Manuscript accepted on :20-10-2019
Published online on: 16-11-2019
Plagiarism Check: Yes
Reviewed by: Mohi Uddin
Second Review by: Cory Whitney
Final Approval by: Prof. em. Hans-Joachim Freisleben
Ananta Swargiary*, Mritunjoy Kumar Roy and Manita Daimari
Department of Zoology, Bodoland University, Kokrajhar, Assam, India, 783370
Corresponding Author E-mail: ananbuzoo101@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1824
Abstract
Ethnobotanical knowledge has been the backbone of rural healthcare since ancient times. Many diseases including helminthiasis are cured by traditional medicine in many parts of the world. The present study aims at exploring the ethnobotanicals used as anthelmintic medicines by the tribal communities of Chirang district of Assam. The present study was conducted in different villages under Chirang district of Assam, India. A face-to-face interview was carried out during survey work along with readymade questionnaire. In our survey work, 20 neighbouring villages were taken as a single cluster and one sample informant was collected. Information regarding the plant and plant parts used, methodology of use as well as informant demography such as age, sex, education was also collected. A total of 20 villages were surveyed and information was gathered from 27 informants, 23 kaviraja and 4 elderly people, 15 male and 12 female. The information collected revealed 43 medicinal plants belonging to 27 families. Lamiaceae was found to be most common family followed by Cucurbitaceae, Fabaceae, Zutaceae and Zingiberaceae. The most highly cited plants were Ananas comosus, Andrographis paniculata, Asparagus racemosus, Alstonia scholaris and Leucas aspera. Leaves, fruits and tubers were found to be the most commonly used plant parts. Except few, most of the herbal medicines were prepared as raw materials and are consumed orally. Documentation of important ethnomedicinal information from the remote areas of Assam will help scientific investigators to look into its scientific aspect leading to the development of new medicines against helminthiasis and many other diseases.
Keywords
Anthelmintic Plants; Chirang District; Ethnomedicine; Tribal Community
Download this article as:| Copy the following to cite this article: Swargiary A, Roy M. K, Daimari M. Survey and Documentation of Ethnobotanicals Used in the Traditional Medicines System of Tribal Communities of Chirang District of Assam Against Helminthiasis. Biomed Pharmacol J 2019;12(4). |
| Copy the following to cite this URL: Swargiary A, Roy M. K, Daimari M. Survey and Documentation of Ethnobotanicals Used in the Traditional Medicines System of Tribal Communities of Chirang District of Assam Against Helminthiasis. Biomed Pharmacol J 2019;12(4). Available from: https://bit.ly/379MNfH |
Introduction
Nature is gifted with rich source of natural vegetations and medicinal plants. Worldwide, approximately 350,000 higher plants are estimated to be present1. The use of plants as a source of medicine has its origin in ethnoveterinary medicines inspired by traditional knowledge system and is an important component of healthcare systems in India and world at large2-4. In many parts of the world, several medicinal plants are traditionally being used against many diseases such as malaria, cholera, dysentery, diabetes, jaundice, wound healing, helminthiasis etc5,6. India is a country bestowed with diversity of flora and fauna. Due to its diverse geographical location and climatic conditions there is rich natural vegetation, making India a storehouse of medicinal plants. This has led to a considerate interest tow
ards locally grown medicinal plants and stimulated a serious study on traditionally used medicinal plants. Agriculture and livestock industry plays a significant role in Indian economy. Helminthiasis is one of the most important animal diseases worldwide, causing heavy economic losses to the farmers6,7. Ethnobotanical studies are significant in revealing locally important plants especially for the discovery of crude drugs8. Study of medicinal plants used by traditional herbal practitioners can form a rich source of new medicines and can contribute immensely in the healthcare system of a nation. Documentation of locally and indigenously used plants not only preserves the indigenous knowledge but also facilitates future research on safety and efficacy of medicinal plants in the treatment of various ailments9. Like many other diseases, the use of medicinal plants for the prevention and treatment of gastro-intestinal parasitism has its origin in ethnoveterinary practices. Many investigators have reported the effectiveness of medicinal plants against helminth parasites10-12. Although a large number of commercial anthelmintic drugs are available, development of resistance capacity among the parasites, the side effects of commercial drugs as well as poor distribution in the rural areas has challenged the effective controlling of helminthiasis13,14. As an alternative to the current situation, plant based medicines provides the safety option for controlling diseases because of its eco-friendliness, less side effect and affordable cost2,15,16. Over the last few decades there is a growing trend of plant-based drug designing research throughout the world.
