Manuscript accepted on :02-12-2019
Published online on: 24-12-2019
Plagiarism Check: Yes
Reviewed by: Vikrant Rai
Second Review by: Luca Fiorillo
Final Approval by: Dr Pallav Sengupta
Ummul Khairi Amsyah1, Mochammad Hatta2*, Hasanuddin Tahir3, Gemini Alam4, Asmawati Asmawati3
1Gradute School, Faculty of Medicine, University of Hasanuddin, Makassar, Indonesia
2Molecular Biology and Immunology Laboratory, Faculty of Medicine, University of Hasanuddin, Makassar, Indonesia
3Departement of Periodontology, Faculty of Dentistry, University of Hasanuddin, Makassar, Indonesia
4Phytochemical Laboratory, Faculty of Pharmacy, University of Hasanuddin, Makassar, Indonesia
Corresponding Author E-mail: hattaram@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1845
Abstract
Periodontitis therapy originating from local natural resources is still lacking so it needs to be researched and developed, one of the natural resources from Soppeng Regency in South Sulawesi, Indonesia is the purple miana leaf. It has not been reported before about the effect of purple miana leaf extract on periodontitis related to IL-10 mRNA expression. This study aims to determine the effect of purple miana leaf extract on IL-10 mRNA expression in rat induced by Aggregatibacter actinomycetemcomitans. Rats were divided into three groups, purple miana leaf extract (PMLE), negative control (aquades), antibiotic (levofloxacin nine mg/kg body weight) as positive control. Rat blood was drawn before (H1) and after induction of 3x108 cfu/ml Aggregatibacter actinomycetemcomitans in the gingival sulcus of the mandibular anterior teeth/after periodontitis (H8) and seven days after intervention (H15). IL 10 mRNA expression was measured by Real-time PCR. The results obtained are processed using SPSS. There were no significant differences in H1-H8 in all groups. IL-10 mRNA expression on H8-H15 has a different pattern between PMLE, negative control and positive control. In the negative control, there was a decrease in IL-10 mRNA expression in H8-H15. In PMLE and positive control, an increase in IL-10 mRNA expression. The treatment of purple miana leaf extract in rat induced with Aggregatibacter actinomycetemcomitans significantly had the same effect as levofloxacin on IL-10 mRNA expression.
Keywords
IL-10; miana leaves; A.actinomycetemcomitans; periodontitis; Real-time PCR
Download this article as:| Copy the following to cite this article: Amsyah U. K, Hatta M, Tahir H, Alam G, Asmawati A. Expression of IL-10 in A.actinomycetemcomitans Induced Rat Treated by Purple Miana Leaves. Biomed Pharmacol J 2019;12(4). |
| Copy the following to cite this URL: Amsyah U. K, Hatta M, Tahir H, Alam G, Asmawati A. Expression of IL-10 in A.actinomycetemcomitans Induced Rat Treated by Purple Miana Leaves. Biomed Pharmacol J 2019;12(4). Available from: https://bit.ly/2EQ77WI |
Introduction
Periodontal disease is an inflammatory disease of the tooth-supporting tissues lead to progressive destruction of the periodontal ligament and alveolar bone, and causes pocket formation, gingival recession, or both,1 are complex bacteria-induced infections characterised by an inflammatory host response to plaque microbiota and their by products,2 is known to influence the systemic condition in various ways and the bacteria and their products such as lipopolysaccharides may spread from the periodontal lesion via the systemic circulation to affect distant organs.3 Periodontal disease is a significant global public health concern and is probably the most common chronic infectious disease of humans.4
Aggregatibacter actinomycetemcomitans is a gram-negative bacterium cocobacillus that can participate in periodontitis (periodontopathogen)5-9 and contribute to destruction the alveolar bone supporting the teeth by triggering the host immune response.10 Elucidation of the virulence in A.actinomycetemcomitans is especially difficult because of this bacterium’s pathogenicity being multifaceted, including both microbial and host factors and the inability to clearly define the disease state of many forms of destructive periodontal disease.4
Periodontal disease involves a natural and adaptive immune system.11 The pathogenic mechanism that regulates periodontal disease is complex and results from a disturbance of the balance between various Th cells and their effector cytokines.12 Th1 cells produce mainly IL-1β, IL-2, IL-12, IFN-y and TNF-α which induce the cellular immune response, Th2 cells produce mainly IL-4,-5,-6,-10, dan -13 which induce the humoral immune response, Th17 cells produces mainly IL-17 which promotes rapid recruitment of neutrophils and is involved in an initial inflammatory response against pathogens and in injury.13 The host response against A.actinomycetemcomitans in periodontitis is characterized by the production of pro-inflammatory cytokines such as IL-1β and IL-8 by gingival epithelial cells.10
Natural products are one of the most popular sources of complementary and alternative medicine for treating inflammatory and immune disorders.14 One of the most commonly used medicinal plants for treatment is purple miana leaves. Research on the efficacy of miana leaves has been carried out by Syamsuri (2018) showing miana leaf extract at a dose of 510 mg/kgbw can significantly reduce TLR 4 expression in Balb/c mice induced with Salmonella thypi. The effects of the Miana Leaf Extract are the same as those of the levofloxacin group.15
Purple Miana leaves as a natural resource will be investigated in this study whether it has the ability of anti-inflammatory effects related to the expression of IL-10 mRNA in rat with periodontitis induced by A.actinomycetemcomitans.
