Manuscript accepted on :26-03-2020
Published online on: 31-03-2020
Plagiarism Check: Yes
Reviewed by: Mostafa Mosalam
Abdulrahman Al-Bazzaz, Kahtan A-Dahir, Abdulsalam Almashhadani and Israa Al-Ani*
Faculty of Pharmacy, Al-Ahliyya Amman University, Jordan
Corresponding Author E-mail : ialani@ammanu.edu.jo
DOI : https://dx.doi.org/10.13005/bpj/1909
Abstract
"Autism spectrum disorder" refers to a range of conditions characterized by challenges with social skills, repetitive behaviors, speech and nonverbal communication. This study aimed to measure the fasting serum levels of glucose, zinc , copper, zinc / copper ratio and their correlations to the measured lipid profile in matched Non-Autistic by age, gender and body mass index with Autistic patients in Jordan. To find a correlations between the studied parameters that might help in understanding the biochemical changes that may lead to autism also to open away for medical management and treatment of autism. Fasting blood samples were taken from both matched groups (35 each with age 4-12 years) then analyzed the above parameters and data was treated statistically by SPPS while Correlations were determined by Pearson’s test. The study revealed that there was significant changes in levels and correlations especially in zinc and zinc/copper ratio in relation to triglycerides and high density lipoprotein in autistic patients.
Keywords
Autism; Lipid profile; Zinc and Copper
Download this article as:| Copy the following to cite this article: Al-Bazzaz A, A-Dahir K, Almashhadani A, Al-Ani I. Estimation of Fasting Serum Levels of Glucose, Zinc, Copper, Zinc /Copper Ratio and Their Relation to the Measured Lipid Profile in Autistic Patients and Non-Autistic Controls in Jordan. Biomed Pharmacol J 2020;13(1). |
| Copy the following to cite this URL: Al-Bazzaz A, A-Dahir K, Almashhadani A, Al-Ani I. Estimation of Fasting Serum Levels of Glucose, Zinc, Copper, Zinc /Copper Ratio and Their Relation to the Measured Lipid Profile in Autistic Patients and Non-Autistic Controls in Jordan. Biomed Pharmacol J 2020;13(1). Available from: https://bit.ly/2Uur3XF |
Introduction
Autistic spectrum disorders (ASDs) are a group of systemic disorders (neuro-developmental disorder) with multiple etiology, including both genetic and environmental / lifestyle factors [1-3].
According to the diagnostic and Statistical Manual of Mental disorder (DSM-5) and the International Statistical Classification of Diseases and Related Health Problem (ICD-10) ,autistic disorder is characterized by impairment in each of the three core areas of social interaction, communication (verbal and non-verbal) and restricted repetitive behaviors, stereotyped behavior, interests and activities [4,5].
The number of diagnosed cases of autism has increased substantially in the last decades where the estimated prevalence of autistic disorder is 21/10,000 [6] ( Garfinkle, 2013), but 1/160 (WHO,2017). While, 1-2 % of population are affected by autism in developed countries [5].
The Centers for Disease Control and Prevention in America found that the autism rate among American children is 1 in 50, knowing that boys outnumber girls with autism by a ratio of 4 or 5 to 1 [7].
There is no known cause for autism, but it is generally accepted that is caused by abnormalities in brain structure or function. The famous causes mentioned in the literature are; genetics [8,9], age of parents [10] , imbalance in neural systems [11] , food Causes [12].
Also, there are many other causes linked with autism for example neuroinflammation and immune dys-regulation [13] and metal metabolism disorders [14,15]
In this context, Kim et al. hypothesize that autism is associated with alterations in the plasma lipid profile and that some lipid fractions in autistic boys may be significantly different than those of healthy boys [16].
The results of Matsuzaki revealed that the serum levels of total cholesterol and triacylglycerol in the male infant subjects with high-functioning autism were significantly lower than those of normal male matched controls [17]. The same results were reached by Herguner & Herguner [18], Ansary and Al-Ayadh[19] .