North-east (NE) India comprising of 8 member states viz., Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Nagaland, Sikkim and Tripura is well known for its diverse ethnic community and rich flora and fauna. The favorable climatic region in this part of India makes it suitable for various flora and fauna, making it one of the major biodiversity hotspots area. With the geographical location of 89°50/ E to 96°10/ E and 24°30/ N to 28°10/ N, Assam is one among the richest biodiversity zones in NE India. Chirang district of Assam is located in the foothills of Bhutan and is blessed with rich flora. Although this part of India is rich in natural vegetation and healthy ethnomedicinal system, lack of proper management and preservation has led to the deterioration of ethnomedicinal knowledge. Today, these traditional knowledge systems is either lost completely or transmitted orally from one generation to the next among traditional health practitioners, are in danger due to poor relations between older and younger generations. The ethnomedicinal plants are under threat due to deforestation, overgrazing and their reckless utilization, indicating the urgent need of their conservation. Conservation of biological resources as well as their sustainable use is important in preservation of traditional knowledge17. Taking this view in mind, the present study was carried out to identify and document the ethnomedicinal plants used by the tribal communities of Chirang district of Assam.
Materials and Methods
Study area description
The present survey work was carried out in Chirang district of Assam which covers an area of 1,923 km², geographical location of 90º21̕ East to 90º56̕ East Longitude and 26º33̕ North to 26º54̕ North Latitude and has a population of 4.81 Lakh. Kalajgaon is the headquarters of the district. The district shares the international boundary with the Bhutan on the North, Kokrajhar district in the West, Bongaigaon and Barpeta district on the South and Baksa district on the East. For administrative purpose Chirang district is divided into 5 small Community Developmental Blocks (CDBs) namely, (1) Borobazar (villages 214), (2) Manikpur (part) (51 villages) (3) Sidli (235 villages), (4) Chakchaka (part) (6 villages) and (5) Gobardhana (part) (2 villages). The entire area of the district is situated at the plains of the northern side of the River Brahmaputra. The climate of the district is sub-tropical in nature with warm and humid summer followed by dry and cool winter. The average annual rainfall is about 1900 mm out of which 75% is received during monsoon months (June to September). According to the recent census report, out of 4.81 lakh populations about 93% live in rural areas and the literacy rate of the district is 62.08% only18.
Data collection and identification of plants
The survey and collection of traditionally used anthelmintic medicinal plants were done in the months of April to December 2018. The information regarding the medicinal plants were collected in CDB-wise manner with the help of village Kaviraja (traditional healer, TH) and elderly village people having knowledge in traditional medicine system. The information was collected in a face-to-face interview manner with the help of ready-made questionnaires. Within every CDB, approximately 20 adjacent villages were taken as a single cluster and one sample was collected from a cluster. Based on the number of villages per CDB, sample size also varied from one CDB to another. Chakchaka and Gobardhana CDBs were not included in the survey work because of less number of villages. The information collected from informants mainly included – informer’s bio-data, plant(s) part(s) used, traditional formulation processes and mode of administration. The plants were collected as per the information given by the informants. A total of 27 informants were interviewed from 20 different villages under Chirang district (Figure 1). Due to the presence of large number of THs 3 persons were interviewed from two villages namely Koptuli and Bwigribari. Sample plants were collected with the help of TH and processed for scientific identification. Herbarium sheet were prepared and submitted to the Department of Botany, Bodoland University for identification of the plants.
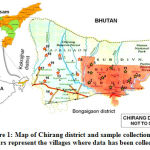 |
Figure 1: Map of Chirang district and sample collection sites. Stars represent the villages where data has been collected. |
Data analysis
All the statistical calculations, graphs etc. were carried out in ms. excel and origin software. The documented data was analyzed by comparing a number of parameters such as number of plant species, families, plant parts used, mode of utilization, habit and habitat of the plant species.
Results
Demography of informants and collection sites
Traditionally prepared herbal medicine is an important part of healthcare system among many tribal communities of India. In our present study, survey was carried out in 20 different villages from 3 CDBs under Chirang district of Assam to collect the putative anthelmintic plants used by tribal communities. Other CDBs were not included because of less number of villages. Out of 20 villages, eight villages were surveyed each from Borobazar and Sidli CDBs while four villages from Manikpur CDB (Table 1). A total of 27 informants were interviewed from the entire district out of which 56% were male and 44% were female informants. Most of the informants (85%) were professional TH and earn some amount of money by selling the herbal preparations while others were elderly people who gained the ethnomedicinal knowledge from their forefathers. Age seems to play a significant role in traditional medicine system. It was found that 74% of the informants were aged above 50 years old followed by 22% in 40-50 years age group while only 4% were below 40 years old. In term of literacy, most of the informants were found illiterate (70%) while 30% were found to have school level education (Table 2). No informants were found to have higher education above school level. We have also reported that none of the informants have any government salaried job. Inhabiting in the rural areas far away from towns and cities, all the informants were mostly cultivators and also spare good amount of time in preparing herbal medicines from neighboring villages. However, except visiting nearby small local markets most of the informants neither have any permanent selling store in any towns or cities nor they practice any kind of business dealings with any pharmaceutical companies. It has also been observed that most of the time TH do not feel secure to give their ethnomedicinal data thinking that secrecy behind the medicinal property will be lost or others will earn money from their knowledge.