Materials and Method
Settings and Design
This study was conducted at the Laboratory of Molecular Biology and Immunology, Faculty of Medicine, Hasanuddin University, Makassar, South Sulawesi, Indonesia. This was a laboratory experimental study of wistar rat animal model with a simple randomized design.
Wistar Rat
Male wistar rats (200-300 grams) were obtained from the Laboratory of Molecular Biology and Immunology, Faculty of Medicine, Hasanuddin University, Makassar, South Sulawesi, Indonesia. They were kept under both 12-h light and 12-h dark periods. Fed and drink sufficiently for fifteen days. They were divided into three groups (n=five/group) based on the intervention; PMLE, negative control (aquades), positive control (antibiotic). Rats were anesthetized with ketamine HCL 80 mg/kg bw (0,22 ml injection of thigh muscle). Blood samples were taken three times, before the induction of A.actinomycetemcomitans (H1), seven days after the induction of A.actinomycetemcomitans in the gingival sulcus of the anterior teeth of mandibular rat had been diagnosed with periodontitis (H8), and seven days after the intervention (H15).
Purple Miana Leaf Extract (PMLE)
Purple miana leaves were taken from Soppeng district, South Sulawesi, Indonesia and extraction was carried out at the Phytochemical Laboratory, Faculty of Pharmacy, Hasanuddin University, Makassar, Indonesia. Purple miana leaf powder macerated with 96% alcohol for 72 hours. The PMLE dose used in this study was 510 mg / kg, dissolved with aquadest 15 w/ v.15
A.actinomycetemcomitans Preparation
A.actinomycetemcomitans bacteria was from the Laboratory of Molecular Biology and Immunology, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia. The amount was 3×108 cfu/ml (Mc Farland Standard).
Nucleic Acid Extraction
The sample volume of about 100 µg/µl blood was fed into 900 µl of L6 solution consisting of 120 g of Guanidium thyocyanate (GuSCN) in 100 ml of 0.1 M Tris HCl, PH 6.4, 22 ml 0.2 M Ethylene Diamine Tetra Acetate (EDTA) pH 8.0 and 2.6g Triton X-100 (Packard, Instrumens) with a final concentration of 50mM Tris HCl, 5 M GuSCN, 20 mM EDTA, 0.1% Triton X-100. Next played at 12,000 rpm. The sediment added a 20 µl diatom suspension consisting of 50 ml H2O and 500 µl 32% (w/v) “Celite” (Jansen Chimica, Beerse, Belgium, 10,846.79), then vortex and centrifuge in a 1.5 ml eppendorf tube at 12,000 rpm for 15 min. The supernatant was removed and the sediment was washed with a solution of “L2” consisting of 120 g of GuSCN in 100 ml of 0.1 M Tris HCl, pH 6.4 by adding 1 ml of the “L2” solution. Then vortex and centrifuged at 12,000 rpm for 15 min, then washing repeated 2 times using “L2” solution, followed by washing with 1 ml of 70% ethanol twice and 1 ml of acetone. The result was then heated in a water bath at temperature