a source of blood proteins enriched with growth factors to promote wound healing to introduction of fibrin glue, PRP, PRF, A-PRF, t-PRF, i-PRF, PRF lysates and CGF in recent years.4 Platelet concentrates used these days are very much efficient than predecessors in terms of biological efficacy and method of preparation.
Histologically diameter of platelets varies from1 to 4 μm; they are colourless, non-nucleated with moderately refractive bodies. Precursor cell of platelet are megakaryocytes, which are extremely large hematopoietic cells present in bone marrow.These megakaryocytes fragment into the small disc shape structures called platelets either in blood stream or in bone marrow itself; From where they squeeze through capillaries. Normal concentration of the platelets in blood is between 150,000 and 300,000 per mm3.5 The average lifespan of a platelet is few days approximately 5 to 10 days. Spleen act as a reservoir for platelets, when needed released by sympathetically-induced contractions of splenic muscle.6
Role of platelets in wound healing: A proinflammatory biochemical environment of wound impairs the healing affiliated to increased protease activity, whichdecreases the concentration of variousGrowth Factors (GFs). As a rich source of Growth Factors Platelet concentrates areused as an interesting alternative for the treatment for wounds and also have mitogenic, angiogenic, and chemotactic properties.7Platelets exert its effects mainly due to three different granules namely α (alpha) granules, lysosomes and dense granules.These granules secretes the growth factors namely Platelet derived growth factor(PDGF), vascular endothelial growth factor (VEGF),Fibroblast Growth Factor (FGF), Insulin like growth factor (ILGF),transforming growth factor (TGF) and epidermal growthfactor (EGF).8PDGF accelerates the wound heling because of the effect on mitosis, angiogenesis and promote the release of other growth factors. TGF play important role in promoting chemotaxis and along with the Insulin ILGFhave role in activation of theOsteoblast. Platelets releases these growth factors within 10 min after clotting and approximately more than 95% of thesepresynthesized growth factor are released within first hour. When direct action of platelets diminish, macrophages arrives through vascular ingrowth stimulated by the platelets and then theybecome responsiblefor woundhealing regulation by secreting their own factors.9So the platelets act as pace setter for wound healing.
Chronological Evolution of Platelet Concentrates
The Journey starts from the development of platelet concentrates originating as key concept for fibrin adhesives development which were quite popular in 1970s in Europe. A list of various platelet concentrates evolved over the time is given in table 1.
Table 1: Platelet Concentrates Evolution and their Drawbacks
| Sr No | Product Name and Year | Description/Technique | Drawbacks |
| 1 | Platelet
Concentrates as fibrin glue in 1970’s10 |
Concentrated fibrinogen, factor XIII and fibronectin from donor plasma was mixed with thrombin and calcium which led to polymerization of fibrinogen | i. Risk of disease transmission due to commercially available products used in preparations |
| 2 | Autologous fibrin adhesive Tayapongsak 199411 | Blood is collected one to three weeks before procedure followed by seprating of one unit of whole blood into RBC component and plasma fraction for using as cryo precipitates thrawed 24 hours before being ready to use. | i. Technique was long and complex.
ii. The amount of concentrate obtained was quite less as compared to the amount of blood collected. iii. Autologous fibrin sealants are generally weaker and have lower resistance than commercial sealants in terms of physical stress
|
| 3 | Platelet rich
Plasma(PRP) by Whitman 199712 |
Double centrifugation of autologous blood is done consisted of a
soft spin (1300 RPM -10 minutes)followed by a hard spin (2000 RPM -10 minutes)after that PRP collected at the bottom part of the tube. |
i. Bovine thrombin which could give rise to life threatening coagulopathies in rare cases.
ii. Higher concentration of thrombin may impede the cell migration during bone healing. iii. Release of growth factors from PRP over a short period of time.
|
| 4 | Plasma rich growth
Factors (PRGF) Anitua & co-workers 199913 |
Venous blood collected in several test tubes with anticoagulant and centrifuged at 460G for 8 mins, resulted in collection of plasma rich growth factors (PRGF) at the bottom of the tube. This PRGF was then taken from the bottom of the tubes and cacl2 is added (0.05ml/
ml of PRGF). This led to coagulation in around 10 minutes and a gelatinous PRGF is obtained |
i. Incomplete activation of platelets and low levels of growth factors release.