Table 1: List of villages where information regarding traditionally used anthelmintic medicinal plants was collected along the geographical location.
| CD Blocks | Villages | Geographical locations |
| 1. Borobazar | 1. Oxiguri No. 2 | 26°37’15”N 90°39’55”E |
| 2. Bawtipara | 26°36’49”N 90°37’31”E* | |
| 3. Batabari (Utarpara No. 1) | 26°30’52”N 90°41’36”E | |
| 4. Malipara No. 1 | 26°34’53”N 90°36’50”E* | |
| 5. Baldi No. 2 | 26°35’26”N 90°34’42”E | |
| 6. Sanja Manikpur | 26°35’26”N 90°33’28”E | |
| 7. Sikapara No. 1 | 26°36’12”N 90°34’38”E | |
| 8. Tangubari No. 2 | 26°33’39”N 90°32’48”E | |
| 2. Manikpur | 9. Koptupli | 26°30’27”N 90°44’38”E** |
| 10. Charagaon No. 2 | 26°31’19”N 90°44’36”E | |
| 11. Bhatipara | 26°32’06”N 90°43’27”E | |
| 12. Chaitianguri No. 1 | 26°30’34”N 90°43’10”E | |
| 3. Sidli | 13. Balapara No. 1 | 26°40’20”N 90°26’52”E |
| 14. Simlaguri | 26°28’50”N 90°29’47”E* | |
| 15. Deosri | 26°46’49”N 90°27’39”E | |
| 16. Bwigribari | 26°37’33”N 90°23’28”E** | |
| 17. Uttar Salbari (Runikhata) | 26°38’25”N 90°25’10”E | |
| 18. Champaguri | 26°41’45”N 90°25’15”E | |
| 19. Madhyam Dakumgaon | 26°38’25”N 90°24’36”E | |
| 20. Hantupara | 26°42’09”N 90°24’27”E |
*villages from where two informants were interviewed.
**villages from where three informants were interviewed.
Table 2: Demography of the informants from different villages of Udalguri district of Assam
| CD Blocks | Kaviraja | Elderly
person |
Male | Female | Age group (in years) | Literacy | ||||
| <40 | 40-50 | >50 | School level | College level | Illiterate | |||||
| Sidli | 7 | 4 | 8 | 3 | 0 | 2 | 9 | 6 | 0 | 5 |
| Borobazar | 10 | 0 | 6 | 4 | 0 | 3 | 7 | 1 | 0 | 9 |
| Manikpur | 6 | 0 | 1 | 5 | 1 | 1 | 4 | 1 | 0 | 5 |
| Total | 23 | 4 | 15 | 12 | 1 | 6 | 20 | 8 | 0 | 19 |
Collection and documentation of anthelmintic plants
Our survey reported a total of 122 plant citations from 27 informants collecting from 20 different sample villages. In an average an informant cited about 5 numbers of medicinal plants. However, in terms of number of citations per male and female informants, it is found that male possesses more ethnomedicinal knowledge (6 citations/informant) while the female has only 3 citations/informants. Due to its popularity and medicinal efficacy, some of the plants have repeated citations by several informants. A total of 20 plant species were found to be repeated more than twice by many informants (Figure 2). Ananas comosus was found to be the most popular plant cited by 17 numbers of informants. Similarly, Andrographis paniculata, Alstonia scholaris, Asparagus racemosus, Leucas aspera, Hypericum japonicum and Clerodendrum infortunatum, all were found to be highly cited plants. Out of the total plant citations made by the informants, 20 repeated plants together constituted 99 citations. On the other hand, 23 plant citations have no repetition among the informants. Thus, a total of 43 plant species were identified from Chirang district of Assam as medicinal plants that act as anthelmintic agents. The scientific name, identification number, family, local name, habitat, parts used, traditional formulation, mode of administration as well as literature available on the plant species is shown in the table 3. The identified 43 plant species belongs to 27 plant families. 10 numbers of plant families namely Lamiaceae, Cucurbitaceae, Fabaceae, Rutaceae, Zingiberaceae, Araliaceae, Apocynaceae, Acanthaceae, Piperaceae, and Rubiaceae contain more than two plant species each with a maximum of four plants under Lamiaceae family (Figure 3). Four plant species were belonged to each of the four families such as Cucurbitaceae, Fabaceae, Rutaceae, and Zingiberaceae. Altogether, the number of plants belonging to 10 families constitutes 60% of all the reported plant species. However, only one plant species was reported from other 17 plant families.
