Manuscript accepted on :15-02-2020
Published online on: 07-03-2020
Plagiarism Check: Yes
Reviewed by: Gundu Rao
Second Review by: hazem shaheen
Final Approval by: Dr. Mohamed Abdel-Daim
Nasrin Nisha A1 , Ruckmani A1
, Ruckmani A1 , Rajasekaran D2
, Rajasekaran D2 , Abinaya E1
, Abinaya E1 , Tanuja Lella1
, Tanuja Lella1 and Arunkumar R1*
and Arunkumar R1*
1Department of Pharmacology, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, Chennai-603103, Tamil Nadu, India.
2Department of General Medicine, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, Chennai-603103, Tamil Nadu, India.
Corresponding Author E-mail : drarunvp@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1886
Abstract
To evaluate the safety and efficacy of Bromocriptine in comparison with Teneligliptin in patients with newly diagnosed type 2 diabetes mellitus with the following outcome measurements. FBS (Fasting blood sugar) PPBS (Post prandial blood sugar-2 hours after breakfast) HbA1c (Glycosylated hemoglobin) BMI (Body mass index) Adverse events and tolerability The study was a prospective, open labelled randomized controlled, clinical evaluation done in 50 newly diagnosed type 2 diabetic patients. The study participants were randomly divided into two groups, Bromocriptine group (25 patients) and Teneligliptin group (25 patients). Bromocriptine was administered at the dose of 0.8 mg in the morning with food which was increased to 1.6 mg from 15th day and was maintained at 1.6 mg till the completion of the study (3 months). Teneligliptin was administered at a dose of 20 mg, once a day in the morning after food, throughout the study (3 months). FBS, PPBS, HbA1c and BMI were assessed at baseline and at the end of 1st, 2nd and 3rd months. Statistical analysis was done using one-way ANOVA (Analysis of variance) between the groups and repeated measures ANOVA within the groups.Bromocriptine and Teneligliptin significantly reduced FBS, PPBS, HbA1c and BMI values (p value < 0.0001). However, Teneligliptin was found to be superior to Bromocriptine in the reduction of FBS, PPBS and HbA1c levels whereas Bromocriptine was found to be superior in reducing BMI levels at the end of 3 months. Teneligliptin was well tolerated by all the patients with no adverse events reported, whereas, 5 patients taking Bromocriptine were found to have nausea as adverse effect.Both the drugs, Teneligliptin and Bromocriptine significantly reduced FBS, PPBS, HbA1c and BMI at the end of 3 months in newly diagnosed type 2 diabetic patients. But Teneligliptin was superior to Bromocriptine in overall efficacy and safety.
Keywords
Blood Sugar; Body Mass Index (BMI); Bromocriptine; Diabetes Mellitus; Teneligliptin
Download this article as:| Copy the following to cite this article: Nisha A. N, Ruckmani A, Rajasekaran D, Abinaya E, Lella T , Arunkumar R. Assessment of Safety and Efficacy of Bromocriptine in Comparison with Teneligliptin in Newly Diagnosed Type 2 Diabetes Mellitus. Biomed Pharmacol J 2020;13(1). |
| Copy the following to cite this URL: Nisha A. N, Ruckmani A, Rajasekaran D, Abinaya E, Lella T , Arunkumar R. Assessment of Safety and Efficacy of Bromocriptine in Comparison with Teneligliptin in Newly Diagnosed Type 2 Diabetes Mellitus. Biomed Pharmacol J 2020;13(1). Available from: https://bit.ly/38By1hC |
Introduction
Diabetes mellitus (DM) is a metabolic syndrome characterized by the presence of hyperglycemia that occurs due to reduction in insulin secretion or increase in insulin resistance in peripheral tissues. Sometimes both reduction in insulin secretion and increased insulin resistance can occur simultaneously (1). International Diabetic Federation (IDF) has estimated that approximately 69.2 million Indians had diabetes mellitus in 2015. This number may increase to 123.5 million by 2040. Of the total diabetic population in India, 90% of patients suffer from type 2 diabetes (2).
The major goals in the management of diabetes are to maintain optimum blood glucose level, alleviate symptoms, prevent complications, reduce morbidity and increase the survival period of life. Life style modification plays a major role in controlling diabetes. Fat free diet, regular physical activity, stress free life and cessation of alcohol and smoking play key roles in managing blood sugar levels. Pharmacological treatment should be started immediately when glycemic control is not achieved with life style modifications or if HbA1C is more than 6.5%, as early initiation of pharmacotherapy would reduce the risk of microvascular complications (3). Cost-effectiveness, ability to reduce HbA1C, potential side effects such as weight gain, hypoglycemia and patient comorbidities should be considered while choosing the pharmacological therapy. Initially, treatment with a single drug should be started along with lifestyle modifications (4). The major classes of oral anti diabetic medications which are available in market are Biguanides, Sulfonylureas, Meglitinides, Thiazolidinediones (TZDs), Dipeptidyl peptidase-4 (DPP-4) inhibitors, Sodium glucose cotransporter-2 (SGLT-2) inhibitors, α-glucosidase inhibitors, Bile acid sequestrants and Dopamine agonists (5).