However, the mechanisms underlying elemental dysregulation and ASD risk are not well understood in spite of thorough researches. There are some contradictory findings regarding the relationship between serum trace elements like Zn and Cu with lipids and lipoproteins. So, the role of these elements needs further elucidation.. It is not known whether this elemental dysregulation is present in fetal and early postnatal life before first clinical symptoms manifest [5].
Faber , Bjorklund and El-Baz showed that Zinc deficiency, excess Cu levels, but low Zn/Cu ratio are common in children diagnosed with an ASD as compared with their matched controls [20-22].
This study aims to highlight the potential importance of the concentration levels of zinc and copper, and it’s correlation to the lipid profile parameters [total cholesterol (TC), high density lipoprotein (HDL), low density lipoprotein (LDL), very low density lipoprotein (VLDL) and triglyceride (TG)] in addition to fasting blood sugar (FBS) in serum of fasted autistic patients with their matched non-autistic controls by age, sex and body mass index (BMI) that might help in understanding in biochemical changes that may lead to autism also to open a way for medical management and treatment of autism. No previous studies investigated this possible relation at the time of starting this study in Jordan and other areas of middle east and north Africa (MENA) in 2013.
Methodology
Patients and Controls
Thirty five (31 male and 4 female) patients with autism spectrum disorder (ASD) matched by age, sex and BMI with thirty five non-autistic controls (31 male and 4 female) participated in this study with age ranged from 4-12 years.
No significant differences in age, sex and BMI between patients and controls.
Autism spectrum disorder (ASD) individuals were referred by specialized clinician’s in Autism Academy of Jordan (AAJ). Diagnoses was confirmed for all ASD patients according to Diagnostic & Statistical Manual of Mental Disorder (DSM-5) & the International Classification of Disorders, tenth edition (ICD-10; WHO, 1994) as criteria, and clinical symptoms of autism; were assessed with the autism diagnostic interview-revised in 35 subjects with available parental informants.
The study was proved by the Institutional review board (IRB) as the “Ethical Committee of Autism Academy of Jordan (AAJ)” and “The Ethical committee” of Al-Ahliyya Amman University at Nov-2013.
Study Design
Blood samples were taken from twelve hours fasted (ASD) patients and their matched controls who stayed in rest position for about 15 minutes before taking the samples. The blood samples were kept for 15 minutes in 4 Co in plane tube then centrifuged to get the serum to measure the levels of glucose, Zn, Cu, and lipid profiles (TC , LDL, HDL,TG & VLDL) . Serum aliquots were transferred into cryostat tubes and stored at -20Co until day of analysis while the fasted serum sugar was measured immediately.
Analysis of Samples
All samples were analyzed in Smart Labs® , Amman/Jordan using standard kits and according to the protocols of the laboratory.
Statistical analysis of data will made by using Statistical Product and Service Solutions (SPSS) (Data will be evaluated as mean and standard deviation by analysis of variance (ANOVA) and t-test, adjusted for multiple comparisons. P-values of <0.05 will be considered statistically significant. Correlations were determined by Pearson’s test.
Result and Discussion
The results of all parameters measured are resented in table (1).
Table 1: Serum glucose, Zn, Cu, Zn/Cu ratio, and lipid profile of the autistic patients and matched controls with results of statistics.
| Fasting Serum level
|
Glucose
mg/dl |
Zn
(µg/dl) |
Cu
(µg/dl) |
Zn/Cu
ratio |
TG
mg/dl |
TC
mg/dl |
LDL
mg/dl |
HDL
mg/dl |
VLDL
mg/dl |
| Autistic patients | 83.29 ± 11.09 | 84.34 ±
18.28 * |
113.82 ±
18.74 |
0.74 1 ±
0.22 * |
74.23 ±
26.46 |
137.14±
22.91 * |
72.02 ±
17.56 |
43.6 3 ±
6.45 * |
14.61± 5.45 |
| Controls | 90.06 ± 7.075 | 95.89 ±
10.91 |
112.49 ±
18.98 |
0.857 ±
0.165 |
83.74 ±
27.47 |
152.08 ±
22.84 |
82.96 ±
18.47 |
53.11 ±
10.11 |
16.75± 5.55 |
*Significant on 5% CI using t-test.
