Manuscript accepted on :21-02-2020
Published online on: 11-03-2020
Plagiarism Check: Yes
Reviewed by: Francesca Gorini
Second Review by: EXBRAYAT Jean-Marie
Abinaya E1 , Meenakshi N2
, Meenakshi N2 , Ruckmani A1
, Ruckmani A1 , Nasrin Nisha A1
, Nasrin Nisha A1 , Tanuja Lella1
, Tanuja Lella1 and Arunkumar R1*
and Arunkumar R1*
1Department of Pharmacology, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, Chennai-603103, Tamil Nadu, India.
2Department of Respiratory Medicine, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, Chennai-603103, Tamil Nadu, India.
Corresponding Author E-mail :drarunvp@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1889
Abstract
To study the efficacy of Metformin add-on therapy to standard ATT in newly diagnosed Pulmonary Tuberculosis patients with the following parameters.Sputum smear conversion Changes in drug resistance pattern. To assess the safety of Metformin add-on therapy. The study was started after obtaining approval from Institutional Human Ethics Committee. It was a prospective, randomised controlled study involving 100 participants. Pulmonary tuberculosis patients who were non-diabetic and positive for sputum AFB were randomly allocated to two groups- Control and Metformin group (50 patients in each group). In control group, patients received standard Anti Tuberculosis Treatment (ATT). In Metformin group, patients were given Metformin 250 mg BD daily along with standard ATT. Sputum smear examination for AFB (Acid fast bacilli) was done every week and resistance pattern (CBNAAT-GeneXpert and / or Line Probe Assay) was assessed at the end of intensive phase. Complete Blood Count (CBC), Random Blood Sugar (RBS), Renal Function Tests (RFT) and Liver Function Tests (LFT) were done at baseline and at end of the study. Adverse events were recorded.Sputum smear conversion: The average time taken for sputum smear conversion was 3.4 weeks in Metformin group and 4.7 weeks in control group. It was significantly less in Metformin group (p = 0.012, unpaired t test).Resistance: Drug resistance pattern at the end of 2 months showed that 1 patient in Metformin group showed resistance to Rifampicin and 4 patients in control group showed resistance (3 patients for Rifampicin and 1 for Isoniazid).Adverse events: 12% of the patients in Metformin group experienced adverse events, and 8% in control group. There were no serious adverse events and most of the adverse events were gastrointestinal related and minor in nature. There were no significant changes noted in RBS, CBC, LFT and RFT parameters.This study provides hands on and preliminary data that supports Metformin added to standard ATT may potentially benefit TB patients by i) significantly reducing the time needed for sputum smear conversion and ii) reducing the occurrence of drug resistance. However larger studies with varied outcome measures are needed to confirm the positive observations noted in this study.
Keywords
Tuberculosis; ATT; Metformin; Drug Resistance; Sputum Smear Conversion
Download this article as:| Copy the following to cite this article: Abinaya E, Meenakshi N, Ruckmani A, Nasrin Nisha A, Tanuja Lella, Arunkumar R. Clinical Evaluation of Efficacy and Safety of Metformin add-on Therapy to Standard ATT in Newly Diagnosed Pulmonary Tuberculosis Patients. Biomed Pharmacol J 2020;13(1). |
| Copy the following to cite this URL: Abinaya E, Meenakshi N, Ruckmani A, Nasrin Nisha A, Tanuja Lella, Arunkumar R. Clinical Evaluation of Efficacy and Safety of Metformin add-on Therapy to Standard ATT in Newly Diagnosed Pulmonary Tuberculosis Patients. Biomed Pharmacol J 2020;13(1). Available from: https://bit.ly/38I5IOe |
Introduction
Tuberculosis (TB) is one of the most common diseases causing mortality worldwide. Nearly one quarter of the world’s population is having latent TB infection and 5 to 15% of the people with latent TB infection have the chance of developing active TB1. According to World Health Organisation global TB report 2018, 10 million people were found to be affected by TB globally. Among them, nearly two-third of the patients were from India, China, Indonesia, Philippines, Pakistan, Nigeria, Bangladesh and South Africa. American and European countries contribute to only 6% of the global TB cases 2. According to the RNTCP (Revised National Tuberculosis Control Programme) report, nearly 27% (27,90,000) of the global TB patients were from India3.