DPP-4 inhibitors act by inhibiting dipeptidyl peptidase enzyme and increase the levels of incretin hormones in gastro intestinal system. Incretin hormones primarily act by stimulating ‘insulin synthesis and secretion’ in a glucose-dependent manner in beta cells and by reducing glucagon secretion from alpha cells of pancreas. They also delay gastric emptying, promote satiety leading to reduced appetite and weight loss. DPP-4 inhibitors are now considered as effective drugs in the management of Type 2 DM due to their efficacy, low risk of hypoglycemia & weight gain and good patient compliance due to once or twice daily dosage (6).
Teneligliptin is a novel DPP-4 inhibitor. It was developed by Mitsubishi Tanabe Pharma Corporation in Osaka Japan. It was first approved in Japan in September 2012 for management of Type 2 Diabetes Mellitus along with life style modification. Teneligliptin was introduced in India in May 2015 after obtaining DCGI (Drug Controller General of India) approval. Teneligliptin given orally for 3 months has shown improvements in left ventricular systolic and diastolic function, endothelial function, and an increase in circulating adiponectin levels in addition to improved glycemic control (7). Advantages of Teneligliptin are weight loss, safety in end stage renal disease, once daily dosing thereby increasing patient adherence to treatment and less risk of hypoglycemia (5). Adverse effects commonly reported with Teneligliptin are hypoglycemia (3%) and constipation (0.9%). Hypoglycemia occurs when Teneligliptin is given along with other hypoglycemic drugs, than Teneligliptin given alone. Incretins cause delayed gastro intestinal motility leading to constipation and sometimes intestinal obstruction. Hence DPP-4 inhibitors should be used cautiously in patients with previous history of intestinal obstruction and abdominal surgery (8).
Bromocriptine Mesylate is a semi synthetic ergot derivative which is a central dopamine receptor (D2) agonist, α1 antagonist and α2 agonist. This drug has been approved by FDA (Food and Drug Administration) in 1978 for the treatment of Parkinson disease, acromegaly, hyperprolactinemia associated dysfunctions like Amenorrhea, galactorrhea, infertility or hypogonadism and prolactin-secreting adenomas. It was approved for the treatment of type 2 DM in adults as an adjunct to diet and exercise to improve glycemic control by FDA in 2009 (9). Bromocriptine has unique mechanism of action which is not fully understood. It acts by resetting circadian rhythm by modulating dopaminergic and sympathetic tone within the central nervous system. Bromocriptine should be given within 2 hours of awakening from sleep. It augments low hypothalamic dopamine levels and inhibits the excessive sympathetic tone within central nervous system. Hepatic glucose production will be suppressed by this effect leading to reduction in PPBS levels. It also inhibits lipolysis and lipogenesis in adipose tissue leading to decrease in free fatty acid (FFA) and triglyceride levels and increases insulin sensitivity (10). Adverse effects noted with Bromocriptine are nausea, rhinitis, head ache, dizziness, fatigue, vomiting, diarrhea, constipation, sinusitis, anorexia, dyspepsia, hypotension, somnolence and psychosis. It is contraindicated in patients suffering from syncopal migraine and psychotic disorders, and patients on neuroleptics and who are breast feeding (11).
Bromocriptine is the first anti-diabetic drug approved by the US FDA, which met cardiovascular safety guidelines released in December 2018. It can be used in moderate renal impairment patients where Metformin is contraindicated. Risk of hypoglycemia associated with Bromocriptine is very low, hence it is superior to sulfonylureas in patients with hypoglycemia risk. It is not associated with edema or risk of CHF (Congestive heart failure) which are common with TZDs (12). Once daily dosing in the morning makes patient compliance better. It causes weight loss and reduction in systolic blood pressure which is advantageous in patients with diabetes and hypertension (13). Resetting circadian rhythm is a novel mechanism of action which is different from other currently used anti-diabetic agents. It also lowers FFA and triglyceride levels, reduces the risk of MI (Myocardial infarction), stroke and other vascular events (14).
Recently approved Teneligliptin and Bromocriptine which act through novel mechanisms have advantages of increased patient adherence, being economical & cardio protective, having low risk of hypoglycemia and being used in end stage renal failure with DM. Many studies for Teneligliptin are done on Japan population and there is lack of clinical data in Indian population. Regarding Bromocriptine, there are studies which compare the effects of Bromocriptine in combination with Metformin and other oral anti diabetic drugs. But the efficacy of Bromocriptine monotherapy in type 2 Diabetes Mellitus was not investigated much. Moreover, though Bromocriptine was approved in 2009 and has a good safety profile, it is not widely used.
Hence, the present study was planned to systematically evaluate the safety and efficacy of Bromocriptine in comparison with Teneligliptin in patients with newly diagnosed type 2 diabetes mellitus.
Aims and Objectives
The aim of the study was to assess the effectiveness of Bromocriptine in the treatment of type 2 Diabetes mellitus in comparison with Teneligliptin.
The objectives were to evaluate the efficacy and safety of bromocriptine in comparison with Teneligliptin in newly diagnosed type 2 Diabetes mellitus with the following outcome measurements.