of 56 ° C for 10 min and added 60 µl of ‘TE’ solution comprising 1 mM EDTA in 10 mM Tris HCL PH 8.0, Then vortex and centrifuge followed at 12,000 rpm for 30 s, then incubate in the oven for 10 min at temperature 56 ° C. Then performed vortex and centrifuge again for 30 sec at a speed of 12,000 rpm and taken supernatant. The supernatant of this process will be obtained by nucleotide extraction and stored at -80 ° C before PCR analysis.16
Real Time Polymerase Chain Reaction
Quantitative Real-time PCR analysis total RNA was extracted from blood using L6 buffer according to the Boom methods, RNA quality and concentration were detected by Nano Drop 2000 (Thermo Scientific Wilmington, DE, U.S.A.). In a reaction volume of 20 µL using M-MLV reverse transcriptase, 2 µg RNA was then reverse transcribed to cDNA using a RT-PCR kit. The mRNA level of the target gene was quantified by real-time PCR using a SYBR® Premixed E x Taq kit on a CFX Connect system, Biorad Laboratories, Real Time PCR 96 well 0.1 ml, USA. The standard PCR conditions were as follows: 95ºC (10 min), 40 cycles of 95ºC (15 s) and 60ºC (1 min), followed by a standard denaturation curve. The Primar pairs IL 10 For: 5′-TGGCCCAGAAATCAAGGAGC-3 ′ and IL 10 Rev: 5′-CAGCAGACTCAATACACACT-3′.16-21
Statistical Analysis
All groups of data are normally distributed with significant value of 0.027-0.200 (Kolmogorov-Smirnov test) and 0.023-0.842 (Shapiro Wilk test). Repeat Anova was used to test the mean difference on mRNA expression of IL-10 between H1, H8 and H15 in each group. All Statistics were performed on IBM SPSS version 25 statistical software.
Results
This study showed no significant difference in IL-10 mRNA expression in H1-H8 for all groups with p> 0.05. Differences in mRNA expression of IL-10 in H8-H15 had a different pattern between PMLE, negative control and the positive control group (figure 1). In the negative control, there was a decrease in IL-10 mRNA expression from H8 to H15 with significance level of p = 0.32, mean difference 19.47 and CI95% -25.54 – 64.48. In the PMLE and the positive control group, there was an increase in IL-10 mRNA expression with mean difference in positive values (figure 1).
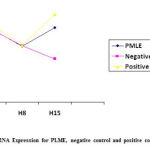 |
Figure 1: Trend IL-10 mRNA Expression for PLME, negative control and positive control in H1, H8 and H15 |
In the positive control group, there was an insignificant increase in IL-10 mRNA expression with significance level of p = 0.49, mean difference 13.96 (CI95% (- 33.88) – (61.80)), had greater increase than the PLME group with mean difference 15.53,p=0.44(CI95% (- 31.49) – (62.56)), whereas in the negative group experienced a decrease in IL 10 mRNA expression with a significance level of p = 0.32, mean difference of 19.47 (CI95% (- 25.54) – (64.47)) (Table 1).