|
|
5 |
Platelet Rich Fibrin (PRF) Choukron et al 200114 | 10 ml of blood sample without anticoagulant is centrifuged atapproximately 400 g(3000 rpm -10 min). |
i. Limited amount of PRF obtained from an autologous blood sample, the quantities produced are low. ii. The systematic utilization of PRF for general surgery is limited. |
Table 2: Advancement in Platelet Concentrates and Procedure
| Advancement in PRF Technology | Procedure |
| Advanced Platelet Rich Fibrin (A-PRF) by Ghanaati in 201430 | 1500 RPM for 14 minutes in sterile plain glass based vaccume tubes |
| Advanced Platelet Rich Fibrin+
(A-PRF+) by Fujoka- kobayashi in 201631 |
1300 RPM for 8 minutes in sterile plain glass based vaccume tubes |
| Injectable Platelet Rich Fibrin
(i-PRF) by Mourao in 201527 |
700 RPM for 3 minutes in plastic tubes |
| Titanium – platelet rich fibrin (T-PRF) Tunali and co-worker 201426 | 2800 RPM for 12 minutes in medical grade Titanium Tubes |
Platelet concentrates;concept was first started in the field of hematology.15 In 1970s the term PRP was coined to describe plasma with a platelet count many folds above that of peripheral blood count, which was earlier used to treat patients with thrombocytopeniaby transfusion products .16 Later in same era Fibrin glue was made by polymerizing fibrinogen with thrombin and calcium. Fibrin Sealants are human plasma derivatives that mimic the final stages of blood coagulation, forming a fibrin clot. It is used as topical haemostatic and tissue sealing agent. However, due to low concentration of fibrinogen in donor plasma, the quality and stability of fibrin glue was suboptimal and these products were associated with a risk of cross infection.
Numerous research works were continued in 1975-1978 which suggested an enhanced healing concept by using blood extracts and called them as plateletfibrinogenthrombin mixtures. During this time period various author designated those products as “GelatinPlatelet Gel Foam”.17In 1986 Knighton et al18was the first to demonstrate that platelet concentrate successfully promote healing and termed it as “Platelet Derived Wound Healing Factors (PDWHF)”, which were successfully used initially for thetreatment ofulcers of skin. Whitman first introduced the Platelet Rich plasma (PRP) in field of oral and maxillofacial surgery.Through the activation of the platelets within the gel there is resultant release of thegrowth factors from secretory granules resulting in enhanced wound healing .12
PRP produced by double centrifugation process consisted of a soft spin at 1300 RPM for 10 minutes followed by which the blood would separate into red corpuscular base, buffy coat and the platelet poor plasma.The last two components were aspirated and re centrifuged at hard 2000 RPM for 10 minutes after which PRP was collected in the bottom of the tube (Figure 1). PRP gained popularity in sinus lifting procedures, Augmentation of Alveolar Ridge, Preservation of Socket, Repair of Alveolar cleft Palate, Oral/nasal fistula repair, Intra bony defects, Reconstruction surgeries of jaws, various Soft tissue procedures likegingival grafts, Connective tissue grafts etc. due to its property of accelerating the healing of soft tissues.19
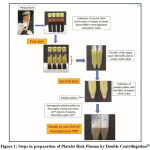 |
Figure 1: Steps in preparation of Platelet Rich Plasma by Double Centrifugation28 |
In 1999 at the same time period,Anitua et al formulated another platelet concentrate utilizing anticoagulants and coined the term Platelet Rich Growth Factor (PRGF). These platelet concentrates were no longer in practice today because of lack of the specific pipetting steps, ergonomy of kit and the significant issues were present with this technique.13Vivostat PRF (Alleroed, Denmark) a widely accepted technique for production of P-PRP was commercialized. However, it is misnamed as it is not a true PRF producing kit instead produces a PRP product. All the products were named as Platelet rich plasma (PRP) or also known as first generation of platelet concentrate.20
Platelet Rich Gel (PRG) proposed to be more active than PRP by Cieslik-Bielecka21 and they defined it as fibrin matrix rich in platelets, leukocytes and relative active molecule which is biologicallymore activated. Sacco22 in 2006 give a new concept of concentrated growth factors. Preparation of concentrated growth factor required venous blood with rpm in range of 2400-2700. Sacco’s membrane shows a complex three-dimensional architecture which makes it’s a biomaterial rich in fibrin, platelets, leukocytes and growth factors very suitable for both patients and doctor. One of the complication associated with PRP handling is its liquid nature, which reduce its application as it required in combination with other biomaterials. The clinical benefits with PRP is limited because of short release of growth factors.Due to this limitation second-generation platelet concentrate termed PRF have been emerged.In 2000Choukroun et al14 introducedPlatelet rich fibrin labelled as PRF, based on the strong fibrin gel polymerization which is another form of Platelet concentrate in France. This is an important turnaround in the evolution of terminology.
