Shilpa Shiragannavar* and Shivakumar Madagi
and Shivakumar Madagi
Karnataka State Akkamahadevi Women’s University, Vijayapura
Corresponding Author E-mail : shilpa.shiragannavar@gmail.comDOI : https://dx.doi.org/10.13005/bpj/1906
Abstract
Mycoplasma hominis is a gram negative bacteria belonging to class Mollicutes, commonly present in men and women of reproductive age. In women it affects the genital tract and is involved in causing Pelvic Inflammatory Disease, ectopic pregnancy, miscarriage, epididymitis in men and sometimes prolonged infection may lead to infertility. At present there is no effective prophylaxis for M.hominis infections. The current work involves insilico reverse vaccinology approach for identifying the immunogens as vaccine candidates that can be effective against reinfections and should be capable of inducing long-term protective immunity against Mycoplasma infections. The study identifies the putative vaccine candidates that are membrane bound with high antigenicity properties which involves identification of T-cell and B-cell epitopes that induce humoral immunity as well as cell-mediated immunity and makes the body stronger against infections and effective for reinfections. The epitope ‘STNYYNLYF’ showed good binding interactions 9.80Kcal/mol with HLA-C*05:01 and maximum population coverage.
Keywords
Antigenicity; Docking; In-Silico; Mycoplasma Hominis ; Vaccine
Download this article as:| Copy the following to cite this article: Shiragannavar S, Madagi S. In Silico Vaccine Design against Mycoplasma hominis Infections. Biomed Pharmacol J 2020;13(1). |
| Copy the following to cite this URL: Shiragannavar S, Madagi S. In Silico Vaccine Design against Mycoplasma hominis Infections. Biomed Pharmacol J 2020;13(1). Available from: https://bit.ly/3bytDBJ |
Introduction
Mycoplasma hominis is a gram-negative bacterium often found in genito-vaginal tracts of women and in sexually active adult males [1]. The human pathogen is transmitted by direct contact during the intercourse. The pathogen can even be transmitted to offspring either during birth or in uterus [2]. Mycoplasma pathogenicity in genital tract of females was confimed in women with postpartum fevers and intra-amniotic infection [3]. The symptoms of the bacterium involve vaginal discharge, genital warts and other inflammatory responses [4].
The epidemiology of the pathogen is associated with colonization in the genitourinary tract and is seen in the patients of young age who are sexually active. The clinical manifestations of the pathogen infection include Pelvic Inflammatory Disease, cervicitis, urethritis, post-partum fever, still birth, brain abscess, pyelonephritis [5]. Pelvic inflammatory disease has minimal symptoms like fever, pelvic and abdominal pain and tenderness of the tissues of uterine, cervix and adnexal [6]. Cervicitis and urethritis lead to vaginal discharge in women and urethral discharge in men and irritation during the urine pass. In men it causes epididymitis, swelling of epididymis underlying the testis within the scrotum [7].
Commonly M.hominis is associated with the other pathogen U. urealyticum [8]. Research confirm the association of the two pathogens with male infertility [9]. Number of studies have reported the involvement of M.hominis in changing parameters of semen such as density and motility of spermatozoa [10]. The current treatment options include commonly prescribed antibiotics, and very few are effective against the bacterial infections. The intracellular pathogen has found to be resistant to potent antibiotics such as ciprofloxacin, macrolides and ofloxacin [11].
Although there are antibiotics available, the mycoplasma vaccine will dramatically reduce the rates of mycoplasma infections hence, mycoplasma vaccine could be more effective in controlling the epidemics of the infections [12, 13]. Unfortunately, in spite of many attempts yet there is no protective vaccine either fully or partially available for the infections [14]. A computational reverse vaccinology technique predicts the surface epitopes which are important in development of a candidate vaccine. The technique identifies B-cell and T-cell lymphocytes that are important in inducing immune responses which activate the humoral and cell-mediated immunity [15]. B-cells identify solvent exposed antigens using B-cell receptors. B-cells upon activation differentiate and secret soluble immunoglobins that mediate adaptive humoral immunity [16]. T-cell epitope prediction identifies short peptides in the antigenic sequences. A peptide binding to the MHC molecules is most important as this determines the selection of T-cell epitopes that can stimulate CD8 or CD4 T-cells [17].