Table 1: Differences Analysis of mRNA expression of IL-10 Rats between before (H1) and seventh Day after (H8) A.actinomycetemcomitans induction and Seventh Day After Intervention (H15)
|
Group |
H1
Mean+SD |
H8 Mean Difference P*
Mean+SD (95%CI)
|
H15 Mean Difference P*
Mean+SD (95%CI)
|
| PLME 11.23±0.81 8.43±0.61 -28.50(-108.96)-(51.96) 0.40 11.19±0.33 15.53(-31.49)-(62.56) 0.44
|
|||
| Negative 11.15±0.78 8.48±0.77 -28.61(-109.02)-(51.80) 0.40 6.52±0.47 19.47(-25.54)-(64.47) 0.32
control
|
|||
| Positive 12.00±0.778. 8.54±0.57 -27.94(-108.69)-(52.81) 0.41 13.18±0.55 13.96(-33.88)-(61.80) 0.49
control
|
|||
P* = paired t test, PMLE = Purple Miana Leaves Extract, H1= before Aa induction, H8 = seventh day after Aa induction, H15 = seventh day after intervention, not significantly different if p>0.05 and significantly different if p<0.05
Discussion
Decreased expression of IL-10 mRNA on H-1 to H-8 occurred in all groups. A.actinomycetemcomitans has virulence factors, namely cytolethal distending toxin (cdtABC), leukotoxins and lipopolysaccharides (LPS) which can modulate the host immune response and play a role in periodontal disease. CdtA and CdtC interact with the host membrane and facilitate CdtB entry into the cell. After CdtB enters the cell, it is transported into the nucleus by an active process that requires amino acid residues in its N terminus. In the nucleus, CdtB causes DNA damage through its DNAse activity and causes induction of apoptosis. In human gingival fibroblast, Aa Cdt is able to stimulate the production of receptor activator of nuclear factor-KB ligand which is involved in pathological bone resorption, characteristic of localized aggressive periodontitis.Leukotoxin producing pores in the target cell by membranolytic activity thus causing osmolysis (water influx into cells) and can cause necrosis and apoptosis. Lipopolysaccharides (LPS) has abroad spectrum of immunological and endotoxic activities, stimulates macrophages to produce interleukin 1α, 1β, tumor necrosis factor, mRNA and protein involved in tissue inflammation, bone resorption and a potent inhibitor of fibroblast proliferation.3,4,22
At H-15, the PMLE group showed an increase in IL-10 mRNA expression the same as in the positive control group. PMLE group dose 510 mg/ kg BW showed IL-10 mRNA expression of 11,199 ng/ml, positive control group (levofloxacin) dose 500 mg / kg BW showed IL-10 mRNA expression of 13.18 ng / mL, whereas the negative control group showed decreased IL-10 mRNA expression (6.52 ng / ml) (Figure 1). Miana is an immunomodulator to enhance immunity (immunostimulant)23 and functions as a complementary and alternative medicine (CAM) in enhancing immunity or modulating the immune response to pathogens or regulating T-Cells.24 Miana leaves as one of the medicinal plants has been used empirically. Previous studies have shown that Miana contains active substances such as alkaloids, saponins, steroids, tannins, triterpenoids and polyphenols which have the potential to be immunomodulators.15 A natural substance containing flavonoids can significantly affect the functioning of the immune system & inflammatory cells.25 Immunomodulatory activity is determined by knowing the ability of plant extracts to induce NO (nitric oxide), cytokine production and activated mitogen protein kinase fosforilase (MAPK)26
Herbs can enhance immunity by changing the balance between inflammatory and anti-inflammatory cytokines and modifying the level and quality of the immune response to T cells, B cells and cytokines.23 Administering of PMLE had an effect on the expression of IL 10 mRNA and subsequently affecting host immunity (rats). IL-10 is an important anti-inflammatory cytokine and polymorphisms of IL-10 gene promoter were involved in the development of periodontal disease, the specific genotypes with low IL-10 expression may aggravate the inflammatory response and cause the overgrowth of gingival.27-30 IL-10 plays a role in suppressing the work of proinflammatory cytokines , inhibits the process of phagocytosis and microbial killing through the production of reactive oxygen species (ROS) and nitrogen intermediates. With decreasing IL-10 levels, the activity of proinflammatory cytokines will increase because they are not suppressed by anti-inflammatory cytokines.31
Immunostimulant stimulation therapy from purple miana leaf extract may be the latest approach for alternative treatment in cases of periodontitis without side effects, given the long-term use of antibiotics has been known to have side effects that can affect the state of patients infected with periodontitis. The nutrient content contained in purple miana leaf extract can induce increased expression of IL-10 mRNA may be able to act as an anti-inflammatory herbal medicine.32
Conclusion
PMLE with dosage 510 mg/kg body weight in rats induced by Aggregatibacter actinomycetemcomitans showed a similar value to positive control on IL-10 mRNA expression. This study represent that PMLE could be a pledging alternative medicine in patients with Aggregatibacter actinomycetemcomitans infection, especially in the case of periodontitis.
Acknowledgment
I am very grateful to all those who have helped in the implementation of this research, especially my supervisor, laboratory staff, and my friends who always support me.
Conflict of interest
The authors declare no conflicts of of interest regarding the publication of this paper.
Source of Funding
Authors their selves
Ethics Statement
From Institutional Research Board of Faculty of Medicine (UH19040207, 2 July 2019), Hasanuddin University, Makassar, Indonesia.