TB treatment
Drugs used in the treatment of drug sensitive tuberculosis include Isoniazid (INH/H), Rifampicin (R), Pyrazinamide (Z), Ethambutol (E) and Streptomycin (S). For the treatment of drug resistant TB, the drugs used are Aminoglycosides (Amikacin, Kanamycin, Capreomycin), Para amino salicylic acid (PAS), Cycloserine, Ethionamide, Fluoroquinolones (Ciprofloxacin/ Levofloxacin/ Moxifloxacin), Clofazimine, Linezolid, Imipenam-Cilastatin/Meropenam, Bedaquiline and Delamanid.4, 5
Drug Resistance
Drug resistance in TB is a serious problem and the treatment of drug resistant TB is extremely difficult. The suffering patients have to take drugs for around 2 years and the drugs used in treatment of drug resistant TB are not as efficacious as first line drugs. WHO report (2018) shows that about 558000 people developed TB that was resistant to the first line anti-tubercular drug Rifampicin and 82% out of these had Multi drug resistant TB (MDR-TB). Among all countries, India, China and Russian federation contributed to nearly 50% of drug resistant TB cases. The percentage of MDR TB in these countries was 24%, 13% and 10% respectively. Among the people who had MDR TB, 8.5% were found to be affected with Extensively drug resistant TB (XDR TB)2. In MDR TB, there is resistance to both Isoniazid and Rifampicin. In XDR TB, in addition to multidrug resistance (Isoniazid and Rifampicin resistance), there is also resistance to any Fluoroquinolone (Ofloxacin, Levofloxacin or Moxifloxacin), and at least one of the second-line injectable drugs (Capreomycin, Kanamycin and Amikacin). Drug resistance in TB may be primary or acquired. Primary drug resistance is defined as the drug resistance in patients who never had anti-tubercular treatment in the past. It occurs due to the infection with drug resistant strains of Mycobacteria. Acquired drug resistance occurs during the course of treatment in patients who were initially sensitive to anti-tubercular drugs6. The factors contributing to drug resistance include poor adherence to therapy, improper selection of drug regimens and incomplete treatment7. Drug resistance in Mycobacteria occurs due to the chromosomal mutations that result in alteration of the drug target8. Hence, in order to overcome the emergence of drug resistance and to improve the efficacy of anti-TB drugs, newer treatment modalities are needed and host directed therapies could be used as adjunct treatment for TB.
Host Directed Therapy for TB
Host directed therapies (HDTs) provide beneficial effect by enhancing the host immune defenses, targeting the inflammatory pathway and interfering with the host mechanisms used by the pathogens to persist in host tissues. HDTs would be useful in TB treatment by shortening the treatment duration, reducing the number of antibiotics, improving the efficacy of anti-tubercular drugs and these drugs do not face resistance from the bacteria9. Recent studies showed that many drugs including Etanercept (Tumor necrosis factor α inhibitor), Statins, vitamin D, Cyclooxygenase inhibitors, Carbamazepine, Bevacizumab (Vascular Endothelial Growth Factor inhibitor), Metformin and many other drugs could be used as HDTs in TB treatment. These drugs alter the host immune response in several ways.10
By disrupting granuloma- they augment drug penetration into the cell
By inducing autophagy of infected cells – intracellular bacteria will be destroyed
By exerting anti – inflammatory response
By enhancing cell mediated immune response
Metformin in TB Treatment
Metformin is the commonly used anti hyperglycemic drug, approved by US FDA (United States Food & Drug Administration) in 1995 for the treatment of type 2 Diabetes mellitus. Metformin reduces the hepatic glucose production by inhibiting gluconeogenesis and exerts some action on mitochondrial respiration by reducing the intracellular levels of ATP (Adenosine triphosphate) and increasing AMP (Adenosine monophosphate) levels. It stimulates AMP dependent protein kinase (AMPK) which in turn stimulates fatty acid oxidation, increases glucose uptake, reduces lipogenesis and gluconeogenesis in the liver.11
Metformin is generally considered to be safe in clinical practice, though gastro intestinal side effects such as nausea, dyspepsia, abdominal bloating, abdominal cramps and diarrhoea are commonly associated with it.12
Apart from diabetes, Metformin is being widely used in many non-diabetic conditions like Polycystic ovarian syndrome (PCOS) and obesity13,14. Metformin is found to have anti-inflammatory and anti-oxidant properties. It is also reported to be beneficial in thyroid disorders, Alzheimer’s disease and various cancers like pancreatic, breast, endometrial, prostate and colorectal cancers15. The beneficial effect of Metformin in tuberculosis patients with diabetes has been evaluated in a few cross sectional and cohort studies. 15,16,17,18 Singhal et al.15 in 2014, investigated the effect of Metformin in TB using in-vitro, in-vivo and clinical experiments. Among these experiments, Metformin increased the production of mitochondrial reactive oxygen species (ROS), inhibited the growth of Mtb and promoted phagocytosis and autophagy in macrophages in in-vitro experiment. In animal models of TB, Metformin, when given alone and in combination with the anti-tubercular drugs, decreased the bacillary load in lungs and spleen of mice and increased the efficacy of anti-tubercular drugs. It was found that Diabetic patients taking Metformin were found to have better clinical outcome and reduced relapse rates in TB when compared to the patients taking other anti-diabetic drugs16. Human studies with Metformin for tuberculosis are very limited and most of them are observational studies involving diabetic patients. The efficacy of Metformin in tuberculosis has not been well studied in non-diabetic patients. Hence, this study was planned to investigate the efficacy and safety of Metformin add-on therapy to standard tuberculosis treatment.