In the present study, an attempt is made to recognize major immunogenic epitopes on Mycoplasma hominis proteins that can be vaccine candidates by using various bioinformatics tools. The results from the study offer novel epitope-vaccine candidates for development of vaccine against Mycoplasma hominis.
Materials and Methods
Retrieval of All the Proteins of Mycoplasma hominis
In the study 529 protein sequence set of M. hominis were retrieved from UniProt Proteome [18] database in FASTA format. www.uniprot.org [The Uniprot Consortium, nucleic Acids Research].
Screening of Proteins Based on Antigenicity, Sub-Cellular Localization and Allergy Study
All the proteins were virtually screened using VaxiJen v2.0 [19] for antigenicity as it induces immune responses. Only the antigenic proteins were considered for further studies. M. hominis lacks cell wall hence the sub-cellular localization prediction for proteins was done using PSORTb 2.0 [20]. Allertop [21] was used to evaluate the allergenicity of proteins. The tool makes predictions based on the physicochemical properties of proteins along with allergen prediction utilizing E-descriptors of amino acids in the protein using several machine learning tools [22]. Allergenic proteins were excluded and the non-allergic proteins were considered for further analysis.
Physicochemical and Functional Analysis of Proteins
Protein structural and functional analysis are important to know their role in the organism’s survival. The physic-chemical and functional analysis of membrane bound, antigenic, non-allergen proteins were studied using ProtParam [23] and Interpro [24]. The parameters like molecular weight, isoelectric point, half-life in vitro and in-vivo, stability, aliphatic index and grand average of hydropathicity (GRAVY) were estimated with ProtParam. Regions of conserved domains and other important sites within the proteins was identified using InterPro.
T-Cell Epitope Identification, Epitope Conservancy Analysis and Population Coverage
Epitopes based on conserved regions were identified from Cytotoxic T lymphocytes (CTL) using NetCTL v1.2 [25]. Prior to the run the peptide length was set to 9.0 and the threshold was fixed at 0.5 while the sensitivity and specificity were set at 0.89 and 0.94. To initiate the immune response the binding of the antigenic peptide to the major histocompatibility complex MHC class I molecules is important. Hence the binding of epitopes to the MHCI analysis was done using immune epitope database IEDB tools [26]. The tool calculates the half maximal inhibitory concentration value (IC50) of the binding epitope to human leukocyte antigen (HLA) molecules. The tool predicts the binding of MHC class I peptides to 12 different HLA supertypes using the stabilized matrix base method [27]. The conservancy of each epitope was also calculated using the conservancy analysis tools at IEDB [28]. The stabilized matrix base method (SMM) tool at IEDB was used to calculate the total score which includes the parameters like processing score, TAP score, proteasomal cleavage score and binding affinity for the MHC-I [29]. Population coverage generally have a crucial role in vaccine design which was calculated using IEDB population coverage tool in this study [30].
Molecular Docking Studies
The 3D structures of epitopes were generated by PEP-FOLD 2.0 web-based server [31]. The server predicts the five most probable structures and the best structure was taken for docking analysis i.e. the lowest energy model. The HLA-C*05:01 allele showed highest binding scores among all the other MHC alleles. Epitope binding evaluation and validation with HLA molecule was done by downloading the structure of HLA-C*05:01 from PDB with id 4VGD. Before performing the docking, the ligand SAE with nine amino acids SER-ALA-GLU-PRO-VAL-PRO-LEU-GLN-LEU epitope [32] was removed using UCSF Chimera [33]. Further the docking was performed with AutoDock Vina to perform the sampling and scoring at each docking round [34].
B-Cell Epitope Identification
B-cell epitopes were identified by using various tools from IEDB. The Bepipred linear epitope prediction analysis [35], Emini surface accessibility prediction [36], Kolaskar and Tongaonkar antigenicity prediction scale [37], Karplus and Schulz flexibility prediction [38], Parker Hydrophilicity Prediction [39] and Chou Fasman [40] beta turn prediction tools were used.
Results
The present investigation focused on predicting the vaccine candidates for Mycoplasma infections by computational reverse vaccinology approach for which a total of 529 protein sequences of Mycoplasma hominis were collected from the UniProt Proteome database in FASTA format. These protein sequences were analysed for their antigenic properties that potentially induce immunogenicity.