References
- Pradeep A. R, Sing S. P, Martande S. S, Naik S. B, Priyanka N, Kalra N and Suke D. K. Clinical and microbiological effects of levofloxacin in the treatment of chronic periodontitis: a randomized, placebo-controlled clinical trial. Journal of investigative and clinical dentistry, 2015; 6(3): 170-178.
- Bascones-Martínez A, Muñoz-Corcuera M, Noronha S, Mota P, Bascones-Ilundain C and Campo-Trapero J. Host defence mechanisms against bacterial aggression in periodontal disease: Basic mechanisms. Med. Oral. Patol. Oral. Cir. Bucal, 2009; 14(12): 680-5.
- Sriraman P, Mohanraj R and Neelakantan P. Aggregatibacter actinomyctemcomitans in periodontal disease. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 2014; 5(406): 406-419.
- Kler S and Malik R. An update on the virulence factor of Actinobacillus actinomycetemcomitans: a systematic review. A Journal of Dentistry, 2010; 1(1): 1-10.
- Ardila C. M, Lopez M. A and Guzman I. C. High resistance against clindamycin, metronidazole and amoxicillin in Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans isolates of periodontal disease. Med. Oral Patol. Oral Cir. Bucal, 2010; 15(6): 947-51.
- Johansson A. Aggregatibacter actinomycetemcomitans leukotoxin: A powerful tool with capacity to cause imbalance in the host inflammatory response. Toxins, 2011; 3(3): 242-259.
- Kachlany S. C. Aggregatibacter actinomycetemcomitans Leukotoksin; from Threat to Therapy. J. Dent. Res., 2010; 89(6): 561-570.
- Psoter W. J, Ge Y, Russel S. L, Chen Z, Katz R. V, Jean-Charles G and Li Y. PCR detection of Streptococcus mutans and Aggregatibacter actinomycetemcomitans in dental plaque samples from Haitian adolescents. Clin. Oral Investig., 2011; 15(4): 461-469.
- Wahasugui T.C, Nakano V. Piazza R. M. F and Avila-Campos M. J. Phenotypic and genotypic features of Aggregatibacter actinomycetemcomitans isolated from patients with periodontal disease. Diagnostic Microbiology and Infectious Disease, 2013; 75: 366-372.
- Ando-Suguimoto E. S, da Silva M. P, Kawamoto D, Chen C, DiRienzo J. M and Mayer M. P. A. The cytolethal distending toxin of Aggregatibacter actinomycetemcomitans inhibits macrophage phagocytosis and subverts cytokine production. Cytokine 66, 2014; 46-53.
- Li Y, Messina C, Bendaoud M, Fine D. H, Schreiner H and Tsiagbe V. K. Adaptive immune response in osteoclastic bone resorption induced by orally administered Aggregatibacter actinomycetemcomitans in a rat model of periodontal disease. Molecular oral microbiology, 2010; Volume 25, issue 4.
- Arun K. V, Talwar A and Kumar T. S. S. T-helper cells in the etiopathogenesis of periodontal disease: A mini review. Journal of Indian Society of Periodontology, 2011; 15(1).
- Andrukhov, Ulm C, Reischl H, Nguyen P. Q, Matejka M and Rausch-Fan X. Serum Cytokine Levels in Periodontitis Patients in Relation to the Bacterial Load. J. Periodontol, 2011; 82(6): 885-892.
- Huang F, Lin S. S, Liao P. H, Young S. C and Yang C. C. The immunopharmaceutical effects and mechanisms of herb medicine. Cell Mol Immunol., 2008; 5(1): 23-31.
- Syamsuri F, Hatta M, Natzir R, Alam G, Massi M. N, Bahar B and Rahardjo S. P. Expression of TLR-4 in Salmonella typhi-Induced Balb/c Mice Treated by Miana Leaves (Coleus scutellaroides (L) Benth. Indian Journal of Public Health Research and Development, 2018; 9(12): 1449-1454.
- Dwiyanti R, Hatta M, Natzir R, Pratiwi S, Sabir M, Yadi Y, Noviyanthi A, Junita A. R, Tandirogang N, Amir M, Fias M, Saning J and Bahar B. Association of Typhoid Fever Severity with Polymorphisms NOD2, VDR and NRAMP1 Genes in Endemic Area, Indonesia. J. Med. Sci., 2017; 17(3): 133-139.