The aim of the study was to evaluate the efficacy and safety of Metformin add-on therapy to standard ATT in newly diagnosed pulmonary tuberculosis patients.
The objectives were
To evaluate the efficacy of Metformin add-on therapy with the following parameters
Sputum smear conversion
Changes in drug resistance pattern
To evaluate the safety of Metformin add-on therapy with the following parameters
Tolerability and adverse events
Complete blood count, Random blood sugar, Renal function tests and Liver function tests
Materials and Methods
The study was initiated after obtaining approval from Institutional Human Ethics Committee. The approval number was 371/IHEC/10-17 dated 23.10.2017. This was a prospective, open labelled, randomised, parallel group study conducted in a tertiary care hospital for a period of 1 year and 6 months (from November 2017 to May 2019) involving 100 tuberculosis patients. Patients were screened and those who fulfilled the selection criteria were included in the study. All the patients were explained about the study in detail and informed consent was obtained from all the participants. After enrolment, patients were randomly allocated to either of the two groups – Control group and Metformin group with 50 patients in each group. The randomisation was simple randomisation applied using computer generated random number tables. In control group, patients received only standard ATT and in Metformin group, patients received Metformin 250 mg twice daily along with standard ATT.
Selection Criteria
Inclusion Criteria
Newly diagnosed sputum smear positive pulmonary tuberculosis patients
Patients sensitive to ATT drugs based on GeneXpert analysis
Age-18 to 65 years
Gender – Males and Females
Patients with normal liver and renal function tests
BMI more than 20 kg/m2
Exclusion Criteria
Patients with other co-morbid conditions including diabetes mellitus and hypertension
Pregnant and nursing women
Patients with significant neurological, cardiac and gastro intestinal disorders
Patients with known hypersensitivity to Metformin and other study medications
Sample Size Calculation
Sample size was calculated using PS power and sample size software version 3.6.1. Prior data indicate that the sputum conversion at the end of 2 months with ATT was 78 % 19. If the sputum conversion rate with Metformin is expected to be 20% more with Metformin and ATT combination when compared to ATT alone, 40 experimental subjects and 40 control subjects should be included to reject the null hypothesis that the sputum conversion rates for experimental and control subjects are equal with probability (power) of 0.8. The Type I error probability associated with this test of null hypothesis was 0.05. Considering non-adherence and dropout rates of 10%, 50 patients were included in each group.
Study Medications
In the control group, patients received the standard anti-tubercular treatment (ATT), i.e. Isoniazid (H), Rifampicin (R), Pyrazinamide (Z) and Ethambutol (E) for the first 2 months (intensive phase) followed by Isoniazid (H), Rifampicin (R) and Ethambutol (E) for next 4 months (continuation phase). The drugs were given as fixed dose combinations based on the weight of the patients. The total duration of treatment was 6 months. In the Metformin group, in addition to the standard ATT, Metformin 250 mg was given twice daily, after food, for 6 months. Patients in both the groups were followed up for a period of 6 months.
Study Assessments at Baseline
All the patients were subjected to the following baseline investigations before starting the study.