Antigenic Protein Identification, Sub-Cellular Localization and Allergy Prediction
Protein screening was done using Vaxijen server which identifies the antigenic and non-antigenic proteins. 0.5 was kept as threshold and the proteins with antigenic score > 0.5 were considered for future analysis. Out of all 529 proteins, 161 proteins were found to be probable non-antigens. Further screening of proteins was done based on the localization of the proteins. PSORTb 2.0 was used to predict the sub-cellular localization of the proteins. 49 proteins out of 368 antigenic proteins were found to be outer membrane proteins which were considered for further analysis. Final protein screening was done based on their probable allergenic properties. Allertop was used for the allergenicity analysis of outer membrane proteins. 8 proteins were outer membrane, antigenic and non-allergenic. These 8 proteins then were subjected to epitope-based analysis.
Protein Physical-Chemical and Functional Analysis
The parameters like molecular weight, theoretical pI, number of amino acids, amino acid composition, atomic composition, extinction coefficient, estimated half-life, aliphatic index, instability index, and grand average of hydropathicity (GRAVY) were obtained from the ProtParam and the functional analysis of proteins was done using InterPro by classifying them into families, predicting domains and other important sites. Two to five conserved domains were observed in the proteins. The table 1 below gives the details of the protein parameters.
Table 1: ProtParam results for the vaccine candidate proteins.
| Protein | Number of amino acids | Molecular weight | Theoretical pI | Extinction coefficients | Estimated half-life | Instability index | Aliphatic index | (GRAVY) |
| Uncharacterized protein | 462 | 54440.09 | 9.06 | 111620 | 30 hours | 25.35 | 74.46 | -0.601 |
| Putative lipoprotein | 577 | 66599.78 | 9.10 | 80110 | 30 hours | 30.03 | 85.30 | -0.436 |
| p120′ protein | 915 | 103639.4 | 5.69 | 143480 | 30 hours | 24.95 | 77.11 | -0.597 |
| Lipoprotein | 808 | 92138.75 | 8.67 | 94090 | 30 hours | 32.58 | 66.05 | -0.857 |
Identification of T-Cell Epitopes and Conservancy Analysis
The 8 proteins were then subjected for NetCTL server that gives the combinatorial score based on MHC-1 binding predictions. the epitopes with IC50 value less than 200 nM were considered for further analysis as this ensures higher affinity for MHC-1 binding. The selected epitope list is given in the table 2.
Table 2: T-cell epitopes identified by the NetCTL server with scores.
| Sl. No. | Epitope | Total Score(nM) |
| 1. | CSTNYYNLY | 3.4358 |
| 2. | FSDDQVKKY | 3.1598 |
| 3. | ETESGKTIY | 2.8097 |
| 4. | FVEKENHKY | 2.7962 |
| 5. | QVEAIANFY | 2.7589 |
| 6. | YTDCVELMH | 2.7527 |
| 7. | WTNSDYRFY | 2.7022 |
| 8. | DTEQGLCHY | 2.678 |
| 9. | FINKSNLDY | 2.5952 |
| 10. | STNYYNLYF | 2.4732 |
| 11. | KSEFVKLGY | 2.4668 |
| 12. | GIDNLSRLY | 2.4439 |
| 13. | NSSDFHKKY | 2.3327 |
| 14. | YSLWTNSDY | 2.3292 |
| 15. | LTAEGKAKY | 2.2817 |
| 16. | NSDFKTALF | 2.2382 |
| 17. | FLELEIFKY | 2.2258 |
| 18. | DSSIAKDFY | 2.213 |
| 19. | KTNLSAYGY | 2.0663 |
These identified peptides were further predicted for effective designing of the T-cell epitopes. The methods like Proteasomal cleavage, TAP score, MHC-I processing and binding affinity scores were from IEDB were considered for T-cell epitope analysis. Higher the score, higher the MHC-I processing capabilities hence, the peptides with higher score are probable T-cell epitopes. All the 19 epitopes were subjected to MHC-I binding predictions using the stabilized matrix base method. The epitopes that showed higher affinity i.e IC50 <200 nM was considered for further analysis 4 out of 19 epitopes were selected based on their IC50 values. The epitope conservancy was performed, higher the conservancy the more the immunogenic are the proteins.