The Difference in Serum Zn between Patients and Control
Figure 1 shows the statistical differences between serum Zn level between the patients and controls. The serum level was significantly decreased in patients with autism using t-test and 5% CI to compare mean serum levels of the two groups.
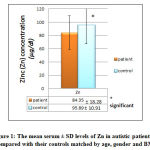 |
Figure 1: The mean serum ± SD levels of Zn in autistic patients as compared |
Zn is an essential trace element, an important antioxidant in spite of not being a free radical scavenger, and a metalloenzyme required for the catalytic activity of at least 300 enzymes [23]. It plays a role in immune system functioning, protein synthesis, DNA synthesis, cell division and many other functions [24]. Prolonged Zn deficiency may therefore cause growth impairment, changes in the intestinal flora and function which are are common in autistic patients [25].It is therefore conceivable that malabsorption due to pathological changes in the intestinal mucosa may play an important role as one of the causes of Zn deficiency in autism. An important clinical point to note is that, because Zn is primarily an intracellular nutrient, serum Zn levels can be normal in states of mild deficiency[26]. So, deficiency of zinc level which is significantly lower in our autistic patient than their matched controls, may play an important roles in the etiology of autism through one or more of the above mentioned causes that help in the development of autism. The changes of zinc levels through progress of age may be influenced by environment which indicates that epigenetics (alteration of gene expression by environmental influences) could be a factor in the pathophysiology of autism [27].
The Difference in Serum Cu between Patients and Control
Figure 2 shows the statistical differences between serum Cu level in µg/dl between the patients and controls. The mean serum levels of both groups were statistically similar using t-test , 5% CI.
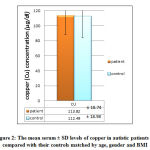 |
Figure 2: The mean serum ± SD levels of copper in autistic patients as compared |
Cu
Is a cofactor required for the activity of the enzyme dopamine-β-hydroxylase that converts dopamine to norepinephrine [28]. Increased norepinephrine levels have been found in autistic individuals[29] .
The variation of the mean of copper in patient group and control group due to genetic or nutritional factors can affects the metabolism of copper [23] . The differences between our result (35 patients) and the studies of Russo and DeVito results may be related to sample size of the study. Zinc deficiency, excess Cu levels, and low Zn/Cu ratio are common in children diagnosed with an ASD [21].
Zn / Cu Ratio
Figure 3 shows the significant difference in Zn/Cu ratio between normal and autistic patients. This significance is related to the change in Zn level.
Rossu et al. suggest that low zinc and high copper may modulate GABA receptors, ultimately changing transmitter concentration [23]. High copper may also be associated with high norepinephrine found in autistic children, and high epinephrine may, in turn, manifest as excitability and hyperactivity associated autistic symptoms.
 |
Figure 3: The mean serum ± SD levels of Zn/Cu ratio in autistic patients as compared |
Lipid Profile
Total Cholesterol
Figure 4 below shows the significant lower level of TC in autistic patients. The results of this study agree with studies of Matsuzaki and Moses. It is likely that in some forms of ASD, the symptoms may be due to interaction of components that are sterol dependent .It is tempting to speculate that dyslipidemia is linked to the pathogenesis of autism because there is some indirect support for such an etiological association [17,30]. Adequate cholesterol levels are crucial for serotonin metabolism and myelination of the brain, both of which have been reported to be abnormal in autism [31,32].
 |
Figure 4: The mean serum ± SD levels of TC in autistic patients as compared |
HDL, LDL and VLDL
Figures 5, 6 and 7 show that patient with autism have significant lower level of both HDL ( 43.63±6.45 mg/dl compared to 53.11±10.11 mg/dl of control group) and LDL (72.03± 17.56 mg/dl compared with 82.96 ± 18.47 mg/dl of control group) while insignificant change in VLDL level (14.61± 17.56 mg/dl compared with 16.75± 5.55 mg/dl of control group) was observed.