Complete blood count – Haemoglobin (Hb), total Red Blood Cell (RBC) count, total White Blood Cell (WBC) count, Differential count, platelet count
Erythrocyte Sedimentation Rate (ESR)
Liver function tests (LFT) – Aspartate transaminase (AST), Alanine transaminase (ALT), Alkaline phosphatise (ALP), Total bilirubin and Direct bilirubin
Renal function tests (RFT) – Blood Urea Nitrogen (BUN) and Creatinine
Fasting Blood Sugar (FBS), Post Prandial Blood Sugar (PPBS) and glycated haemoglobin (HbA1c)
Sputum smear examination
Drug sensitivity testing using GeneXpert
Chest X-ray
Follow up Assessments
Sputum smear examination
Random blood sugar
GeneXpert and / or Line Probe Assay (LPA)
Tolerability and adverse events
Patients were advised for follow up every week for initial 2 months and once in 15 days thereafter. ATT and Metformin 250 mg tablets were dispensed to the patients once a week for first 2 months and once in 15 days for the remaining period. During the follow up period, sputum smear examination was done once weekly till it became negative and at the end of intensive phase. Those patients who were still sputum positive after intensive phase, continued to receive the same treatment, if they were drug-sensitive and their sputum was tested every week till it became negative, whereas they were removed from the study if they became drug-resistant at the end of intensive phase.
Random blood sugar was assessed once in 15 days for the first two months and once a month thereafter. Tolerability and adverse events were recorded during each follow up visit. The patients were asked to report immediately in case of any adverse event. At the end of intensive phase, drug sensitivity testing was done using GeneXpert and / or LPA.
Drug Resistance
All the subjects, at the time of enrolment, were subjected to drug sensitivity analysis using GeneXpert. Only those subjects who were drug-sensitive were enrolled in the study and drug-resistant patients were excluded. The subjects who remained sputum smear positive after 2 months were again subjected to GeneXpert analysis. If they were still drug-sensitive, same treatment was continued and those who were found to be drug-resistant (with GeneXpert) after 2 months, were subjected to LPA analysis and removed from the study and appropriate alternate drug regimens were provided to them.
Study Assessments at the End of 6 Months
Complete blood count – Hb, total RBC count, total WBC count, Differential count, platelet count
Erythrocyte Sedimentation Rate
Liver function tests – AST, ALT, ALP, total bilirubin and direct bilirubin
Renal function tests – BUN & Creatinine
Statistical Analysis
Graph pad instat software version 3.0 was used for analysing the data generated in the study. All the variables were subjected to descriptive analysis and summary statistics was generated. Chi square test was used for analysing drug resistance and sputum conversion. All continuous variables were tested for significant differences by using paired t test within group and unpaired t test between groups. Average duration for sputum conversion between groups was statistically compared by using unpaired t test. Blood sugar values obtained during periodic assessments were analysed using repeated measures ANOVA within group and one-way ANOVA between the groups.
Results
Demographic Data
The mean age of the patients in control group was 43 (±12.8) years and in Metformin group, it was 39.3 (±12.1) years. In control group, there were 37 males and 13 females and in Metformin group, 35 males and 15 females. There was no significant difference seen in age and gender distribution of the patients between two groups, as evidenced by the p value more than 0.05 in unpaired t test for age and chi square test for gender. Hence, both the groups were comparable in terms of age and gender.
Sputum Smear Conversion
Sputum smear examination was done at baseline and once a week till it became negative. Weekly sputum smear assessment showed that significant number of patients attained smear negativity in the Metformin group compared to the control group. The number of patients who attained sputum smear conversion in both the groups is shown in table 1 and represented graphically in figure 1. In metformin group, one patient remained sputum positive after completion of intensive phase and in control group 9 patients remained sputum positive.
The average time taken for sputum smear conversion was significantly lower in the Metformin group in comparison with the control group (p = 0.012, unpaired t-test). It was about 3.4 (±1.74) weeks in Metformin group while it was 4.7 (±2.31) weeks in the control group.
Drug Resistance Pattern
Drug susceptibility testing was performed at the end of intensive phase for patients who remained sputum positive, in both the groups using GeneXpert. In Metformin group, one patient who remained sputum positive had resistance for Rifampicin. In control group, out of 9 patients who remained sputum positive, three patients had resistance for Rifampicin and one patient had indeterminate result in GeneXpert. The sputum of the patient who had indeterminate result in GeneXpert was analysed in LPA and found to have INH resistance. The other five patients in control group who were sputum positive showed sensitivity to the standard ATT and hence they were continued on the same medications and eventually they became sputum negative. The difference in the development of drug resistance between the two groups was not statistically significant (p value=0.358, chi square test). The drug resistant patients were removed from the study and appropriate alternate drug regimens were provided to them.