Table 3: Interaction, binding, and conservancy scores of the identified T-cell epitopes.
| Epitope | MHC-I allele interaction total score (proteasome score, TAP score, MHC-I score, processing score) | Epitope conservancy (%) |
| FSDDQVKKY | HLA-C*12:03(4.945) HLA-C*05:01(14.476)
HLA-C*07:01(44.07) HLA-A*01:01(64.35) HLA-B*15:02(89.58) HLA-C*06:02(127.00) HLA-C*08:02(131.87) HLA-C*14:02(163.9) |
62.58% |
| STNYYNLYF | HLA-C*05:01(11.18) HLA-C*14:02(106.0)
HLA-C*12:03(117.54) HLA-C*15:02(123.7) HLA-B*15:02(125.38) HLA-*32:01(129.10) HLA-B*58:01(155.34) |
50.00% |
| KTNLSAYGY | HLA-A*12:03(28.85) HLA-A*30:02(28.93)
HLA-C*05:01(31.67) HLA-B*58:01(91.26) HLA-C*15:02(103.37) HLA-A*29:02(114.48) HLA-C*32:01(172.16) |
75.00% |
|
WTNSDYRFY |
HLA-A*12:03(29.80) HLA-A*03:03(38.60)
HLA-C*05:01(50.66) HLA-A*29:02(102.50) HLA-A*30:02(123.15) HLA-C*07:02(173.48) |
50.00% |
Population Coverage
Each epitope that were recognized as optimum for the MHC-I binders were then subjected for the population coverage analysis. The epitopes showed 91.17% coverage in Europe. The population coverage analysis in other area are tabulated in the table 4.
Table 4: Population coverage analysis for the proposed epitopes against Mycoplasma hominis.
| Population | Coverage (%)A | Average hitB | PC90C |
| Central Africa | 55.05% | 3.56 | 1.11 |
| East Africa | 61.77% | 4.12 | 1.31 |
| East Asia | 48.85% | 3.05 | 0.98 |
| Europe | 91.17% | 8.28 | 5.16 |
| India | 63.33% | 4.43 | 1.36 |
| North Africa | 70.41% | 5.09 | 1.69 |
| North America | 77.36% | 5.76 | 2.21 |
| Northeast Asia | 56.38% | 3.76 | 1.15 |
| Oceania | 51.89% | 3.33 | 1.04 |
| South Africa | 62.41% | 4.37 | 1.33 |
| South America | 51.15% | 3.16 | 1.02 |
| South Asia | 65.33% | 4.64 | 1.44 |
| Southeast Asia | 51.75% | 3.31 | 1.04 |
| Southwest Asia | 70.60% | 5.13 | 1.7 |
| West Africa | 64.29% | 4.44 | 1.4 |
| West Indies | 72.40% | 5.07 | 1.79 |
A) Projected population coverage. B) Average number of epitope hits/HLA combinations recognized by the population. C) Minimum number of epitope hits/HLA combinations recognized by 90% of the population.
Molecular Docking Studies
To validate the epitopic potential of the identifed peptides molecules, molecular docking studies was performed. As all the selected epitopes showed good binding affinity with allele HLA-C*05:01, its structure was downloaded from PDB with ID 4VGD. The epitope structures were predicted by PEPFold and the molecular docking was performed for the HLA-C*05:01against the predicted epitopes as ligands using Autodock vina. The XYZ coordinates were set to x=7.1, y=85.5, z=14.5. The binding energies and the RMSD values for the epitopes is given in the table 5.