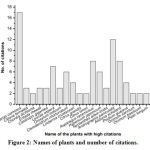 |
Figure 2: Names of plants and number of citations. |
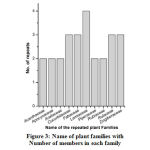 |
Figure 3: Name of plant families with number of members in each family |
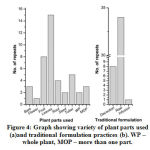 |
Figure 4: Graph showing variety of plant parts used (a) and traditional formulation practices (b). WP – whole plant, MOP – more than one part. |
Different plant parts are generally used in the preparation of herbal medicines. Figure 4 showed the nature of plant parts used and methodology of traditional formulation practiced by THs of Chirang district. Almost all the common parts of a tree such as leaves, flowers, barks, fruits, seeds, etc. are found to be used in the traditional herbal preparations. Leaves are found to be the most commonly used plant parts (39%) followed by fruits (18%), tubers (12%), roots (9%), barks (7%), seeds (5%) and flower (2%) (Figure 4a). Whole plant parts were found to be used in 2 plants namely Oldenlandia corymbosa and Hypericum japonicum. Similarly, in Solanum torvum, Nyctanthes arbor-tristis and Psidium guajava more than one part is used to formulate herbal medicines. The preparation of herbal medicine is formulated by 3 different traditional formulations namely, decoction, infusion and raw. In our study we have found that most of the traditional healers prepare the herbal product in the form of raw material (74%). Herbal products are formulated in the form of grinded pallet mixed with other plants or sometime direct consumption by quizzing the juice of the plant part [Figure 4b]. However, it has also been seen that traditional healers do not follow any precise and standard method of herbal formulation and doses are altered based on the nature of infection and ages of the person. In most cases the concentrations of doses were prepared in terms of the number of leaves, seeds, fruits or flower. While for barks or stems etc. length seems to be the main measurement system for preparing the different doses of herbal medicine.
Discussion
The miraculous properties of medicinal plants are known to the whole world. It has been the life savior of many since time immemorial. Percolated from one generation to the next through oral tradition, with no written document, traditional medicine system attracts first priority for curing many common diseases, especially to the people living in the lowest rung of the society. Moreover, there is a natural tendency and faith attached to it that make people rely on traditional medicine. India is a country of folk and tradition where one of the oldest traditional medicine systems ‘Ayurveda’ was originated19. Attempts have been made over the last few decades by many scientific researchers to retrieve the once forgotten traditional knowledge. Today, plant-based drug discovery is a hot soup for sprouting investigators. Despite of its great medical importance, many reports seems to suggest that most of the times ethnomedicinal knowledge is owned by poor, uneducated rural people. Only a handful of them possess formal education20-22. Similarly, we also revealed that about 30% informants were having formal education. However, the knowledge of ethnomedicine is so popular that the secret of its formulation is also known to people other than professional healers. In our survey we found that 15% of the informants were not traditional healers but possesses the knowledge. It is also observed that male folks used to be the major traditional knowledge bearers. Age and experience seems another factor in traditional medicine system whereby people bestow their belief. Similar to our findings, most of the survey reports revealed that village kavirajas are mostly aged (above 50 years old) and they have a family history of practicing such ethnomedicine since long time23,24. In terms of plant parts used, leaves are found to be the most commonly used plant parts used by traditional healers25,27. It may be because traditional herbalist uses ordinary equipments such as stone or small iron rode to grind or make paste of the plant parts. Since leaves seem to be easier to collect and grind, most of the herbalist prefer to use leaves. Similarly, most of the traditional herbal formulations are orally consumed as raw materials. Very few formulations are found where a slightly laborious practice such as decoction or infusion methods is practiced28.
Today, science and scientific discoveries have overtaken many aspects of our lives. Any practice without scientific reasoning seems to be neglected by the people. In an attempt to verify whether the traditional knowledge owned by kavirajas have any scientific logic or not we did literature survey to the plants reported during our survey. It has been seen that out of 43 plants about 50% have scientific evidence about the anthelmintic property. The mostly cited plants such as Ananas comosus and Andrographis paniculata were investigated by many researchers confirming their anthelmintic property29-32. Similarly other plants such as Leucas aspera, Oroxylum indicum, Clerodendrum infortunatum, etc. were also highly cited and possess good number of scientific evidences33-35. Meanwhile, some popular traditional medicines such as Alstonia scholaris, Hypericum japonicum, Lindernia crustacean, Calotropis gigantean, etc. do not have any scientific experimental data against helminth infection. The absence of any scientific data may be because of the reason that most of these plants are endemic to India and few tropical countries (www.indiabiodiversity.org). As a follow up of the work, we are looking forward to study the anthelmintic property of those plants that do not have any scientific data.
Table 3: List of plant species, parts used and mode of traditional formulation along with identification number used in the traditional medicine system of Bodo community of Chirang district to control helminth infection.