These results agree with some studies that found that deficiency in HDL of ASD children is due to some kind of lipid metabolism disorder associated with autism [16,33], and it is plausible that low blood levels of HDL and omega-3 fatty acids observed in autistic children at an early age may be an indicator of impaired fatty acid metabolism [33]. Low levels of HDL can be attributed to a number of causes ranging from poor diet, lack of exercise, genetics and vitamin deficiency. Low blood levels of HDL and omega-3 fatty acids observed in autistic children at an early age may be an indicator of impaired fatty acid metabolism. HDL may reduce atherosclerosis through several different mechanisms [34] which include, increasing reverse cholesterol transport, inhibiting physical and chemical modifications of LDL and thus reducing foam cell formation, protecting against endothelial dysfunction, inhibiting chronic inflammation by suppressing adhesion molecules and macrophage chemotactic proteins, and reducing arterial lipoprotein retention.
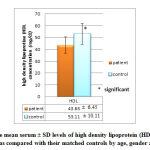 |
Figure 5: The mean serum ± SD levels of high density lipoprotein (HDL) in autistic |
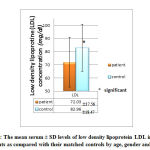 |
Figure 6: The mean serum ± SD levels of low density lipoprotein LDL in autistic |
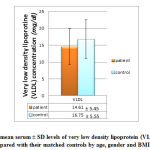 |
Figure 7: The mean serum ± SD levels of very low density lipoprotein (VLDL) |
TG
The study demonstrate insignificant (p = 0.147) lower mean serum ± SD TG level (74.23± 26.46 mg/dl) in autistic patients compared with the mean serum ± SD TG, (83.74 ± 27.79 mg/dl) of controls matched for age, sex, and BMI, although both of these mean levels within the normal reference ranges as shown in figure 8.
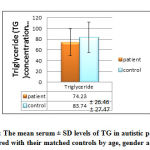 |
Figure 8: The mean serum ± SD levels of TG in autistic patients as |
A study by International Society for Autism Research in 2010 found that the serum levels of TG and VLDL in the infant subjects with high-functioning autism were significantly lower than those of normal control subjects. But our study showed same results except that the TG and VLDL were insignificant. Kim et al.(2010)mentioned that presence of dyslipidemia in boys with autism and suggest a possibility that dyslipidemia might be a marker of association between lipid metabolism and autism .Triglyceride provided fatty acids to the body when degraded. Vitamins A, D, E, and K are fat-soluble vitamins, which mean the body must have fat to absorb them. These vitamins are transported through the vessels by the chylomicrons Vitamins E, D, and K are also stored in the fat. If there is too little dietary fat, or if a medical problem exists that interferes with the body’s ability to absorb fat, then vitamin deficiency occurs(a state observed in some autistic children) [35].
Fasting Blood Sugar (FBS)
Our study showed significant (p= 0.004) lower mean serum ± SD glucose (FBS) level (83.29 ± 11.09 mg/dl) in autistic patients as compared with the mean serum mean ± SD glucose (90.06 ± 7.075 mg/dl) of controls, although both of these mean levels within the normal reference range as shown in figure 9.
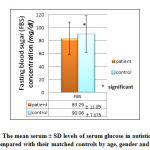 |
Figure 9: The mean serum ± SD levels of serum glucose in autistic patients |
The results agreed with the result of Moses et al which revealed that ASD had significantly lower fasting serum glucose. There is growing evidence that nutritional therapy can really make a big difference to children with autism [30].
Woeller mentioned that low blood glucose levels on blood tests are something to be seen from time to time in children on the Autism spectrum [36] Campbell-McBride mentioned that abnormal absorption or digestion of the glucose or other toxins due to abnormal gut flora which may be seen in autistic patients this lead the child misses that window of opportunity of learning and starts developing autism depending on the mixture of toxins, depending on how severe the whole condition is, and how severely abnormal the gut flora is in the child [37].
Correlation of Zn, Cu and Zn/Cu ratio with lipid profile and FBS in autistic patients
Statistical treatment of data aimed also to examine the possible correlation between each parameter of lipid profile and FBS with Zn, Cu and Zn/Cu ratio in autistic patients.