According to Everts et al23platelet concentrates has been found in two forms, which include nonactivated and activated forms(on the basis of leukocyte component). The inactivated form was called as “platelet-leukocyte rich plasma (P-LRP) and the activated form was called platelet-leukocyte-gel” (PLG). In 2010by Sohn24concept of sticky bone was introduced in which autologous fibrin glue was mixed with bone graft. Later on,APRF (advanced PRF) introducedin 2014 by Choukroun et alwhich contain more monocytes.25Later Titanium prepared PRF (T-PRF) by Tunali et al26 introduced. In 2015 Mourao et al27 gave detailed infotmation on preparation of i-PRF (injectable PRF).
Protocol of PRF Preparations and its Advancement
Dr. Joseph Choukroun the research pioneer has led to the development of a second-generation platelet concentrate, in this anticoagulation factors have not been utilized. A platelet concentrate without coagulation factors can be gathered (750 g) from the superficial layer of centrifugation tubes following single centrifugation cycle (2,700 rpm,12 minutes). This formulation was called Platelet rich fibrin and it contained a fibrin matrix after centrifugation. The composition of PRF include concentrate of white blood cells, platelets, and fibrin. It has been shown that the initially developed Platelet rich fibrin (also termed L-PRF) composed of 97% platelets and more than 50% leukocytes in a high-density fibrin network when compared to whole blood.28
The major drawback of PRF is its preparation along with storage. The factor affecting the potential benefit of PRF includes its quick handling between the blood collection and the centrifugation as PRF preparation does not include any anticoagulants. One of the other important benefit include the dehydration which cause decreased growth factor content in PRF and leukocyte viability will be adversely affected altered its biologic properties. Platelet rich fibrin is obtained as a gel form which is not conducive to be injected. To overcome these limitations several modifications are done and newer forms of PRF were introduced.29
Later the advanced PRF matrices in solid form was developed having the (LSCC) Low Speed Centrifugation Concept. This improved preparation protocol for advanced PRF (A-PRF) is reducing the applied RCF to 208 g. The structure of advanced fibrin clot is a more porous with a larger interfibrous space when compared to PRF. For formation of A-PRF, slower speed (1500 rpm) and more time (14 mins) is used in the sterile plain glass-based vacuum tubes.30
A-PRF shows more neutrophilic granules in distal part mostly at red blood cells-buffer coat interface. Another modification of A-PRF has been suggested by Kobayashi & co-workers in 2016 where they have reduced the centrifugation time to 1300 rpm for 8 minutes. They called this modification as A-PRF+ and suggested that less time would result in a decrease in the amount of forces that the cells of the blood would be exposed to & hence, would increase the number of cells contained in the PRF matrix.31
The PRF so obtained is in gel form which is not conducive to be injected. To overcome this limitation i-PRF is introduced. For producing i-PRF, blood is drawn without anticoagulant in plastic tubes without any coatings and centrifuged at around 700 for 3 minutes.27 The time is considerably shorter than other two protocols (i.e. L-PRF & A-PRF). This can be attributed to the fact that for i-PRF only the separation of blood components is desired which happens in initial 2-4 minutes. Plastic tubes are used for this process as it have a hydrophobic surface and do not efficiently activate the coagulation process. Hence, all the blood components that are required to form a good platelet concentrate (plasma containing all clotting factors & platelets) reach the top of the tube under the centrifugation force in the first 2-4 minutes. Currently, it has been used for mixing with bone grafts, which forms a gel-putty consistency with the graft particles incorporated in the graft. The graft thus formed has a good workable consistency, leads to decreased leaching of the graft as it is tightly encapsulated in the fibrin matrix.32