Table 5: Binding energy and RMSD values and vaxijen scores for the epitopes with the HLA-C*05:01
| Epitope | Binding energy (kcal/mol) | RMSD Ao | Vaxijen score |
| FSDDQVKKY | -8.30 | 0.00 | 0.5772 |
| STNYYNLYF | -9.80 | 0.00 | 1.3297 |
| KTNLSAYGY | -9.40 | 0.00 | 0.5027 |
| WTNSDYRFY | -9.80 | 0.00 | 0.6230 |
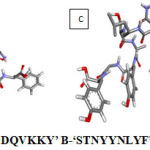 |
Figure 1: Structures of epitopes A-‘FSDDQVKKY’ B-‘ STNYYNLYF’ C-‘ KTNLSAYGY’ D-‘ WTNSDYRFY’ |
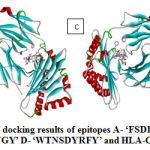 |
Figure 2: Visualization of docking results of epitopes A- ‘FSDDQVKKY’ B- ‘STNYYNLYF’ |
B-Cell Epitope Prediction
The protein epitopes were further evaluated for B cell specific epitope nature by tools in IEDB. To evaluate the physicochemical properties of the amino acids in the protein to be as B cell epitopes was performed by Kolaskar and Tongaonkar antigenicity prediction tool. The peptides FSDDQVKKY, STNYYNLYF, KTNLSAYGY and WTNSDYRFY showed acceptable score ranging from 1.023, 0.983, 0.976, 1.015 and 0.960 respectively. The Emini surface accessibility prediction was carried out for assessing the surface accessibility of the epitopes. The Emini surface accessibility prediction scores for the selected peptides was ranging from 0.967 to 2.261 indicating good surface accessibility. Parker hydrophilicity prediction tool was utilized to check the hydrophilic regions of the proteins. Identification of surface accessible hydrophilic region is essential as these regions are likely to evoke the B cell immune responses. Results indicated that the selected peptides are hydrophilic.
Studies show the correlation between the localization of antigenic sites and the presence of turns in proteins hence, Beta-turns prediction in proteins was performed as these regions are involved in initiating antigenic properties [41]. Chou-Fasman beta-turn prediction was performed to find the beta-turn regions in the proteins. Karplus Schulz flexibility prediction tool was used to know the flexible regions in the proteins. The regions of the peptide locations were considerably in the most favourable regions of the flexibility prediction analysis. The peptides showed good flexibility scores ranging from 0.983 to 1.009. Further the linear B-cell epitopes were predicted using Bepipred, hidden Markov model-based machine learning process. All most all the regions in the proteins showed most favourable regions in Bepipred linear epitope predictions. By analysing the results obtained from the B-cell epitope prediction tools, the peptides that were selected as T-cell epitopes were found to satisfy requisites required for inducing B-cell immune responses. The results of B-cell epitope analysis using different tools is shown in Figure 3.
 |
Figure 3: B-cell epitope epitope prediction for the protein. |
Discussion
Antigenic proteins are important in deciding the virulence of the pathogen invasion hence more antigenic proteins that are membrane bound were considered for the study. A distinctive T & B-cell vaccination initiates both humoral and cell-mediated immunity effective on controlling the reinfection of the pathogen. Epitope based vaccine design approach has been reported for Francisella tularensis [42], Haemophilus influenza [43], Dengue virus [44], human coronaviruses [45], chikungunya virus [46], Ebola [47].
The proteome of Mycoplasma hominis was retrieved from UniProt Proteome Database. The proteins were further analysed for antigenic properties using vaxijen, the antigenic proteins with antigenic score > 0.5 were then subjected for localization prediction using Psortb. The outer membrane proteins with higher antigenicity scores were then evaluated for allergic properties using Allertop. The conserved domain analysis was done using InterPro. The membrane bound proteins with higher antigenic scores, non-allergens with maximum conserved domains were selected for further immunoinformatic study. The Proteins putative lipoprotein, Membrane protein, Uncharacterized protein, Serine/threonine-protein kinase, Bacteriophage MHoV1 protein HtpH, p120′ protein and Lipoprotein were the proteins finally considered for further analysis. The proteins were subjected to NetCTL T-cell epitope analysis around 19 epitopes were selected from the proteins based on IC50 values. Peptides with highest scores have highest processing capabilities.
An effective peptide epitope should be conserved among the host proteins, good processing capabilities and binding affinities with the MHC alleles with higher population coverage. Four peptides were selected from 19 epitopes based on the epitope conservancy, and binding with the MHC alleles. All the four epitopes showed good conservancy and interactions with the HLA alleles. However, the epitope STNYYNLYF showed maximum antigenicity score 1.3297 and interacting with seven HLA variants. Further population coverage analysis showed maximum coverage in most regions. It calculates the percentage of people showing potential responses to the query epitopes among the people living in that region. The identified epitopes were then subjected for molecular docking analysis and the results showed good binding interactions with the HLA-C allele. The epitope STNYYNLYF showed the lowest binding energy of -9.80 Kcal/mol and RMSD 0.00Ao. B cell epitope identification was also performed using various tools of IEBD, these epitopes induce primary and secondary immunity. After analysing the results, it was found that the selected T-cell epitopes satisfied the potential to B-cell epitopes also.