- Hatta M, Surachmanto E. E, Islam A. A and Wahid S. Expression of mRNA IL-17F and sIL-17F in atopic asthma patients. BMC Res. Notes, 2017; 10(1): 202.
- Kamelia E, Islam A. A, Hatta M, Kaelan C, Patellongi I, Massi M. N, Tammasse J, Nasrullah, Hardjo M, Bintang M and Miko H. Evaluation of Caspase-3 mRNA Gene Expression Activity in Amyloid Beta-induced Alzheimer’s Disease Rats. J. Med. Sci., 2017; 17(3): 117-125.
- Kamelia E, Islam A. A, Hatta M, Kaelan C and Patelongi I. The effect of administration of ethanol extract from Musa paradisiaca L (MPL) fruit on the caspase-3 mRNA gene expression in rat amyloid beta induced an Alzheimer’s disease model. Asian J. Pharm. Clin. Res., 2018; 11(4): 298-302.
- Sirait R. H, Hatta M, Ramli M, Islam A. A and Arief S. K. Systemic lidocaine inhibits high-mobility group box 1 messenger ribonucleic acid expression and protein in BALB/c mice after closed fracture musculoskeletal injury. Saudi J. Anaesth., 2018; 12: 395‑8.
- Yajima T, Yagihashi A, Furuya D and Kameshim H. Quantitative reverse transcription-PCR assay of the RNA component of human telomerase using the TaqMan fluorogenic detection system. Chem., 1998; 44(12): 2441-5.
- Herbert B. A, Novince C. M and Kirkwood K. L. Aggregatibacter actinomycetemcomitans, a potent immunoregulator of the periodontal host defense system and alveolar bone homeostasis. Mol. Oral Microbiol., 2016; 31(3): 207-227.
- Venkatesha S, Rajaiah R and Berman B. Immunomodulation of Autoimmune Arthritis by Herbal CAM. Evidence Based Complementary and Alternative Medicine, 2011.
- Cooper A. M, Mayer-Barber K. D and Sher A. Role of innate cytokines in mycobacterial infection, review. Mucosal Immunology, 2011; 4: 232-260.
- Kumar S and Pandey A. K. Chemistry and Biological Activities of Flavonoids: An Overview. The Scientific World Journal, 2013; 1-16.
- Kouakou K, Schepetkin I, Jun S and Kirpotina L. N. Immunomodulatory activity of Polysaccharides isolated from Clerodendumsplendes: Beneficial effect in experimental autoimmune BMC Compl. and Alter Med., 2013; 13(149): 1-19.
- Passoja A, Puijola I, Knuuttila M, Niemela O, Karttunen R, Raunio T and Tervonen T. Serum levels of interleukin-10 and tumour necrosis factor-α in chronic periodontitis. J. Clin. Periodontol., 2010; 37: 881-887.
- Jaradat S. M, Ababneh K. T, Jaradat S and Haddad H. Association of interleukin-10 gene promoter polymorphisms with chronic and aggressive periodontitis. Oral Diseases, 2012; 18(3): 271-279.
- Sabat R, Grutz G, Warszawska K, Kirsch S, Witte E, Wolk K and Geginat J. Biology of interleukin-10. Cytokine & Growth Factor Reviews 21, 2010; 331-344.
- Zhang Q, Chen B, Yan F, Guo J, Zhu X, Ma S and Yang W. Interleukin-10 inhibits bone resorption: a potential therapeutic Strategy in periodontitis and other bone loss diseases. Biomed Rest In, 2014.
- Redford P. S, Boonstra A, Read S, Pitt J, Graham C, Stavropoulos E, Bancroft G. J and O’Garra A. Enhanced protection to mycobacterium tuberculosis infection in IL-10-decefient mice is accompanied by early and enhanced TH1 responses in the lung. J. Immunol, 2010; 40: 2200-2210.
- Karo M, Hatta M, Salma W, Patellongi I and Natzir R. Effects of Miana (Coleus scutellariodes (L) Benth) to Expression of mRNA IL-37 in Balb/c Mice Infected Candida albicans. J, 2018; 10(1): 16-9.