| Species name & Identification number | Family | Local name (Bodo) | Parts used | Traditional
Formulation |
Mode of use | References (anthelmintic activity) |
| Ananas comosus (L.) Merr. (BUBH2018025) | Bromeliaceae | anaras | L | raw | Oral | Yes20,29,32 |
| Clitoria ternatea L. (BUBH2018050) | Fabaceae | aphim, neel | L | raw | Oral | Yes36,37 |
| Phlogacanthus tubiflorus Nees. (BUBH2018028) | Acanthaceae | basikhor | F | decoction | Oral | No |
| Curcuma caesia Roxb. (BUBH0000008) | Zingiberaceae | haldi gaswm | T | raw | Oral | No |
| Oldenlandia corymbosa L. (BUBH0000123) | Rubiaceae | daousri ateng | WP | raw | Oral | No |
| Cynodon dactylon (L.) Pers. (BUBH2018032) | Poaceae | dubri hagra | R | raw | Oral | Yes38,39 |
| Chordia dichtoma (L.) Merr. (BUBH0000124) | Bogarinaceae | dobakharu | B | infusion | Oral | No |
| Momordica charantica Linn. (BUBH0000086) | Cucurbitaceae | fwrla tita/ tita kerela | F | raw | Oral | Yes40,41 |
| Entada rheedii Spreng. (BUBH0000125) | Fabeceae | gila | F | raw | Oral | No |
| Calotropis gigantea (L.) W. T. Aiton (BUBH2018072) | Apocynaceae | gogondo | L | raw | Oral | No |
| Curcuma longa L. (BUBH2018002) | Zingiberaceae | haldi | T | raw | Oral | Yes42,43 |
| Curcuma caesia Roxb. (BUBH2018008) | Zingiberaceae | kala haldi | T | raw | Oral | No |
| Gymnopetalum chinense (Lour.) Merr. (BUBH0000102) | Cucurbitaceae | khaila | F | raw | Oral/ Aroma | No |
| Momordica dioica Roxb. ex Willd (BUBH0000126) | Cucurbitaceae | Khangkhrikhola | F | raw | Oral | No |
| Leucas aspera (Willd.) Link. (BUBH2018010) | Lamiaceae | kansinsa | L | raw | Oral | Yes32 |
| Pongamia pinnata (L.) Pierre (BUBH0000127) | Fabeacea | kharangsu bifang | S | raw | Oral | Yes44 |
| Oroxylum indicum (L.) Kurz (BUBH2018012) | Bignoniaceae | kharaokhandai | B | decoction | Oral | Yes35 |
| Solanum torvum Sw. (BUBH2018018) | Solanaceae | khunthai nara | F, R | raw | Oral | Yes31 |
| Paederia foetida L. (BUBH2018015) | Rubiaceae | khiphi bendwng | L | decoction | Oral | Yes45 |
| Mikania micrantha Kunth. (BUBH2018058) | Asteraceae | lewa bengdwn | L | raw | Oral | No |
| Houttuynia cordata Thunb. (BUBH0000128) | Saururaceae | maisundari | L | raw | Oral | Yes46 |
| Hydrocotyle sibthorpioides Lam. (BUBH2018019) | Araliaceae | manimuni fisa | L | raw | Oral | No |
| Hydrocotyle asiatica L. (BUBH2018020) | Araliaceae | manimuni gidir | L | raw | Oral | No |
| Carica papaya L. (BUBH0000109) | Caricaceae | mwdwmful | R | decoction | Oral | No |
| Clerodendrum infortunatum L. (BUBH2018047) | Lamiaceae | mwkhwna | L | raw | Oral | Yes34,47 |
| Lindernia crustacean (L.) F. Muell. (BUBH2018048) | Linderniaceae | na bikhi | L | decoction | Oral | No |
| Citrus grandis (L.) Osbeck (BUBH2018064) | Rutaceae | nareng jumbra | F | raw | Oral/ Aroma | No |
| Citrus limon (L.) Osbeck (BUBH0000107) | Rutaceae | nareng asugur,
nareng asi |
S | raw | Oral | Yes48 |
| Asparagus racemosus Willd. (BUBH2018063) | Asparagaceae | nilikhor | R | raw | Oral | Yes49 |
| Murraya koenigii (L.) Spreng. (BUBH2018055) | Rutaceae | nwrsing | L | raw | Oral | Yes47,50 |
| Bryophyllum pinnatum (Lam.) Oken. (BUBH2018057) | Crassulaceae | patgaja | L | raw | Oral | Yes51 |
| Hypericum japonicum Thunb. (BUBH0000129) | Hypericaceae | rupafuli, sonafuli | WP | raw | Oral | No |
| Nyctanthes arbor-tristis L. (BUBH2018084) | Oleaceae | sefaliful | F, S | raw | Oral | No |
| Peliosanthes bakeri Hook. f. (BUBH2018039) | Liliaceae | sikho bifang | T | raw | Oral | No |
| Terminalia chebula Retz. (BUBH2018062) | Combretaceae | silikha | F | raw | Oral | Yes52,53 |
| Piper longum L. (BUBH2018085) | Piperaceae | simfri | F | decoction | Oral | Yes55 |
| Andrographis paniculata (Burm.f.) Nees (BUBH2018009) | Acanthaceae | sirota tita | L | decoction | Oral | Yes30,31 |
| Alstonia scholaris (L.) R. Br. (BUBH2018040) | Apocynaceae | sithona | B | raw | Oral | No |
| Psidium guajava L. (BUBH2018041) | Myrtaceae | sofari | S, L | raw | Oral | Yes50,55 |
| Melastoma malabatricum L. (BUBH0000130) | Melastomataceae | tinkur bedor | T | raw | Oral | No |
| Ocimum sanctum L. (BUBH2018045) | Lamiaceae | tulsi | L | raw | Oral | Yes 56 |
| Ocimum basilicum L. (BUBH0000111) | Lamiaceae | tulsi ram | R | raw | Oral | Yes 57 |
| Piper nigrum L. (BUBH0000035) | Piperaceae | jabrang | F | decoction | Oral | No |
L – leaves, R – Roots, B – Barks, F – flowers, T – tubers, S – seeds, St – stems, wp – whole plant
Conclusion
The present study shows the richness of ethnobotanical knowledge and diversity of plants used as remedies to helminth infection. Such plants are very useful to people who cannot afford modern medicines and healthcare facilities. In the arena of modern synthetic medicine the knowledge of herbal medicine is still a part of life and culture to many endogenous people of the world. However, there is a sense of rising threat to the traditional medicine system due to mass deforestation, habitat destruction and unwillingness to practice herbal medicine. This calls for an urgent preservation of the integrity of the forest and indigenous knowledge of herbal medicine use. Therefore, documentation of plants and traditional methodology of herbal preparation will definitely contribute in the development of newer and better drug of tomorrow.