Complete Blood Count (CBC)
The blood parameters such as Haemoglobin, total RBC count, total WBC count, Differential count and platelet count were measured at the baseline and at the end of the study. The difference noted between the values observed before and after treatment was not statistically significant between Metformin and control groups. The analysis was done by using unpaired t test (between group analysis) and the p value was more than 0.05. Within group analysis was done by using paired t test which showed that there was a reduction in total WBC count and ESR within control and Metformin groups and the reduction was statistically significant (p-value less than 0.05). The other parameters did not show significant changes in the within group analysis.
Renal Function Tests (RFT)
Renal function tests which include Blood urea nitrogen (BUN) and serum creatinine did not show any significant differences within the groups and between the groups.
Liver Function Tests (LFT)
Liver function tests showed significant increase in the liver enzymes- AST, ALT and ALP, at the end of the study when compared with baseline values. The increase was seen in both control and Metformin groups but inter group comparison did not show any statistically significant difference in the enzyme levels. There was no significant difference in the total and direct bilirubin values both within the groups and between the groups.
Random Blood Sugar
All the subjects enrolled in the study were non-diabetics. At the time of enrollment, their fasting and post prandial blood sugar and HbA1c values were measured and only those who were having normal values were selected for the study. The mean fasting blood sugar was 95.5±9.8mg/dl and 91.2±12.4mg/dl and the mean sugar values at post prandial state was 126.22±25.15 mg/dl and 124.98±31.11 mg/dl in control and Metformin groups respectively at the time of enrollment. In control group, the baseline HbA1c was 4.72±0.51 % and it was 4.85±0.73 % in Metformin group.
After the initiation of treatment, random blood sugar was measured once in 15 days for first two months and once in a month thereafter. Within group analysis was done using repeated measures ANOVA and between group analysis was done by using one-way ANOVA to detect the differences in random blood sugar values. There was no statistically significant difference noted in the RBS values within the groups in both control and Metformin groups. When RBS values of control and Metformin groups were compared, it showed significant difference between the groups (p<0.001). Though statistically significant, there was no clinical significance as the mean values were within the normal range.
Adverse Events
Adverse events were seen in 4 patients (8%) in control group and 6 patients (12%) in Metformin group. The difference was not statistically significant (p value = 0.818, chi square test). All of the adverse events were only minor in nature and gastrointestinal related problems like nausea, vomiting and gastritis.
Table 1: Sputum smear conversion (positive to negative)
| Week | Control | Metformin | p-value |
| 1 | 3 (6%) | 8 (16%) | 0.11 |
| 2 | 7 (14%) | 16 (32%) | 0.032* |
| 3 | 13 (26%) | 23 (46%) | 0.037* |
| 4 | 26 (52%) | 38 (76%) | 0.012* |
| 5 | 33 (66%) | 42 (84%) | 0.064 |
| 6 | 38 (76%) | 47 (94%) | 0.025* |
| 7 | 40 (80%) | 49 (98%) | 0.01* |
| 8 | 41 (82%) | 49 (98%) | 0.019* |
No. of patients attained sputum smear negativity
Control Vs Metformin group- Chi-square test
*p value <0.05-statistically significant
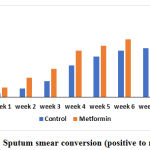 |
Figure 1: Sputum smear conversion (positive to negative) |
 |
Table 2: Complete Blood Count & ESR |
Discussion
Sputum smear examination is the test which is usually done to assess the treatment outcome in pulmonary tuberculosis patients. It is an inexpensive and easy method when compared to sputum culture. Sputum smear examination is usually done at the end of intensive phase and if it becomes negative, it indicates good prognosis. If the sputum smear remains positive despite treatment, it might result in treatment failure, relapse and increase the chance of drug resistance20,21. Sputum smear positive patients are highly infectious and one of the important goals of anti-tubercular therapy is to render the patients non-infectious as a smear positive patient can infect more than 10 persons annually.22
In our study, the average time taken for smear conversion in control group was 4.7 weeks, which was almost similar to the results obtained from a prospective study done by Parikh et al., in 2012. In that study, the average time required for sputum smear conversion was 5 weeks in patients who were on category I DOTS treatment19. In Metformin group, the average time taken for sputum conversion was 3.4 weeks, which was significantly less when compared to control group and Parikh et al.19
In this study, Metformin added to standard therapy was found to have significant effect on sputum smear conversion. The number of patients who had become smear negative was significantly high in the Metformin group when compared to control. This difference was observed every week and at the end of 8 weeks, 49 patients (98%) in Metformin group attained smear negativity as against 41 patients (82%) in the control group.