FBS (Fasting blood sugar)
PPBS (Post prandial blood sugar -2 hours after breakfast)
HbA1c (Glycosylated hemoglobin)
BMI (Body mass index)
Adverse reactions and tolerability
Materials and Methods
The study was initiated after obtaining approval from the Institutional Human Ethics Committee (IHEC). The approval number is 03 / IHEC / 03 – 18. CTRI (Clinical trial registry-India) registration number is CTRI/2018/05/013591
It was a prospective, open labelled, randomized controlled study done in 50 newly diagnosed type 2 diabetic patients. Sample size was calculated by using the formula:
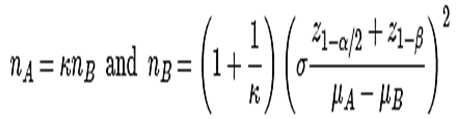
µA = mean reduction in blood sugar value for group 1 (assumed 37 mg/dl – Bromocriptine), µB = mean reduction in blood sugar value for group 2 (assumed 45 mg/dl – Teneligliptin), S.D (σ) = 10, sampling ratio (ⱪ) = 1, α = 5% & 1- β = 0.80
Simple randomization was done at the ratio of 1:1. 25 subjects in Bromocriptine group and 25 in Teneligliptin group were randomized by using online randomization table generator. Total study duration was 1 year (May 2018 – May 2019) and drug treatment period was 3 months.
Inclusion Criteria
Newly diagnosed type 2 diabetic patients
FBS between 140 mg/dl and 240 mg/dl and PPBS between 200 mg/dl and 350 mg/dl
Hb1Ac between 7% and 10%
Age – 30 to 60 years
Gender – Males and Females
BMI between 22 and 40 kg/m2
Exclusion Criteria
Patients with complications of diabetes mellitus
Pregnant and nursing women
Patients with known hypersensitivity to Bromocriptine, ergot related drugs or Teneligliptin.
Patients with migraine headache.
Patients with the history of significant disorders of Cardio vascular system, Gastro intestinal system, Respiratory system and Central Nervous system.
50 subjects who fulfilled inclusion and exclusion criteria were enrolled in the study. Their demographic data was recorded. As per the randomization code, they were dispensed with the study medications, either Bromocriptine or Teneligliptin. Initially, they were followed up for once in a week for one month and then once in a month for the next 2 months
All the subjects were advised to follow diet and life style modifications along with drug treatment.
If any subject was found to be having FBS more than 250 mg/dl and / or PPBS more than 300 mg/dl after 1 month of treatment, there was a scope to exclude those subject(s) and offer rescue medications for effective control of blood sugar. In this study, none of the subjects had their FBS and PPBS beyond 250 mg/dl and 300 mg/dl respectively after one month of treatment.
Dose of Study Medications
Tablet Bromocriptine – 0.8 mg orally once a day in the morning with food. For those patients who had tolerated 0.8 mg dose, the dose was increased to 1.6 mg from 15th day and maintained at 1.6 mg till the completion of the study (3 months)
Tablet Teneligliptin – 20 mg orally once a day after food in the morning (3 months)
Study Assessments
FBS, PPBS, HbA1c and BMI levels were estimated at baseline and once in a month for 3 months
Tolerability and adverse events were recorded during every visit
In case of adverse events, they were managed as per the standard medical practice.
Statistical Methods
Statistical analysis was done using GraphPad InStat version 3.0 and Microsoft Excel 2013.
Descriptive statistics of demographic data and endpoints were carried out within the group.
Mean and Standard Deviation (SD) were derived for the results of FBS, PPBS, HbA1C and BMI.
Baseline characteristics were compared using unpaired t-test, between groups.
Comparative statistics were done for the efficacy variables within the group by repeated series ANOVA and between the groups by one-way ANOVA.
p value < 0.05 was considered significant
Results
In this study, the safety and efficacy of Bromocriptine was investigated in comparison with Teneligliptin in patients with newly diagnosed type 2 diabetes mellitus. 50 patients were enrolled and randomized at the ratio of 1:1 by simple randomization, 25 in Bromocriptine group and 25 in Teneligliptin group. All 50 patients completed the study entirely and there were no drop outs.
The following parameters were assessed in Bromocriptine and Teneligliptin groups to assess the safety and efficacy of the study drugs.
FBS – monthly once
PPBS (2 hours after breakfast) – monthly once
HbA1c – monthly once
BMI – monthly once
Adverse reactions and tolerability – during every visit
Baseline Data
Baseline demographic and laboratory variables were age, FBS, PPBS, HbA1c and BMI. The mean values observed between the two groups were not statistically significant. In Bromocriptine group there were 12 males and 13 females and in Teneligliptin group, there were 13 males and 12 females. No significant difference was seen between the two groups (p = 1.000, chi square test).
Baseline demographic and laboratory data are shown in table 1.
Table 1: Baseline demographic and laboratory data
| Bromocriptine
Mean (SD) |
Teneligliptin
Mean (SD) |
p value
(unpaired t test) |
|
| Age (years) | 47.28 (7.38) | 49.32(7.97) | 0.352 |
| FBS (mg/dl) | 184.32 (29.29) | 182.4 (25.95) | 0.807 |
| PPBS (mg/dl) | 263.32 (34.53) | 264.72 (38.22) | 0.892 |
| HbA1c (%) | 8.4 (0.7) | 8.8 (0.8) | 0.063 |
| BMI (kg/m2) | 27.3 (2.5) | 27.1 (3.1) | 0.86 |
Statistics
Bromocriptine Vs Teneligliptin group – unpaired t test
p value < 0.05 was considered significant
Analysis of Fasting Blood Sugar
At the end of 1st month, the mean FBS in Bromocriptine group was 173.36 mg/dl (± 30.69) and Teneligliptin group was 151.48 mg/dl (±25.31). Mean reduction in FBS was 10.96 mg/dl (5.9%) in Bromocriptine group and 30.92 (17%) in Teneligliptin group. Mean reduction was found to be statistically significant in both groups (p < 0.001). The difference observed between the Teneligliptin group and the Bromocriptine group was statistically significant (p value < 0.001), indicating Teneligliptin was better than Bromocriptine.