Acknowledgment
Authors would like to acknowledge the village people for providing ethnomedicinal data without which the present work would have been a futile exercise. We are very much thankful to Department of Botany, Bodoland University for scientific identification of the sample plants. Authors also acknowledge the infrastructural facilities provided by Department of Zoology, Bodoland University.
Source of support
The work is supported by DST-SERB, Government of India, in the form of Research Grant under Empowerment and Equity Opportunities for Excellence in Science (File no. EEQ/2017/000071).
Conflict of Interest
Authors declare no conflict of interest.
References
- Heywood V. Ethnopharmacology, food production, nutrition and biodiversity conservation: towards a sustainable future for indigenous peoples. J Ethnopharmacol., 137: 1-15 (2011).
- Shikova A, Pozharitskaya O, Makarov V, Wagner H, Verpoorte R and Heinrichd M. Medicinal plants of the Russian pharmacopoeia; their history and applications. J Ethnopharmacol., 154: 481-536 (2014).
- Swargiary A. Recent trends in traditionally used medicinal plants and drug discovery. Asian Journal of Pharmacy and Pharmacology, 3: 111-20 (2017).
- Umair M, Altaf M and Abbasi A.M. An ethnobotanical survey of indigenous medicinal plants in Hafizabad district Punjab, Pakistan. Plos One, 12: 1-22 (2017).
- Stepek G, Lowe A.E, Buttle D.L, Duce I.R and Behnke J.M. The anthelmintic efficacy of plant derived cysteine proteinases against the rodent gastrointestinal nematode, Heligmosomoide spolygyrus, in vivo. Parasitology, 134: 103-112 (2007).
- Tandon V, Yadav A.K, Roy B and Das B. Phytochemicals as cure of worm infections in traditional medicine systems, In: Srivastava, U.C, Kumar, S. Emerging Trends in Zoology. Narendra Publishing House, New Delhi, 1-27 (2011).
- Akhtar M.S, Iqbal Z, Khan M.N and Lateef M. Anthelmintic activity of medicinal plants with particular reference to their use in animals in the Indo-Pakistan subcontinent. Small Rumin Res., 38: 99-107 (2000).
- Muthee J.K, Gakuya D.W, Mbaria J.M, Kareru P.G, Mulei C.M and Njonge F.K. Ethnobotanical study of anthelmintic and other medicinal plants traditionally used in Loitoktok district of Kenya. J Ethnopharmacol., 35: 15-21 (2011).
- Tugume P, Kakudidi E.K, Buyinza M, Namaalwa J, Kamatenesi M, Mucunguzi P and Kalema J. Ethnobotanical survey of medicinal plant species used by communities around Mabira Central Forest Reserve, Uganda. J Ethnobiol Ethnomed., 12: 5 (2000).
- Williams A.R, Soelberg J and Jager A.K. Anthelmintic properties of traditional African and Caribbean medicinal plants: Identification of extracts with potent activity against Ascaris suum in vitro. Parasite, 23: 24 (2016).
- Giovanelli F, Mattellini M, Fichi G, Flamini G and Perrucci S. In vitro anthelmintic activity of four plant-derived compounds against sheep gastrointestinal nematodes. Vet Sci., 5: PII78 (2018).
- Esteban-Ballesteros M, Sanchis J, Gutierrez-Corbo C, Balana-Fouce R, Rojo-Vazquez F, Gonzalez-Lanza C and Martinez-Valladares M. In vitro anthelmintic activity and safety of different plant species against the ovine gastrointestinal nematode Teladorsagia circumcincta. Res Vet Sci., 123: 153-158 (2019).
- Idris O.A, Wintola O.A and Afolayan A.J. Helminthiases; prevalence, transmission, host-parasite interactions, resistance to common synthetic drugs and treatment. Heliyon, 5: e01161 (2018).
- Preston S and Gasser R.B. Working towards new drugs against parasitic worms in a public-development partnership. Trends Parasitol., 34: 4-6 (2018).
- Verma R.K. An ethnobotanical study of plants used for the treatment of livestock diseases in Tikamgarh District of Bundelkhand, Central India. Asian Pac J Trop Biomed., 4: S460-67 (2014).
- Li G and Lou H.X. Strategies to diversify natural products for drug discovery. Med Res Rev., 38: 1255-1294 (2018).