Conclusion
The untreated M.hominis infection have a negative impact on the reproductive health of human. In the present study, we made an attempt in designing a peptide-based vaccine for the pathogen Mycoplasma homins which causes infertility in both men and women. Through in silico approach using bioinformatics tools we identified novel therapeutic epitope vaccine candidates. Both T-cell and B-cell epitopes are identified that can offer long term immunity. The identified peptides show both B and T-cell selectivity, wide range of population coverage, good epitope conservancy and significant binding interactions with the MHC-1 HLA allele. The predicted epitopes are supposed to offer protective immunity for long term against M.hominis. However, in-vitro studies have to be performed in order to validate the predicted vaccine candidates.
Conflıct of Interest
The authors Shilpa Shiragannavar, Shivakumar Madagi declare that they have no conflict of interest.
Acknowledgments
The authors thank DBT-BIF Center, Karnataka State Akkamahadevi Women’s University, Vijayapura for providing a platform for the research work.
Funding Source
There are no any financial assistance
Research Involving Human Participants and/or Animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
The article does not contain any studies in patients by any of the authors.
References
- Nagata Y, Iwasaka T, & Wada T. Mycoplasma infection and infertility. Fertility and sterility, 31(4), 392-395. (1979).
- Arya OP, Tong C Y W, Hart C.A, Pratt B.C, Hughes S, Roberts P, & Goddard A D. Is Mycoplasma hominis a vaginal pathogen? Sexually transmitted infections, 77(1), 58-62. (2001).
- Cunningham, S. A., Mandrekar, J. N., Rosenblatt, J. E., & Patel, R. Rapid PCR Detection of Mycoplasma hominis, Ureaplasma urealyticum, and Ureaplasma parvum. International journal of bacteriology. (2013).
- Horner, P., Donders, G., Cusini, M., Gomberg, M., Jensen, J. S., & Unemo, M. Should we be testing for urogenital Mycoplasma hominis, Ureaplasma parvum and Ureaplasma urealyticum in men and women? –a position statement from the European STI Guidelines Editorial Board. Journal of the European Academy of Dermatology and Venereology, 32(11), 1845-1851. (2018).
- Bradshaw, C. S., Jensen, J. S., Tabrizi, S. N., Read, T. R., Garland, S. M., Hopkins, C. A., & Fairley, C. K. Azithromycin failure in Mycoplasma genitalium urethritis. Emerging infectious diseases, 12(7), 1149. (2006).
- Gdoura, R., Kchaou, W., Ammar‐Keskes, L., Chakroun, N., Sellemi, A., Znazen, A & Hammami, A. Assessment of Chlamydia trachomatis, Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis, and Mycoplasma genitalium in semen and first void urine specimens of asymptomatic male partners of infertile couples. Journal of andrology, 29(2), 198-206. (2008).
- Huang, C., Zhu, H. L., Xu, K. R., Wang, S. Y., Fan, L. Q., & Zhu, W. B. Mycoplasma and ureaplasma infection and male infertility: a systematic review and meta‐ Andrology, 3(5), 809-816. (2015).
- Taylor‐Robinson, D., & Lamont, R. F. Mycoplasmas in pregnancy. BJOG: An International Journal of Obstetrics & Gynaecology, 118(2), 164-174. (2011).
- Liu, J., Wang, Q., Ji, X., Guo, S., Dai, Y., Zhang, Z & Lee, Y. Prevalence of Ureaplasma urealyticum, Mycoplasma hominis, Chlamydia trachomatis infections, and semen quality in infertile and fertile men in China. Urology, 83(4), 795-799. (2014).
- Kılıç, D., Başar, M. M., Kaygusuz, S., Yılmaz, E., Başar, H., & Batislam, E. Prevalence and Treatment of Chlamydia trachomatis, Ureaplasma urealyticum and Mycoplasma hominis in patients with Nongonococcal Urethritis. Jpn J Infect Dis, 57(1), 17-20. (2004).