At the end of 2nd month, mean FBS in Bromocriptine group was 165.08 mg/dl (± 30.01) and Teneligliptin group was 133.88 mg/dl (±21.09). Mean reduction in FBS was 19.24 mg/dl (10.4%) in Bromocriptine group and 48.52 mg/dl (26.6%) in Teneligliptin group. Mean reduction was found to be statistically significant in both groups (p < 0.001). The difference observed between Teneligliptin group and Bromocriptine group was statistically significant (p value < 0.001), indicating Teneligliptin was better than Bromocriptine.
At the end of 3rd month, mean FBS in Bromocriptine group was 154.32 mg/dl (± 31.23) and Teneligliptin group was 118.04 mg/dl (±17.57). Mean reduction in FBS was 30 mg/dl (16.3%) in Bromocriptine group & 64.36 mg/dl (35.3%) in Teneligliptin group. Mean reduction was found to be statistically significant in both groups (p < 0.001). The difference observed between Teneligliptin group and Bromocriptine group was statistically significant (p value < 0.001), indicating Teneligliptin was better than Bromocriptine. At 95 % confidence interval, Bromocriptine and Teneligliptin will reduce the FBS by 25.6 – 34.3 mg/dl and 55.2 – 73.5 mg/dl respectively at the end of 3 months of treatment.
The mean FBS of Bromocriptine and Teneligliptin groups is provided in table 2 and the same is graphically represented in figure 1.
The mean reduction in FBS of Bromocriptine and Teneligliptin groups is provided in table 3.
Table 2: Mean FBS of study population
| Mean FBS (mg/dl) | ||||||
| Group | Baseline | 1st month end | 2nd month end | 3rd month end | p value
(ANOVA) |
Confidence interval |
| Bromocriptine
Mean (SD) |
184.32 (29.29) | 173.36 (30.69) | 165.08
(30.41) |
154.32
(31.23) |
*<0.0001 | 25.6 – 34.3 |
| Teneligliptin
Mean (SD) |
182.4
(25.95) |
151.48
(25.31) |
133.88
(21.09) |
118.04
(17.57) |
*<0.0001 | 55.2 – 73.5 |
Statistics
* Bromocriptine & Teneligliptin groups (within the group) – Repeated measures ANOVA
p value < 0.05 was considered significant
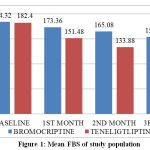 |
Figure 1: Mean FBS of study population |
Table 3: Mean reduction of FBS in study population
| Mean reduction in FBS (mg/dl) | |||
| Group | 1st month end | 2nd month end | 3rd month end |
| Bromocriptine
Mean (SD) |
10.96 (7.77) | 19.24 (8.52) | 30.00 (10.83) |
| Teneligliptin
Mean (SD) |
30.92 (15.10) | 48.52 (16.81) | 64.36 (22.48) |
| p value | *<0.001 | *<0.001 | *<0.001 |
| C.I | 8.0 – 31.8 | 17.3 – 41.1 | 22.4 – 46.2 |
Statistics
* Bromocriptine Vs Teneligliptin groups – one way ANOVA, Post hoc analysis done by Tukey-Kramer Multiple Comparisons Test
p value < 0.05 was considered significant
Analysis of Post Prandial Blood Sugar
At the end of 1st month, the mean PPBS in Bromocriptine group was 251.16 mg/dl (±33.98) and Teneligliptin group was 227.52mg/dl (±34.45). Mean reduction in PPBS was 12.16 mg/dl (4.6%) in Bromocriptine group and 37.2 mg/dl (14.1%) in Teneligliptin group. Mean reduction was found to be statistically significant in both groups (p < 0.001). The difference observed between Teneligliptin group and Bromocriptine group was statistically significant (p value < 0.001), indicating Teneligliptin was better than Bromocriptine.
At the end of 2nd month, mean PPBS in Bromocriptine group was 240.16 mg/dl (±34.96) and Teneligliptin group was 197.72 mg/dl (±27.71). Mean reduction in PPBS was 23.16 mg/dl (8.8%) in Bromocriptine group & 67 mg/dl (25.3%) in Teneligliptin group. Mean reduction was found to be statistically significant in both groups (p < 0.001). The difference observed between Teneligliptin group and Bromocriptine group was statistically significant (p value < 0.001), indicating Teneligliptin was better than Bromocriptine.
At the end of 3rd month, mean PPBS in Bromocriptine group was 231.44 mg/dl (±33.73) and Teneligliptin group was 172.04 mg/dl (±23.39). Mean reduction in PPBS was 31.88 mg/dl (12.1%) in Bromocriptine group and 92.68 mg/dl (35.0%) in the Teneligliptin group. Mean reduction was found to be statistically significant in both groups (p < 0.001). The difference observed between Teneligliptin group and Bromocriptine group was statistically significant (p value < 0.001), indicating Teneligliptin was better than Bromocriptine. At 95% confidence interval Bromocriptine and Teneligliptin will reduce the mean PPBS by 28.0 – 35.6 mg/dl and 77.7 – 107.6 mg/dl respectively at the end of 3 months of treatment.
The mean PPBS of Bromocriptine and Teneligliptin groups is provided in table 4 and the same is graphically represented in figure 2.
The mean reduction in PPBS of Bromocriptine and Teneligliptin groups is provided in table 5.