- Datta T, Patra A.K and Ghosh Dastidar S. Medicinal plants used by tribal population of Coochbehar district, West Bengal, India-an ethnobotanical survey. Asian Pac J Trop Biomed., 4: S478-82 (2014).
- District census handbook of Chirang. www.chirang.gov.in
- Jaiswal Y.S and Williams William L.L. A glimpse of Ayurveda: The forgotten history and principles of Indian traditional medicine. J Tradit Complement Med., 7: 1-4 (2017).
- Ahmed M, Laing M.D and Nsahlai I.V. In vivo effect of selected medicinal plants against gastrointestinal nematodes of sheep. Trop AnimHealth Prod., 46: 411-417 (2014).
- Ahmad L, Semotiuk A, Zafar M, Ahmad M, Sultana S, Liu M.P, Ul Abidin S.Z and Yaseen G. Ethnopharmacological documentation of medicinal plants used for hypertension among the local communities of DIR Lower, Pakistan. J Ethnopharmacol., 175: 138-146 (2015).
- Fayaz M, Jain A.K, Bhat M.H and Kumar A. Ethnobotanical survey of Daksum forest range of Anantnag District, Jammu and Kashmir, India. J Herbs Spices Med Plants., 25: 1-13 (2019).
- Ritter R.A, Monteiro M.V.B, Monteiro F.O.B, Rodrigues S.T, Soares M.L, Silva J.C.R, et al. Ethnoveterinary knowledge and practices at Colares island, Para State, Eastern Amazon, Brazil. J Ethnopharmacol., 114: 346-352 (2012).
- Teklehaymanot T. An ethnobotanical survey of medicinal and edible plants of Yalo Woreda in Afar regional state, Ethiopia. J Ethnobiol Ethnomed 2017;13:40.
- Sharma J, Gairola S, Sharma Y.P and Gaur R.D. Ethnomedicinal plants used to treat skin diseases by Tharu community of district Udham Singh Nagar, Uttarakhand, India. J Ethnopharmacol., 158: 140-206 (2014).
- Choudhury R.C, Dutta Choudhury M, Ningthoujam S.S, Das D, Nath D and Das Talukdar A. Ethnomedicinal plants used by traditional healers of North Tripura district, Tripura, North East India. J Ethnopharmacol., 166: 135-148 (2015).
- Komoreng L, Thekisoe O, Lehasa S, Tiwani T, Mzizi N, Mokoena N, Khambule N, Ndebele S and Mdletshe N. An ethnobotanical survey of traditional medicinal plants used against lymphatic filariasis in South Africa. S Afr J Bot., 111: 12-16 (2017).
- Ahmad K.S, Hamid A.H, Nawaz F, Hameed M, Ahmad F, Deng J, Akhtar N, Wazarat A and Mahroof S. Ethnopharmacological studies of indigenous plants in Kel village, Neelum Valley, Azad Kashmir, Pakistan. J Ethnobiol Ethnomed., 13: 68 (2017).
- Hordegen P, Cabaret J, Hertzberg H, Langhans W, Maurer V. In vitro screening of six anthelmintic plant products against larval Haemonchus contortus with a modified methyl-thiazolyl-tetrazolium reduction assay. J Ethnopharmacol., 108: 85-89 (2006).
- Singh S, Mehta A, John J and Mehta P. Anthelmintic potential of Andrographis paniculata, Cajanus cajan and Silybum marianum. Phcog J., 1: 243-245 (2009).
- Kamaraj C, Rahuman A.A, Elango G, Bagavan A and Abduz Z.J. Anthelmintic activity of botanical extracts against sheep gastrointestinal nematodes, Haemonchus contortus. Parasitol Res., 109: 37-45 (2011).
- Domingues L.F, Giglioti R, Feitosa K.A, Fantatto R.R and Rabelo M.D. In-vitro and in-vivo evaluation of the activity of pineapple (Ananas comosus) on Haemonchus contortus in Santa Ines sheep. Vet Parasitol., 197:263-270 (2013).
- Agarwal S, Jacob S, Chettri N, Bisoyi S, Badarinath D.K, Vedamurthy A.B, Krishna V and Hoskeri H.J. Evaluation of in vitro anthelminthic activity of Leucas aspera Phcog J., 3: 77-80 (2011).
- Das J.K, Choudhury S, Adhikary S.B, Das B, Samanta S, Mandal S.C and Dey S.P. Anthelmintic activity of Clerodendrum viscosum., 11: 119-122 (2011).
- Deori K and Yadav A.K. Anthelmintic effects of Oroxylum indicum stem bark extract on juvenile and adult stages of Hymenolepis diminuta (Cestoda), an in vitro and in vivo study. Parasitol Res., 115: 1275-1285 (2016).
- Khadatkar SN, Manwar J and Bhajipale N.S. In vitro anthelmintic activity of root of Clitoria ternatea Phcog Mag., 4: 148-150 (2008).
- Gollen B, Mehla J and Gupta P. Clitoria ternatea Linn: A Herb with potential pharmacological activities: Future prospects as therapeutic Herbal Medicine. Pharmacol Rep., 3: 141 (2018).