Another modification of PRF is T-PRF i.e. Titanium -PRF, obtained by centrifugation of blood at 2800 RPM for 12 minutes in Titanium tubes. T-PRF provide more tightly woven and thicker fibrin than classic L-PRF, as has titanium better hemocompatibility compared to glass and potentially led to the formation of a more polymerized fibrin.26
Conclusion
Platelet concentrates has been used in various application of dentistry since many years. Technological advancement in this field shows promising result in use of platelet rich fibrin (PRF) in Periodontal regeneration. Various studies have been conducted to determine the utilization of PRF in various procedures i.e periodontology, oral surgery, and implant dentistry and encouraging results obtained in both soft and hard tissue regeneration. Various factors like speed, duration of centrifuge, temperature, blood haematocrit influence the quality of fibrin scaffold. Lastly, the prominent role of leukocytes or fibrin in PRF scaffolds is discussed as potential avenues for future research.
Acknowledgement
I express my profound sense of gratitude and sincere regards to Dr.Priyanka Chopra and Dr.Vidushi Sheokand for their meticulous supervision, valuable guidance, encouragement, and critical appreciation. I would also like to thank my husband Maj (Dr) Sachin Saini, my little daughter Vedika and all family members for their unconditional love and support during this work.
Financial support and sponsorship
Nill
Conflict of Interest
I declared that there is no conflict of interest with regard to publication of my research work.
References
- ZhuW, Ma X, GouM, Mei D, Zhang K, Chen S. 3D printing of functional biomaterials for tissue engineering. Current opinion in biotechnology. 2016;40:103–12
CrossRef - WikesjoUME, Selvig K. Periodontal wound healing and regeneration; Periodontol 2000 1999;19:19-31.
CrossRef - BarbeckM, Najman S, Stojanovic S, Mitic Z, Zivkovic JM, Choukroun J, et al. Addition of blood to a phycogenic bone substitute leads to increased in vivo vascularization. Biomedical materials (Bristol, England). 2015;10(5):055007.
CrossRef - Agarwal AA. Evolution current status and evidences in application of platelet Concentrates in periodontology and implantology.World J Clin Cases 2017;5:159-71.
CrossRef - Jameson CA. Autologus Platelet Concentrates for the production of platelet gel. Lab med 2007;38:39-42.
CrossRef - Guyton AC, Hall JE. Physiology of Blood: Platelets. Textbook of medical physiology. 2005;5:345-56.
- Montero CE, Alves R, Grimalt R (eds). PRP in wound healing: Clinical Indications and Treatment Protocols with Platelet-Rich Plasma in Dermatology. Barcelona, Ediciones Mayo, 2016, pp 59-72.
- Ghoshal K, Bhattacharya M. Overview of Platelet Physiology: Its Hemostatic and Nonhemostatic Role in Disease Pathogenesis. Scientific World J 2014;3:1-16.
CrossRef - Kaur P, Kumar P, Dahiya V. Platelet rich plasma: a novel bioengineering concept. Trends Biomater 2011;25(2):86-90.
- Chhabra S, Chhabra N, Vaid P. Platelet concentrates: Anew alternative to Bone Regenration. Int j Exp Dent Sci 2013;2:118-21.
CrossRef - Tayapongsak P, O’ Brien DA, Monteiro CB, Arceo-Diaz LY. Autologus fibrin adhesive in mandibular reconstruction with particulate cancellous bone marrow. J Oral MaxillofacSurg 1994;52(2):161-5.