Table 4: Mean PPBS of study population
| Mean PPBS (mg/dl) | ||||||
| Group | Baseline | 1st month end | 2nd month end | 3rd month end | p value
(ANOVA) |
Confidence interval |
| Bromocriptine
Mean (SD) |
263.32 (34.53) | 251.16
(33.98) |
240.16
(34.96) |
231.44
(33.73) |
*<0.0001 | 28.0 – 35.6 |
| Teneligliptin
Mean (SD) |
264.72
(38.22) |
227.52
(34.45) |
197.72
(27.71) |
172.04
(23.39) |
*<0.0001 | 77.7 – 107.6 |
Statistics
* Bromocriptine & Teneligliptin groups (within the group) – Repeated measures ANOVA
p value < 0.05 was considered significant
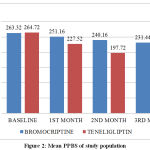 |
Figure 2: Mean PPBS of study population |
Table 5: Mean reduction of PPBS in study population
| Mean reduction in PPBS (mg/dl) | |||
| Group | 1st month end | 2nd month end | 3rd month end |
| Bromocriptine
Mean (SD) |
12.16 (6.06) | 23.16 (8.80) | 31.88 (8.65) |
| Teneligliptin
Mean (SD) |
37.2 (21.30) | 67.00 (31.58) | 92.68 (36.48) |
| p value | *<0.001 | *<0.001 | *<0.001 |
| C.I | 5.6 – 44.4 | 24.4 – 63.2 | 41.3 – 80.2 |
Statistics
* Bromocriptine Vs Teneligliptin group – one way ANOVA, Post hoc analysis done by Tukey-Kramer Multiple Comparisons Test
p value < 0.05 was considered significant
Analysis of Glycosylated Hemoglobin
At the end of 1st month, the mean HbA1c in Bromocriptine group was 8.1% (±0.7) and Teneligliptin group was 8.4% (±0.8). Mean reduction in HbA1c was 0.3% in Bromocriptine group & 0.4% in Teneligliptin group. Mean reduction was found to be statistically significant in both groups (p < 0.001). The difference observed between Teneligliptin group and Bromocriptine group was statistically significant (p < 0.05), indicating Teneligliptin was better than Bromocriptine.
At the end of 2nd month, the mean HbA1c in Bromocriptine group was 7.9% (±0.7) and Teneligliptin group was 8.0% (±0.8). Mean reduction in HbA1c was 0.5% in Bromocriptine group & 0.8% in Teneligliptin group. Mean reduction was found to be statistically significant in both groups (p < 0.001). The difference observed between Teneligliptin group and Bromocriptine group was statistically significant (p < 0.001), indicating Teneligliptin was better than Bromocriptine.
At the end of 3rd month, the mean HbA1c in Bromocriptine group was 7.6% (±0.7) and Teneligliptin group was 7.5% (±0.8). The mean reduction in HbA1c was 0.8% in Bromocriptine group & 1.3% in Teneligliptin group. Mean reduction was found to be statistically significant in both groups (p < 0.001). The difference observed between Teneligliptin group and Bromocriptine group was statistically significant (p value < 0.001), indicating Teneligliptin was better than Bromocriptine. At 95% confidence interval Bromocriptine and Teneligliptin will reduce the mean HbA1c by 0.6 – 0.7 % and 0.6 – 1.8 % respectively at the end of 3 months of treatment.
The mean HbA1c of Bromocriptine and Teneligliptin groups is provided in table 6 and the same is graphically represented in figure 3.
The mean reduction in HbA1c of Bromocriptine and Teneligliptin groups is provided in table 7.
Table 6: Mean HbA1c of study population
| Mean HbA1c (%) | ||||||
| Group | Baseline | 1st month end | 2nd month end | 3rd month end | p value
(ANOVA) |
Confidence interval |
| Bromocriptine
Mean (SD) |
8.4 (0.7) | 8.1 (0.7) | 7.9 (0.7) | 7.6 (0.7) | *<0.0001 | 0.6 – 0.7 |
| Teneligliptin
Mean (SD) |
8.8 (0.8) | 8.4 (0.8) | 8.0 (0.8) | 7.5 (0.8) | *<0.0001 | 0.6 – 1.8 |
Statistics
* Bromocriptine & Teneligliptin group (within the group) – Repeated measures ANOVA
p value < 0.05 was considered significant
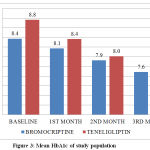 |
Figure 3: Mean HbA1c of study population |
Table 7: Mean reduction of HbA1c in study population
| Mean reduction in HbA1c (%) | |||
| Group | 1st month end | 2nd month end | 3rd month end |
| Bromocriptine
Mean (SD) |
0.3 (0.1) | 0.5 (0.2) | 0.8 (0.2) |
| Teneligliptin
Mean (SD) |
0.5 (0.1) | 0.8 (0.2) | 1.3 (0.3) |
| p value | *<0.05 | *<0.001 | *<0.001 |
| C.I | 0.2 – 0.3 | 0.1 – 0.4 | 0.3 – 0.7 |
Statistics
* Bromocriptine Vs Teneligliptin group – one way ANOVA, Post hoc analysis done by Tukey-Kramer Multiple Comparisons Test
p value < 0.05 was considered significant
Analysis of Body Mass Index
Bromocriptine and Teneligliptin showed statistically significant reduction in BMI at the end of three months. On comparing the two groups, Bromocriptine showed statistically significant reduction in BMI, indicating Bromocriptine was better than Teneligliptin
The mean BMI of Bromocriptine and Teneligliptin groups is provided in table 8
Table 8: Mean BMI of study population
| Mean BMI (kg/m2) | ||||||
| Group | Baseline | 1st month end | 2nd month end | 3rd month end | p value
(ANOVA) |
Confidence interval |
| Bromocriptine
Mean (SD) |
27.3 (2.5) | 26.9 (2.5) | 26.4 (2.5) | 26.2 (2.6) | *<0.0001 | 0.95 – 1.24 |
| Teneligliptin
Mean (SD) |
27.1 (3.1) | 26.9 (3.1) | 26.7 (3.2) | 26.5 (3.3) | *<0.0001 | 0.32 – 0.85 |
| p value
ANOVA |
0.86# | >0.05# | #<0.01 | #<0.01 | ||
Statistics
*Bromocriptine & Teneligliptin group (within the group) – Repeated measures ANOVA
#Bromocriptine Vs Teneligliptin (between the groups) – one way ANOVA, Post hoc analysis by Tukey-Kramer Multiple Comparisons Test.