- Mihai M, Valentin N, Bogdan D, Carmen CM, Coralia B, Demetra S. Antibiotic susceptibility profiles of Mycoplasma hominis and Ureaplasma urealyticum isolated during a population-based study concerning women infertility in northeast Romania. Brazilian Journal of Microbiology.42(1):256-60 (2011).
- Pereyre S, Bébéar CM, Bébéar C. Mycoplasma hominis, M. genitalium and Ureaplasma spp. Antimicrobial Therapy and Vaccines (3rd Edition). Appel Trees Production, NY, USA. (2015).
- Grandi, G. Antibacterial vaccine design using genomics and proteomics. Trends in biotechnology, 19(5), 181-188. (2001).
- Sette, A., & Fikes, J. Epitope-based vaccines: an update on epitope identification, vaccine design and delivery. Current opinion in immunology, 15(4), 461-470. (2003).
- De Temmerman ML, Rejman J, Demeester J, Irvine DJ, Gander B and De Smedt,SC 2011. Particulate vaccines: on the quest for optimal delivery and immune response. Drug discovery today, 16(13-14), pp.569-582. (2011).
- Rees W, Bender J, Teague TK, Kedl RM, Crawford F, Marrack P and Kappler J. An inverse relationship between T cell receptor affinity and antigen dose during CD4+ T cell responses in vivo and in vitro. Proceedings of the National Academy of Sciences, 96(17), pp.9781-9786. (1999).
- Vialle RA, Tamuri AU and Goldman N. Alignment modulates ancestral sequence reconstruction accuracy. Molecular biology and evolution, 35(7), pp.1783-1797 (2018).
- Apweiler, R., Bairoch, A., Wu, C. H., Barker, W. C., Boeckmann, B., Ferro, S., & Martin, M. J. UniProt: the universal protein knowledgebase. Nucleic acids research, 32(suppl_1), D115-D119. (2004).
- Doytchinova, I. A., & Flower, D. R. VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC bioinformatics, 8(1). (2007).
- Gardy JL, Laird MR, Chen F, Rey S, Walsh CJ, Ester M, Brinkman FS. PSORTb v. 2.0: expanded prediction of bacterial protein subcellular localization and insights gained from comparative proteome analysis. Bioinformatics.Oct 22;21(5):617-23. (2004).
- Dimitrov I, Bangov I, Flower, D.R. and Doytchinova, I. AllerTOP v. 2—a server for in silico prediction of allergens. Journal of molecular modeling, 20(6), p.2278. (2014).
- Awad Elkareem MA, Ahmed Osman S, Mohamed HA. Prediction and conservancy analysis of multiepitope based peptide vaccine against merkel cell polyomavirus: an immunoinformatics approach. Immunome Research.;13(134):2. (2017).
- Gasteiger, E., Hoogland, C., Gattiker, A., Wilkins, M. R., Appel, R. D., &Bairoch, A. Protein identification and analysis tools on the ExPASy server. In The proteomics protocols handbook (pp. 571-607). Humana press. (2005).
- Finn, R. D., Attwood, T. K., Babbitt, P. C., Bateman, A., Bork, P., Bridge, A. J., … & Gough, J. InterPro in 2017—beyond protein family and domain annotations. Nucleic acids research, 45(D1), D190-D199. (2016).
- Larsen MV, Lundegaard C, Lamberth K, Buus S, Lund O and Nielsen M. Large-scale validation of methods for cytotoxic T-lymphocyte epitope prediction. BMC bioinformatics, 8(1), p.424. (2007)
- Hoof I, Peters B, Sidney J, Pedersen LE, Sette A, Lund O, Buus S. and Nielsen M. NetMHCpan, a method for MHC class I binding prediction beyond humans. Immunogenetics, 61(1), p.1. (2009)
- Peters B and Sette A 2005. Generating quantitative models describing the sequence specificity of biological processes with the stabilized matrix method. BMC bioinformatics, 6(1), p.132. (2005).
- Bui HH, Sidney J, Li W, Fusseder N and Sette A. Development of an epitope conservancy analysis tool to facilitate the design of epitope-based diagnostics and vaccines. BMC bioinformatics, 8(1), p.361. (2007).