CrossRef - Whitman DH, Berry RL, Green DM. Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg1997;55:1294-9.
CrossRef - Anitua E. Plasma rich in growth factors: Preliminary results of use in preparations of future sites for implants.Int J Oral Maxillofac Implants1999;14:529-35.
- Choukroun J, Adda F, Schoeffer C, Vervelle A. PRF: An opportunity in perio implantology. Implantodontie 2001;42:55-62.
- Andia I, Abate M. Platelet rich plasma: underlying biology and clinical correlates. Regen Med 2013;8:645-58.
CrossRef - Andia I: Platelet-rich plasma biology; in Alves R, Grimalt R (eds): Clinical Indications and Treatment Protocols with Platelet-Rich Plasma in Dermatology. Barcelona, Ediciones Mayo 2016:3;3-15..
- Rosenthal AR, Egbert PR, Harbury C, Hopkins JL, Rubenstein E. Use of platelet-fibrinogen-thrombin mixture to seal experimental penetrating corneal wounds. Albrecht Von Graefes Arch Klin Exp Ophthalmol 1978;207:111-5.
CrossRef - Knighton DR, Ciresi KF, Fiegel VD, Austin LL, Butler EL. Classification and treatment of chronic nonhealing wounds. Successful treatment with autologous platelet-derived wound healing factors (PDWHF). Ann Surg 1986;204:322-30.
CrossRef - Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral RadiolEndod 1998;85:638-46.
CrossRef - Marx RE. Platelet-Rich Plasma (PRP): What Is PRP and What Is NotPRP? Implant Dent 2001;1:104-10.
CrossRef - Cieslik-Bielecka A, Gazdzik TS, Bielecki TM, Cieslik T. Why the platelet-rich gel has antimicrobial activity? Oral Surg Oral Med Oral Pathol Oral RadiolEndod 2007;103:303-5
CrossRef - Sacco L. Lecture, International academy of implant prosthesis and osteoconnection. Lecture 2006.
- Everts PA, Hoffmann J, Weibrich G, Mahoney CB, Schönberger JP, van Zundert A, Knape JT. Differences in platelet growth factor release and leucocyte kinetics during autologous platelet gel formation. Transfus Med 2006;16:363-8.
CrossRef - Sohn DS. Lecture Entitled with Sinus and Ridge Augmentation with CGF and AFG. Symposium on CGF and AFG 2010.
- Choukroun J. Advanced PRF and i-PRF: Platelet concentrate or blood concentrate? J Periodontal Med Clin Pract 2014;1:1-4.
- Tunalı M, Ozdemir H, Kucukodacı Z, Akman S, Fıratlı E. In vivo evaluation of titanium-prepared platelet-rich fibrin (T-PRF): a new platelet concentrate. Br J Oral MaxillofacSurg 2013;51:438-43.
CrossRef - Mourao CF, Valiense H, Melo ER, Mourão NB, Maia MD. Obtention of injectable platelets rich-fibrin (i-PRF) and its polymerization with bone graft: technical note. Rev Col Bras Cir 2015;42:421-3.
CrossRef - Dhurat R, Sukesh M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. J Cutan Aesthetic Surg. 2014;7:189-97.
CrossRef - Chandran P, Sivadas A. Platelet-rich fibrin: Its role in periodontal regeneration. J Dent Res 2014;5:117–22.
CrossRef - Ghanatti S, BoomsP,Orlowska A, Kubesch A, Lorenz J, Rutkowski J et al. Advanced platelet rich fibrin a new concept of cell based tissue engineering by means of inflammatory cells. J Oral Implantol 2014;6:679-89.
CrossRef - Kobayashi E, Fluckiger L, Fujioka-Kobayashi M, Sawada K, Sculean A,Schaller B, et al. Comparative release of growth factors from PRP, PRF,and advanced-PRF+. Clin Oral Investig 2016;2:23-30.
CrossRef - Shah R. An Update on the Protocols and Biologic Actions of Platelet Rich Fibrin in Dentistry. European J Prosthodont Restorative Dent2017;25:64–72.