p value < 0.05 was considered significant
Adverse Reactions and Tolerability
Teneligliptin was well tolerated by all the patients with no adverse events reported, whereas 5 patients (20%) in Bromocriptine group reported nausea. Nausea was observed during the escalating dose of bromocriptine from 0.8 mg to 1.6 mg which resolved spontaneously with reassurance and no subject was withdrawn due to adverse event in Bromocriptine group.
Discussion
The study was planned to evaluate the efficacy and safety of Bromocriptine in comparison with Teneligliptin in newly diagnosed type 2 diabetic patients.
Bromocriptine is a semi-synthetic ergot derivative that has a novel mechanism of action. It resets the circadian rhythm and thereby controls hyperglycemia (15). All the existing medications have mostly peripheral actions, either they increase insulin secretion or reduce insulin resistance or both. Bromocriptine, has been available in the market for more than a decade in India with the specific approval from CDSCO (Central drugs standard control organization) for diabetic indication, but it has never been a common drug chosen by the clinicians while they treat diabetes. This is the reason for conducting the present study to evaluate its efficacy and safety in diabetes mellitus.
In this study, Bromocriptine has significantly reduced FBS, PPBS, HbA1c and BMI in 25 diabetic patients at 1.6 mg daily dose. The dose was not increased further to the maximum of 4.8mg which was the recommended maximum dose for Bromocriptine in the treatment of diabetes mellitus (15). However, 1.6 mg dose was able to demonstrate statistically significant and clinically relevant reductions in FBS, PPBS, HbA1c and BMI and if the dose had been increased to 4.8 mg, the significance could have been much stronger and the reductions might have been matching the efficacy of Teneligliptin. It is pertinent to note here that Teneligliptin was superior to Bromocriptine in the present study in both efficacy and safety.
In the present study, Bromocriptine at 1.6 mg/day has reduced FBS by 30 mg/dl, PPBS by 31.88 mg/dl and HbA1c by 0.7%. The response observed in the present study is better than Ramteke KB et al. (16) who reported that Bromocriptine reduced FBS by 16.09 mg/dl, PPBS by 14.38 mg/dl and HbA1c by 0.4% with 2.4 mg administered for the same duration of 12 weeks.
Cincotta AH et al (17) observed in their study of assessing the efficacy of Bromocriptine monotherapy in diabetes mellitus that Bromocriptine at the dose of 4.8 mg/day reduced FBS by 31 mg/dl, PPBS by 37 mg/dl and HbA1c by 0.4%. Their observations were from a randomized controlled double-blind trial comparing Bromocriptine with placebo, conducted for the duration of 24 weeks. But the results of the present study were better than Cincotta AH et al.
While analyzing the data of Teneligliptin, it clearly outweighed the benefits of Bromocriptine by way of demonstrating much significant reductions in FBS (64.36 mg/dl), PPBS (92.68 mg/dl) and HbA1c (1.2%). However the reductions observed in PPBS and HbA1c with Teneligliptin were less when the data were compared with Glimepiride. Ramachandran A et al (18) conducted a randomized controlled, open labelled clinical trial in newly diagnosed type 2 diabetic patients. The patients were randomized into 4 groups. In Group 1, patients followed only diet and exercise, in Group 2, patients were given Glimepiride, 1-2 mg/day, in Group 3, they were given Metformin 250 – 850 mg/day and in Group 4, they were given Pioglitazone 15 – 30 mg/day. At the end of 12 weeks, there were significant reductions in FBS (0.3, 2.8, 1.6, 2.5 mmol/L in group 1, 2, 3 & 4 respectively), PPBS (2.0, 7.1, 5.0, 6.0 mmol/L in group 1, 2, 3 & 4 respectively) and HbA1c (0.3, 2.5, 1.4, 2.6 % in group 1, 2, 3 & 4 respectively). Regarding BMI, reduction was observed in group 1 (0.2 kg/m2), group 3 (0.2 kg/m2) and group 4 (0.4 kg/m2) while group 2 showed increase in BMI (1.7 kg/m2). On converting the mmol/L data of blood sugar to mg/dl by multiplying with numerical factor 18 (19), it can be seen that Glimepiride reduced FBS by 52 mg/dl and PPBS by 127 mg/dl while its ability to reduce HbA1c was by 2.5%. Hence it can be derived that Teneligliptin was better in reducing FBS than glimepiride while it was performing less than Glimepiride in reducing PPBS and HbA1c.