The role of Metformin in tuberculosis has been studied only in diabetic patients so far. Singhal et al., in their study found that tuberculous patients, who were taking Metformin for Diabetes showed reduced number of pulmonary cavities when compared to the patients who were on other anti-diabetic medications15.Ye-Jin Lee et al, in their retrospective study found that pulmonary tuberculosis patients with cavitatory TB taking Metformin for Diabetes showed significantly higher sputum culture conversion rates at the end of two months16.Y. Ma et al (2018), in their retrospective cohort study involving TB patients with Diabetes, found out that Metformin treatment had a favourable effect on treatment success rate, sputum culture conversion at the end of two months and also the relapse rates when compared to the diabetic patients who were not on Metformin.18
In the present study, drug resistance pattern also showed changes between the control and Metformin group. Drug sensitivity testing was done using the molecular methods, GeneXpert and/or LPA at the end of 2 months. It was observed that 4 patients (8%) in the control group showed drug resistance, 3 patients became resistant to Rifampicin, identified using GeneXpert and 1 patient to Isoniazid, identified using LPA. In Metformin group, drug resistance was seen in only one patient (2%) who demonstrated resistance for Rifampicin.
One of the reasons for antibiotic resistance in tuberculosis is the formation of persister phenotypes of Mycobacteria which can survive even in the presence of antibiotics. These are slow growing and genetically similar to susceptible bacteria.23 The main mechanism of persister formation is utilisation of the NAD (Nicotinamide adenine dinucleotide) pathway and NDH-I (NADH dehydrogenase-I) for ATP synthesis. NDH-I is similar to human mitochondrial complex-I. Metformin is an inhibitor of mitochondrial complex-I and hence it could also inhibit NDH-I of Mycobacteria and prevent the formation of persister phenotypes, thereby preventing resistance.24
Along with antibiotics, host immune mechanisms are very important in destroying the TB bacilli. In animal models of TB, Metformin treatment increased the production of CD4+ and CD8+ T-lymphocytes and there are also an increased percentage of Interferon-γ secreting CD8+ cells. By inhibiting mitochondrial complex-I, Metformin increases the production of mitochondrial ROS and damages the bacterial cell.15 Mycobacteria, on entering the host cells by phagocytosis, prevents the maturation of phagosome and starts replicating within the cell. Phagosome maturation is essential for eliminating the pathogen. Autophagy is a defense mechanism which involves the formation of autophagosome, a double membrane vesicle engulfing the cellular components along with the microbes and this autophagosome then fuses with the lysosome, leading to degradation of the cellular components.25 Metformin was found to induce autophagy and phagolysosome fusion in the host cells.15
In the present study, adverse drug reactions were seen in 4 patients (8%) in the control group and 6 patients (12%) in the Metformin group and the difference noted between the groups was not statistically significant. The adverse reactions seen in both the groups were only mild and most of them were gastrointestinal related symptoms like nausea, vomiting and gastritis. These adverse events are not specific to Metformin and could occur with anti TB drugs also. Hypoglycaemia was not reported in any of the patients in the Metformin group.
When the incidence of adverse events in this study was compared to literature reported data, it was noted that the occurrence of adverse events in the present study was less. Singh A et al. (2015), in their review article, stated that the incidence of adverse events in patients taking ATT was between 2.3% to 17%, commonly involving hepatobiliary and gastrointestinal system. The authors of the review article further quoted an Indian study done by Shinde et al., which reported the incidence of GI adverse effects like nausea, vomiting and abdominal pain as 12.5% with ATT26. Bray GA et al., in their article, reported that gastrointestinal adverse effects were common with Metformin and the incidence was around 28%27. However in the present study, the incidence of adverse events was less, 8% in control group and 12% in metformin group.
In summary, Metformin added to the standard ATT in pulmonary tuberculosis patients produced beneficial effects – significant reduction in the time needed for sputum smear conversion and decreased the occurrence of drug resistance. It was also well tolerated by the tuberculosis patients.