- Tenzer S, Peters B, Bulik S, Schoor O, Lemmel C, Schatz MM, Kloetzel PM, Rammensee HG, Schild H. and Holzhütter HG. Modeling the MHC class I pathway by combining predictions of proteasomal cleavage, TAP transport and MHC class I binding. Cellular and Molecular Life Sciences CMLS, 62(9), pp.1025-1037. (2005).
- Bui HH, Sidney J, Dinh K, Southwood S, Newman MJ and Sette A. Predicting population coverage of T-cell epitope-based diagnostics and vaccines. BMC bioinformatics, 7(1), p.153. (2006).
- Maupetit J, Derreumaux P, Tuffery P. PEP-FOLD: an online resource for de novo peptide structure prediction. Nucleic acids research. 11;37(suppl_2):W498-503. (2009).
- Kaur G, Gras S, Mobbs JI, Vivian JP, Cortes A, Barber T, Kuttikkatte SB, Jensen LT, Attfield KE, Dendrou CA, Carrington M. Structural and regulatory diversity shape HLA-C protein expression levels. Nature communications. 2017 Jun 26;8:15924. (2017).
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC and Ferrin TE. UCSF Chimera—a visualization system for exploratory research and analysis. Journal of computational chemistry, 25(13), pp.1605-1612. (2004).
- Dhanik A, McMurray JS and Kavraki LE. DINC: a new AutoDock-based protocol for docking large ligands. BMC structural biology, 13(1), p.S11. (2013).
- Jespersen MC, Peters B, Nielsen M, Marcatili P. BepiPred-2.0: improving sequence-based B-cell epitope prediction using conformational epitopes. Nucleic acids research. May 2;45(W1):W24-9. (2017).
- Emini EA, Hughes JV, Perlow DS, Boger J 1985. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J Virol 55:836-839. (1985).
- Kolaskar AS. and Tongaonkar A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS letters, 276(1-2), pp.172-174. (1990).
- Karplus PA and Schulz Prediction of chain flexibility in proteins. Naturwissenschaften, 72(4), pp.212-213. (1985).
- Parker JMR, Guo D and Hodges RS. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry, 25(19), pp.5425-5432. (1986).
- Chou PY and Fasman GD 1978. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol 47: 45–148. Chou4547Adv. (1978).
- Pellequer JL, Westhof E, Van Regenmortel MH. Correlation between the location of antigenic sites and the prediction of turns in proteins. Immunology letters.s Apr 1;36(1):83-99. (1993).
- Whelan AO, Flick-Smith HC, Homan J, Shen ZT, Carpenter Z, Khoshkenar P, Abraham A, Walker NJ, Levitz SM, Ostroff GR. and Oyston PC. Protection induced by a Francisella tularensis subunit vaccine delivered by glucan particles. PloS one, 13(10), p.e0200213. (2018).
- Zahroh H, Ma’rup A, Tambunan USF and Parikesit AA. Immunoinformatics approach in designing epitope-based vaccine against meningitis-inducing bacteria (Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type b). Drug target insights, 10, pp.DTI-S38458. (2016).
- Chakraborty S, Chakravorty R, Ahmed M, Rahman A, Waise, TM, Hassan F, Rahman M. and Shamsuzzaman S. A computational approach for identification of epitopes in dengue virus envelope protein: a step towards designing a universal dengue vaccine targeting endemic regions. In silico biology, 10(5, 6), pp.235-246. (2010).
- Oany AR, Emran AA and Jyoti TP. Design of an epitope-based peptide vaccine against spike protein of human coronavirus: an in-silico approach. Drug design, development and therapy, 8, p.1139. (2014).
- Islam R, Sakib MS and Zaman A. A computational assay to design an epitope-based peptide vaccine against chikungunya virus. Future Virology, 7(10), pp.1029-1042. (2012).
- Srivastava PN, Jain R, Dubey SD, Bhatnagar S and Ahmad N. Prediction of epitope-based peptides for vaccine development from coat proteins GP2 and VP24 of Ebola virus using immunoinformatics. International journal of peptide research and therapeutics, 22(1), pp.119-133. (2016).