The challenging aspect of providing treatment in diabetes mellitus is weight management. Among the drugs that control hyperglycemia in an effective manner, the most efficacious drugs usually increase body weight. For example, Sulfonylureas, Meglitinides, Insulin and Insulin analogues increase body weight (18, 20). Pioglitazone also increases body weight (21). Metformin is the only anti diabetic agent that significantly reduces body weight (5).
The present study shows that Bromocriptine reduced body mass index by 1.1 kg/m2 and Teneligliptin by 0.6 kg/m2 with 3 months of treatment. Statistical analysis showed that the reduction in the BMI was significant within groups, and on comparing between groups using one way ANOVA, Bromocriptine was found to be superior to Teneligliptin. Though Teneligliptin was superior to Bromocriptine on all other fronts, Bromocriptine even at the dose of less than half (1.6 mg/day) of the maximum dose of 4.8 mg/day was able to produce significant reductions in the body mass index. This is really encouraging as it may help to tackle the weight gain associated with other high efficacy anti diabetic drugs. And it will be interesting to study further whether addition of Bromocriptine with drugs like Glimepiride, Glibenclamide or Insulin will be able to reduce BMI while increasing the anti-diabetic efficacy. Cincotta AH and Meier AH (22) in 1996 reported, in their publication, that Bromocriptine significantly reduced the body weight to the extent of 6.3 kg at “1.6 to 2.4 mg/day” dose administered for the duration of 18 weeks. As BMI is the better mass criteria than body weight, in the present study only BMI was critically considered, though Bromocriptine reduced body weight by 3.8 kg at the end of 3 months of treatment, while Teneligliptin reduced it by 2.1 kg.
With regard to safety of Bromocriptine, in the present study, 5 patients out of 25 who were treated with Bromocriptine had nausea while the patients who were treated with Teneligliptin did not have any adverse event. Hence it is very clear that Teneligliptin was better tolerated than Bromocriptine. However, Bromocriptine is not the only anti-diabetic agent which is associated with nausea and other gastro intestinal side effects. A clinical review article published by Arun Chaudhury et al (23) in 2017 stated that 30% of subjects who were started on Metformin treatment supposedly had nausea, dyspepsia and diarrhea as adverse events. Nausea is also associated with GLP 1 analogues and SGLT 2 inhibitors (56). Similarly, Madiha Fatima et al (24) in their review article stated that Metformin was associated with diarrhea (62.1%), heart burn (52.1%), nausea (47.4%), abdominal pain (35.5%), bloating (35.2%) and retching (21.1%) and 20 to 30% of patients consuming Metformin were having their quality of life significantly affected due to Metformin related gastro intestinal side effects. Metformin is considered to be the 1st line of drug in the management of diabetes though it is associated with such high prevalence of GI adverse effects and hence Bromocriptine can still be a useful medication in diabetes in spite of having gastro intestinal adverse events.
Limitations of the Study
This study was an open labelled experiment and not a double blinded one. Double blinding was not possible as Bromocriptine arm involved revision of the dose from 0.8 mg to 1.6 mg after 14 days of treatment, while Teneligliptin arm did not involve revision of dose. As double blinded, controlled studies are the gold standard design for such studies, it is a limitation for the present study. This design could have been converted to a double blinded and double dummy in order to overcome this limitation, but it was not possible due to limitation of resources.
Limitations of biomarkers measured in the study – The other potential biomarkers to evaluate the mechanisms of the action of study medications were not measured in the present study due to financial constraints. These bio markers are C peptide, serum Insulin, HOMA-β and HOMA- IR.
Detailed safety parameters such as CBC, RFT and LFT were not assessed at the end of the study, though they were measured at the baseline and only those subjects who had normal blood parameters were enrolled. These medications have established safety profile in the respective blood parameters and hence they were not assessed at the end of the study.
The initial dose of Bromocriptine in diabetes mellitus is 0.8 mg which will be gradually increased every week to the maximum of 4.8 mg per day. In the present study, Bromocriptine dose was increased only up to 1.6 mg and this may be the reason for Bromocriptine, being inferior to Teneligliptin though both the drugs have significant efficacy in ‘within group analysis’. The dose of bromocriptine was not increased beyond 1.6 mg due to the fear of increased gastro intestinal adverse events. Hence further studies with the maximum dose of Bromocriptine may be required to confirm whether Teneligliptin is really superior to Bromocriptine.
Conclusion
In this study, we investigated the safety and efficacy of Bromocriptine in comparison with Teneligliptin in patients with newly diagnosed type 2 diabetes mellitus. 50 patients who have fulfilled inclusion and exclusion criteria were enrolled in the study. They were randomized at the ratio of 1:1 by simple randomization, 25 patients in the Bromocriptine group and 25 patients in the Teneligliptin group and all of them completed the study with no drop outs.
Bromocriptine was given at a dose of 0.8 mg in the morning with food. For those patients who had tolerated 0.8 mg dose, the dose was increased to 1.6 mg from 15th day and maintained at 1.6 mg till the completion of the study (3 months). Teneligliptin was given at a dose of 20 mg once a day after food in the morning for 3 months. Initially, they were followed up once in a week for one month and then once a month for the next 2 months. FBS, PPBS, HbA1c and BMI levels were estimated at baseline and once a month while tolerability and adverse events were recorded during every visit.