Results showed that there was insignificant correlation between mean serum levels of Zn and mean serum levels of lipid profile and fasting blood sugar in control and autistic groups except a significant positive (P= 0.018) correlation between Zn and HDL in autistic group . The current researcher did not find any study linked between Zn and HDL in autistic patients. Cellular Zn controls the gene that makes heart-protective HDL [38]. This may be the cause of a significant correlation between zinc and HDL in patients of current study.
Results also show no significant correlation between mean serum levels ± SD of Cu and the mean serum levels ± SD of lipid profile and fasting blood sugar in controls group and autistic group.
Regarding Zn/Cu ratio, no significant correlation between mean serum levels ± SD of Zn/Cu ratio and mean serum levels ± SD of lipid profile and fasting blood sugar in controls and autistic groups except a significant positive (p = 0.013) correlation between Zn/Cu ratio and (HDL) in autistic group as shown also in table (1).
Conclusion
The study revealed that there is no statistically significant correlation of the variables (total cholesterol, high density lipoprotein , low density lipoprotein, very low density lipoprotein, triglyceride and fasting blood sugar ) of patients to control subjects, but only statistically significant correlation between zinc and zinc/copper ratio with high density lipoprotein (HDL) and TC in autistic patients.
Acknowledgment
The researchers would like to thank Autism Academy of Jordan and SmartLabs® for their endless support and cooperation in this research.
References
- Kidd Autism, An extreme challenge to integrative medicine. Part 1: The knowledge base alternative. Med. Rev. 2002;7(4):292-316.
- Lakshmi Priya MD, Geetha A. A biochemical study on the level of proteins and their percentage of nitration in the hair and nail of autistic children. Clinica Chimica Acta 2011;16(5):216–222.
- Blaurock -Busch E, Amin O, Dessoki H, Rabah T. Toxic Metals and Essential Elements in Hair and Severity of Symptoms among Children with Autism. Maedica 2012;7(1):38-48.
- World health statistics in 2017 on: https://www.who.int/gho/publications/world_health_statistics/2017/en/
- Curtin P, Christine A, Austen C, et al. Dynamical features in fetal and postnatal zinc-copper metabolic cycles predict the emergence of autism spectrum disorder. Sci Adv. 2018;4(5): 1293:1298.
- Garfinkle AN, Emerson J.Montana’s Children’s Autism Waiver (CAW) Report:Initial Outcomes of Cohort One (Full Report)(p.8). University of Montana : Developmental Disabilities Program/ Department of Public Health and Human Services. 2013.
- Center for Disease Control and Prevention.( 2012). CDC Division of News & Electronic Media on: http://www.cdc.gov/media/releases/2012/p0329_autism_disorder.html.
- Rodier PM. The early origins of autism. Sci. Am 2000; 282:56–63.
- Ratajczak HV. Theoretical aspects of Autism. J. Immunotoxicol.2011; 8(1);68-79.
- Shelton JF, Tancredi DJ, Hertz-Picciotto I (2010). Independent and dependent contributions of advanced maternal and paternal ages to autism risk. Autism Res. 2010;3(1):30-9.
- Polleux F, Lauder JM (2004) Toward a developmental neurobiology of autism. Dev. Disabil. Res. Rev. 2004;10:303–317.
- Adams JB .Summary of Dietary, Nutritional, and Medical Treatments for Autism Research Institute (ARI) Publication 40:10-21.
- Suzuki K, Sugihara G, Ouchi Y, et al. Microglial activation in young adults with autism spectrum disorder. JAMA Psychiatry. 2013;70(1):49-58.
- Bernard S, Enayati A, Redwood L et al. Autism ,a novel form of mercury poisoning. Med Hypotheses. 2001;56(4):462-71.
- Geier DA, Audhya T, Kern JK (2010) Blood mercury levels in autism spectrum disorder: Is there a threshold level? Acta Neurobiol Exp. 2010;70(2):177-86.