The goal of WHO’s End-TB strategy is to cut down the incidence of TB to 80% and mortality rate to 90% by 20302. To achieve these goals, it is important to reduce the transmission of TB in the community. As smear positive patients pose major risk by way of transmitting the disease to other people, any treatment modality providing early smear conversion is encouraging from public health point of view.
Limitations of the Study
This study was done in newly diagnosed smear positive, pulmonary tuberculosis. Inclusion of smear negative tuberculous patients, patients with extra pulmonary tuberculosis and complicated TB would have added more value to this study.
The primary efficacy parameters assessed in this study were sputum smear conversion and drug resistance. But the previous studies assessing the usefulness of Metformin in TB had varied and wide outcome measures such as sputum culture conversion, pulmonary cavities in Chest X-rays, long term follow up and relapse rates.16, 17, 18, 19 These parameters were not studied in the present study. Though chest X-ray was taken for all the subjects at baseline and at the end of 2 months and 6 months, the radiological findings were not included in the analysis.
Some of the previous studies on Metformin in TB had in-vitro assessments explaining the favorable role of Metformin on T-cell immune responses by measuring CD4+/CD8+ cell counts in Mtb infection and its anti-inflammatory effect by measuring the levels of pro-inflammatory cytokines.15, 28 These assessments, if carried out in the present study, would have added more value.
The present study was done with 50 patients in each group. Though sputum smear conversion and drug resistance were in favor of Metformin group, a larger sample size and multi center patient enrollment would have supported the outcome with much more clinical and statistical strength.
In spite of these limitations, this study was able to demonstrate the beneficial effects of Metformin add-on therapy to the existing antibiotics in pulmonary tuberculosis patients in terms of earlier sputum conversion and decrease in emergence of drug resistance. Since smear positive pulmonary tuberculosis patients are the major source of infection, the attainment of sputum negativity in short time render the patient non-infectious to others and the transmission could be reduced.
Conclusion
This study was done to evaluate the efficacy and safety of Metformin add-on therapy to standard ATT in newly diagnosed pulmonary tuberculosis patients. It was observed that the average time taken for sputum smear conversion was 3.4 weeks in Metformin group and 4.7 weeks in control group. Drug resistance pattern at the end of 2 months showed that 1 patient in Metformin group became resistant to Rifampicin and 4 patients in control group showed resistance, 3 patients for Rifampicin and 1 for Isoniazid. There were no serious adverse events and most of the adverse events were gastrointestinal related and minor in nature.
This study provides preliminary data that supports Metformin added to standard ATT potentially benefiting TB patients as evidenced by (i) significant reduction in the time needed for sputum smear conversion and (ii) reduction in the occurrence of drug resistance. However, further studies with large sample size and with varied outcome measures are needed to confirm the observations noted in this study.
Acknowledgement
The authors are grateful to Chettinad Hospital and Research Institute (CHRI), Chettinad Academy of Research and Education (CARE) for supporting the study.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- https://www.who.int/news-room/fact-sheets/detail/tuberculosis
- World Health Organization. Global tuberculosis report 2018. World Health Organization; 2018.
- India TB. Revised National Tuberculosis Control Programme Annual Status Report. Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare. New Delhi: Central TB Division. 2018.
- Revised National Tuberculosis Control Programme (RNTCP), 2016. Technical and operational guidelines for tuberculosis control in India.
- Chaudhuri AD. Recent changes in technical and operational guidelines for tuberculosis control programme in India-2016: A paradigm shift in tuberculosis control. The Journal of Association of Chest Physicians. 2017 Jan 1;5(1):1.
- Van Rie A, Warren R, Richardson M, Gie RP, Enarson DA, Beyers N, Van Helden PD. Classification of drug-resistant tuberculosis in an epidemic area. The Lancet. 2000 Jul 1;356(9223):22-5.
- Liang L, Wu Q, Gao L, Hao Y, Liu C, Xie Y, Sun H, Yan X, Li F, Li H, Fang H. Factors contributing to the high prevalence of multidrug-resistant tuberculosis: a study from China. Thorax. 2012 Jul 1;67(7):632-8.
- Gillespie SH. Evolution of drug resistance in Mycobacterium tuberculosis: clinical and molecular perspective. Antimicrobial agents and chemotherapy. 2002 Feb 1;46(2):267-74.
- Palucci I, Delogu G. Host directed therapies for tuberculosis: futures strategies for an ancient disease. Chemotherapy. 2018;63(3):172-80.