The results had shown both Bromocriptine and Teneligliptin produced significant reductions in FBS, PPBS, HbA1c and BMI values at the end of 3 months. However, Teneligliptin was found to be superior to Bromocriptine in reduction of FBS, PPBS and HbA1c levels. Bromocriptine was found to be superior to Teneligliptin in reducing BMI.
Teneligliptin was well tolerated by all the patients with no adverse events reported, whereas 5 patients in Bromocriptine reported nausea.
However, further studies with larger sample size, patient enrollment in multiple sites and long-term follow up are needed to reaffirm these observations made in the present study.
Acknowledgement
The authors are grateful to Chettinad Hospital and Research Institute (CHRI), Chettinad Academy of Research and Education (CARE) for supporting the study.
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding Source
Nil
References
- Punthakee Z, Goldenberg R, Katz P. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Canadian journal of diabetes. 2018 Apr 1;42:S10-5.
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nature Reviews Endocrinology. 2018 Feb;14(2):88.
- DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Diabetes Epidemiology, Genetics, Pathogenesis, Diagnosis, Prevention, and Treatment. 2018:181-253.
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019 Jan;42(Suppl 1):S13.
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes care. 2015 Jan 1;38(1):140-9.
- Umpierre D, Ribeiro PA, Kramer CK, Leitão CB, Zucatti AT, Azevedo MJ, Gross JL, Ribeiro JP, Schaan BD. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. Jama. 2011 May 4;305(17):1790-9.
- Chaudhury A, Duvoor C, Dendi R, Sena V, Kraleti S, Chada A, Ravilla R, Marco A, Shekhawat NS, Montales MT, Kuriakose K. Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Frontiers in Endocrinology. 2017 Jan 24;8:6.
- Crasto W. Type 2 diabetes: pharmacological management strategies. Simulation. 2018 Nov 5;11:15
- Viollet B, Guigas B, Garcia NS, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clinical science. 2012 Mar 1;122(6):253-70.
- Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. Jama. 2014 Dec 24;312(24):2668-75.
- Dicker D. DPP-4 inhibitors: impact on glycemic control and cardiovascular risk factors. Diabetes care. 2011 May 1;34(Supplement 2):S276-8.
- Nauck MA, Meier JJ. The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology, and response to therapeutic interventions. The lancet Diabetes & endocrinology. 2016 Jun 1;4(6):525-36.
- Nabeno M, Akahoshi F, Kishida H, Miyaguchi I, Tanaka Y, Ishii S, Kadowaki T. A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. Biochemical and biophysical research communications. 2013 May 3;434(2):191-6.
- Chen XW, He ZX, Zhou ZW, Yang T, Zhang X, Yang YX, Duan W, Zhou SF. Clinical pharmacology of dipeptidyl peptidase 4 inhibitors indicated for the treatment of type 2 diabetes mellitus. Clinical and Experimental Pharmacology and Physiology. 2015 Oct;42(10):999-1024.
- DeFronzo RA. Bromocriptine: a sympatholytic, D2-dopamine agonist for the treatment of type 2 diabetes. Diabetes Care. 2011 Apr 1;34(4):789-94.
- Ramteke KB, Ramanand SJ, Ramanand JB, Jain SS, Raparti GT, Patwardhan MH, Murthy M, Ghanghas RG. Evaluation of the efficacy and safety of bromocriptine QR in type 2 diabetes. Indian journal of endocrinology and metabolism. 2011 Jul;15(Suppl1):S33.
- Cincotta AH, Meier AH, Cincotta Jr M. Bromocriptine improves glycaemic control and serum lipid profile in obese Type 2 diabetic subjects: a new approach in the treatment of diabetes. Expert opinion on investigational drugs. 1999 Oct 1;8(10):1683-707.
- Ramachandran A, Snehalatha C, Salini J, Vijay V. Use of glimepiride and insulin sensitizers in the treatment of type 2 diabetes—a study in Indians. JAPI. 2004 Jun;52:459
- Garg A, Grundy SM. Cholestyramine therapy for dyslipidemia in non–insulin-dependent diabetes mellitus: A short-term, double-blind, crossover trial. Annals of internal medicine. 1994 Sep 15;121(6):416-22
- Russell‐Jones D, Khan R. Insulin‐associated weight gain in diabetes–causes, effects and coping strategies. Diabetes, Obesity and Metabolism. 2007 Nov;9(6):799-812.
- Aghamohammadzadeh N, Niafar M, Dalir Abdolahinia E, Najafipour F, Mohamadzadeh Gharebaghi S, Adabi K, Dalir Abdolahinia E, Ahadi H. The effect of pioglitazone on weight, lipid profile and liver enzymes in type 2 diabetic patients. Therapeutic advances in endocrinology and metabolism. 2015 Apr;6(2):56-60.
- Cincotta AH, Meier AH. Bromocriptine (Ergoset) reduces body weight and improves glucose tolerance in obese subjects. Diabetes care. 1996 Jun 1;19(6):667-70.
- Chaudhury A, Duvoor C, Dendi R, Sena V, Kraleti S, Chada A, Ravilla R, Marco A, Shekhawat NS, Montales MT, Kuriakose K. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Frontiers in endocrinology. 2017 Jan 24;8:6.
- Fatima M, Sadeeqa S, Nazir SU. Metformin and its gastrointestinal problems: A review. Biomedical Research. 2018;29(11):2285-9.