- Kim EK, Neggers YH, Shin CS et al. Alterations in lipid profile of autistic boys: a case control study. Nutr Res. 2010;30(4):255-60.
- Nakamura K, Sekine Y, Ouchi Y. Brain serotonin and dopamine transporter bindings in adults with high-functioning autism. Arch Gen Psychiatry. 2010;67(1):59-68.
- Hergüner S, Hergüner A. Cholesterol Levels In Children And Adolescents With Autistic Disorder. Selçuk Üniv Tıp Derg 2011;27(4):226-228.
- Ansary A, Al-Ayadh L. Lipid mediators in plasma of autism spectrum disorders. Lipids Health Dis 2012;11;160.
- El-Meshad GM, Abd El-Nabi SA, Moharam NM, Abou El-Khair MS. The plasma zinc/serum copper ratio as a biomarker in children with autism spectrum disorders. 2017; 30(3): 727-733.
- Bjorklund G (2013) The role of zinc and copper in autism spectrum disorders. Acta Neurobiol Exp 2013; 73: 225–236
- Dekhil O, Hajjdiab H, Shalaby A, et al. Using resting state functional MRI to build a personalized autism diagnosis system. PLoS ONE. 2018; 13(10): e0206351. https://doi.org/10.1371/journal.pone.0206351
- Russo AJ. Plasma copper and Zinc concentration in individuals with autism correlate with selected symptom severity. Nutr Metab Insights.2012; 5: 41–47.
- Prasad AS. Discovery of human zinc deficiency: 50 years later. J Trace Elem Med Biol. 2012;26 (2-3):66-69.
- Derrick F. MacFabe, MD. Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb.Ecol. Health Dis.2012; 24:23-47.
- Salgueiro MJ, Krebs N, Zubillaga MB et al. Zinc and diabetes mellitus: is there a need of zinc supplementation in diabetes mellitus patients? Biol. Trace Elem. Res. 2001; 81(3): 215–228.
- Deans, E. (2012). Zinc and Autistic Spectrum Disorders. On: http://www.psychologytoday.com/blog/evolutionary-psychiatry/201208/zinc-and-autistic-spectrum-disorders
- Rahman K, Rahman F, Rahman T et al.Dopamine- β-hydroxylase (DBH), its cofactors and other biochemical parameters in the serum of neurological patients in Int. j Biomed.Sci.2009; 5(4): 395–401.
- Lake CR, Ziegler MG, Murphy DL.Increased norepinephrine levels and decreased dopamine-beta-hydroxylase activity in primary autism. Arch Gen Psychiatry. 1977;34(5):553-556.
- Moses L, Kats N, Weizman A(2013). Metabolic profiles in adults with autism spectrum disorder and intellectual disabilities. Eur Psychiat.2013;29(7): 397-401.
- Herbert MR, Ziegler DA, Makris N, et al (2004) Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55(4):530-40.
- Chugani DC. Serotonin in autism and pediatric epilepsie Epilepsy and the Development of Autism. Dev. Disabil. Res. Rev. 2004;10(2):112–116.
- Kim EK, Neggers YH, Shin CS et al. Alterations in lipid profile of autistic boys: a case control study. Nutr Res. 2010 ;30(4):255-60.
- Assmann G, RochNofer J. Atheroprotective Effects of High-Density Lipoproteins. Annu. Rev. Med.2003; 54;321-341.
- Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: relevance for autism. FASEB Journal 2014; 28:1-15.
- Hoirisch-Clapauch S, Antonio N (2019) Autism spectrum disorders: let’s talk about glucose? Transl. Phychiatry 2019; 9:51-57.
- Finegold SM. State of the art; microbiology in health and disease. Intestinal bacterial flora in Autism. Anaerobe. 2011;17(6):367-8.
- Beattie J, Gordon M, Duthie S (2012). Suboptimal dietary zinc intake promotes vascular inflammation and atherogenesis in a mouse model of atherosclerosis. Mol Nutr Food Res. 2012 ;56(7):1097-105.
(Visited 618 times, 1 visits today)