- Kolloli A, Subbian S. Host-directed therapeutic strategies for tuberculosis. Frontiers in medicine. 2017 Oct 18;4:171.
- Bristol‐Myers Squibb Company. GLUCOPHAGE®(metformin hydrochloride) tablets and GLUCOPHAGE® XR (metformin hydrochloride) extended-release tablets prescribing information.
- Motta AB. Mechanisms involved in metformin action in the treatment of polycystic ovary syndrome. Current pharmaceutical design. 2009 Sep 1;15(26):3074-7.
- Ning HH, Le J, Wang Q, Young CA, Deng B, Gao PX, Zhang HQ, Qin SL. The effects of metformin on simple obesity: A meta-analysis.
- Elamin Abdelgadir RA, Rashid F, Bashier A. Effect of metformin on different non-diabetes related conditions, a special focus on malignant conditions: review of literature. Journal of clinical medicine research. 2017 May;9(5):388.
- Singhal A, Jie L, Kumar P, Hong GS, Leow MK, Paleja B, Tsenova L, Kurepina N, Chen J, Zolezzi F, Kreiswirth B. Metformin as adjunct antituberculosis therapy. Science translational medicine. 2014 Nov 19;6(263):263ra159-.
- Lee YJ, Han SK, Park JH, Lee JK, Kim DK, Chung HS, Heo EY. The effect of metformin on culture conversion in tuberculosis patients with diabetes mellitus. The Korean journal of internal medicine. 2018 Sep;33(5):933.
- Marupuru S, Senapati P, Pathadka S, Miraj SS, Unnikrishnan MK, Manu MK. Protective effect of metformin against tuberculosis infections in diabetic patients: an observational study of south Indian tertiary healthcare facility. Brazilian Journal of Infectious Diseases. 2017 Jun;21(3):312-6.
- Ma Y, Pang Y, Shu W, Liu YH, Ge QP, Du J, Li L, Gao WW. Metformin reduces the relapse rate of tuberculosis patients with diabetes mellitus: experiences from 3-year follow-up. European Journal of Clinical Microbiology & Infectious Diseases. 2018 Jul 1;37(7):1259-63.
- Parikh R, Nataraj G, Kanade S, Khatri V, Mehta P. Time to sputum conversion in smear positive pulmonary TB patients on category I DOTS and factors delaying it. J Assoc Physicians India. 2012 Aug;60(22):6.
- Singla R, Bharty SK, Gupta UA, Khayyam KU, Vohra V, Singla N, Myneedu VP, Behera D. Sputum smear positivity at two months in previously untreated pulmonary tuberculosis patients. International journal of mycobacteriology. 2013 Dec 1;2(4):199-205.
- Kim J, Kwak N, Lee HY, Kim TS, Kim CK, Han SK, Yim JJ. Effect of drug resistance on negative conversion of sputum culture in patients with pulmonary tuberculosis. International Journal of Infectious Diseases. 2016 Jan 1;42:64-8.
- Ekinci GH, Karakaya E, Ongel EA, Haciomeroglu O, Yilmaz A. Patient and doctor delays in smear-negative and smear-positive pulmonary tuberculosis patients attending a referral hospital in Istanbul, Turkey. The Scientific World Journal. 2014;2014.
- Zhang Y, Yew WW, Barer MR. Targeting persisters for tuberculosis control. Antimicrobial agents and chemotherapy. 2012 May 1;56(5):2223-30.
- Vashisht R, Brahmachari SK. Metformin as a potential combination therapy with existing front-line antibiotics for tuberculosis.
- Bento CF, Empadinhas N, Mendes V. Autophagy in the fight against tuberculosis. DNA and cell biology. 2015 Apr 1;34(4):228-42.
- Singh A, Prasad R, Balasubramanian V, Gupta N, Gupta P. Prevalence of adverse drug reaction with first-line drugs among patients treated for pulmonary tuberculosis. Clinical Epidemiology and Global Health. 2015 Jan 1;3:S80-90.
- Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes care. 2012 Apr 1;35(4):731-7.
- Lachmandas E, Eckold C, Böhme J, Koeken VA, Marzuki MB, Blok B, Arts RJ, Chen J, Teng KW, Ratter J, Smolders EJ. Metformin alters human host responses to Mycobacterium tuberculosis in healthy subjects. The Journal of infectious diseases. 2019 Feb 12;220(1):139-50.