- Pal D and Pandab K. Evaluation of anthelmintic activities of aerial parts of Cynodon dactylon Anc Sci Life., 30: 12-13 (2013).
- Vijaya and Yadav A.K. In vitro anthelmintic assessment of selected phytochemicals against Hymenolepis diminuta, a zoonotic tapeworm. J Parasit Dis., 40: 1082-1086 (2014).
- Pereira C.A.J, Oliveira L.L.S, Coaglio A.L, Santos F.S.O, Cezar R.S.M, Mendes T, Oleivera F.L.P, Conzensa G and Lima W.S. Anti-helminthic activity of Momordica charantia against Fasciola hepatica eggs after twelve days of incubation in vitro. Vet Parasitol., 228: 160-166 (2016).
- Rashid M.M, Ferdous J, Banik S, Islam M.R, Uddin A.H and Robel F.N. Anthelmintic activity of silver-extract nano particles synthesized from the combination of silver nano particles and charantia fruit extract. BMC Complement Altern Med., 16: 242 (2016).
- Bazh E.K and El-Bahy N.M. In vitro and in vivo screening of anthelmintic activity of ginger and curcumin on Ascaridia galli. Parasitol Res., 112: 3679-3676 (2013).
- Nasai N.B, Abba Y, Abdullah F.F, Marimuthu M, Tijjani A, Sadiq M.A, Mohammed K, Chung E.L and Omar M.A. In vitro larvicidal effects of ethanolic extract of Curcuma longa on Haemonchus larval stage. Vet World., 9: 417-420 (2016).
- Sunilson J.A.J, Jayaraj P, Varatharajan R, Anandarajagopal K, Rejitha G and Suraj R. Anthelmintic activity of aqueous extract of Pongamia pinnata Asian J Chem., 22: 761-764 (2010).
- Chanda S, Sarethy I.P, De B and Singh K. Paederia foetida — a promising ethno-medicinal tribal plant of northeastern India. J Forest Res., 4: 801-808 (2013).
- Yadav A.K and Nath P. Anthelmintic effects and toxicity of Cynodon dactylon (L.) Pers. in rodent models. J Intercult Ethnopharmacol., 6: 407-413 (2017).
- Swargiary A, Daimari A, Daimari M, Basumatary N and Narzary E. Phytochemicals, antioxidant, and anthelmintic activity of selected traditional wild edible plants of lower Assam. Ind J Pharmacol., 48: 418-423 (2016).
- Munne S, Parwate D, Ingle V and Nagpurkar V. Evaluation of the anthelmintic activity of citrus limon juice sacs. International Journal of Pharma Professional’s Research., 2: 235-237 (2011).
- Gupta S, Baweja S, Dubey R, Singh D, Singh S. Combinational effect of Asparagus racemosus, Andrographis paniculata and Discorea villosa for anthelmintic and antimicrobial potential. International Journal of Pharmaceutical Sciences Review and Research., 16: 104-107 (2012).
- Molla S.H and Bandyopadhyay P.K. In-vitro anthelmintic activity of Psidium guajava against sheep gastrointestinal nematode, Haemonchus contortus. Environment and Ecology., 32: 616-621 (2014).
- Lunkad A.S, Agrawal M.R and Kothawade S.N. Anthelmintic activity of Bryophyllum pinnatum. Research Journal of Pharmacognosy and Phytochemistry, 8: 21-24 (2016).
- Dwivedi S, Dwivedi A, Kapadia R and Kaul S. Anthelmintic activity of alcoholic and aqueous extract of fruits of Terminalia chebula Ethnobotanical Leaflets, 12: 741-743 (2008).
- Behera D.R and Bhatnagar S. Assessment of macrofilaricidal activity of leaf extracts of Terminalia sp. against bovine filarial parasite Setaria cervi. Journal of Infection and Public Health., 11: 643-647 (2018).
- Koorse K.G, Samraj S, John P, Narayanan P.M, Devi S.S, Usha P.T.A, Sunilkumar S and Gleeja V.L. Anthelmintic activity of fruit extract and fractions of Piper longum in vitro. Phcog J., 10: 333-340 (2018).
- Pina-Vázquez D.M, Mayoral-Pena, Gómez-Sanchez M, Salazar-Olivo LA and Arellano-Carbajal. Anthelmintic effect of Psidium guajava and Tagetes erecta on wild-type and levamisole-resistant Caenorhabditis elegans J Ethnopharmacol., 202: 92-96 (2017).
- Kanojiya D, Shanker D, Sudan V, Jaiswal A.K and Parashar R. Anthelmintic activity of Ocimum sanctum leaf extract against ovine gastrointestinal nematodes in India. Research in Veterinary Science., 99: 165-170 (2015).
- Pessoa LM, Morais SM, Bevilaqua CM, Luciano JHS. Anthelmintic activity of essential oil of Ocimum gratissimum Linn. and eugenol against Haemonchus contortus. Vet Parasitol., 109: 59-63 (2002).







