Manuscript accepted on :13-11-2019
Published online on: 12-12-2019
Plagiarism Check: Yes
Reviewed by: Helena Prado Felgueiras
Second Review by: Pankaj dwivedi
Final Approval by: Dr Ayush Dogra
Anjali Devi B.S1, Venugopal Reddy Bovilla2 and SubbaRao V. Madhunapantula2,3*
1Department of Biochemistry, JSS Medical College, JSS Academy of Higher Education and Research, Mysuru - 570015, Karnataka, India
2Center of Excellence in Molecular Biology and Regenerative Medicine (CEMR) Laboratory, JSS Medical College, JSS Academy of Higher Education and Research, Mysuru - 570015, Karnataka, India
3Special Interest Group in Cancer Biology and Cancer Stem Cells (SIG-CBCSC), JSS Medical College, JSS Academy of Higher Education and Research, Mysuru - 570015, Karnataka, India
Corresponding Author E-mail: mvsstsubbarao@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1798
Abstract
It is now well-established that Human Papilloma Viruses (HPV) are responsible for causing >90% of cervical cancers. Existing evidences have also demonstrated a key role for HPV in a portion of Head and Neck cancers as well as carcinomas of Vulva, Vagina, Penis and Anus. Therefore studies aiming at developing highly sensitive diagnostic methods have become high-priority in the recent years. To date, an estimated 202 types of HPV have been identified, of which only a small percentage viruses are involved in carcinogenesis. Among the carcinogenic HPV, the most predominant ones are HPV 16 and HPV 18. The other high risk types are 31, 33, 34, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 70. In general, HPV is detected by polymerase chain reaction (PCR) and hybrid capture (HC) methods. However, the existing PCR methods specifically identify only HPV 16 and HPV 18, but not the other types. More over, the PCR protocols are known for errors, and are not suitable for on-field screening procedures, hence, a suitable much quicker and sensitive method is required at the earliest. More over, the PCR and HC methods are expensive, hence, require more funds to conduct public health screening campaigns. Therefore, in this book chapter we have made an attempt to address the recent developments in HPV screening methods, and covered various advanced procedures available in the literature to identify HPV in patient specimens. For example, a section is dedicated to cover the “Biosensors”, which have been developed in detecting the HPV DNA in body fluids. These devices are much simpler compared to sequencing technologies and likely to be the next-generation detection devices for HPV
Keywords
HPV; NGS; Vaccines; Biosensors and Cancers
Download this article as:| Copy the following to cite this article: Devi B. S. A, Bovilla V. R, Madhunapantula S. V. Current Perspectives in Human Papilloma Virus: Where We are and What We Need? Biomed Pharmacol J 2019;12(4). |
| Copy the following to cite this URL: Devi B. S. A, Bovilla V. R, Madhunapantula S. V. Current Perspectives in Human Papilloma Virus: Where We are and What We Need? Biomed Pharmacol J 2019;12(4). Available from: https://bit.ly/2sjdUoL |
Introduction
Cancer is an uncontrolled growth of cells. Morphologically, cancer cells differ from normal cells in that they are poorly differentiated and possess abnormal membrane structures (1-3). Other features of cancer cells include: (a) Self sufficiency in growth signals; (b) Non-responsive to inhibitory signals; (c) Loss of contact inhibition and anchorage dependence; (d) Evasion of apoptosis; (e) Pluri-potency; (f) Angiogenesis; (g) Ability to invade tissues, cell migration and metastasis. In general, cancers are caused by (a) genetic mutations; (b) consumption of alcohol; (c) Smoking; (d) Obesity; (e) Prolonged exposure to sun; (f) Poor dietary practices; (g) Lack of exercise; (h) Presence of toxic food additives; (i) Drugs, pesticides and viral infections. Cervical cancer caused by infection with HPV is one best example for virus infection-induced cancer types. In this section, a summary on the cancers caused by viruses is made and for more information the readers can refer (4-7)
Viruses causing cancer
Viruses are small subcellular organisms which posess either DNA or RNA as genetic material (8, 9). Viruses infect only one type of cell and use the cells own machinery for replication. Infections with some of the viruses cause cancers (Table -1) (10-14).
Table 1: List of viruses that can induce cancers in humans\
| Name of the virus | Genetic material | Primary infection | Cancer caused due to infection |
| Human Papilloma Virus (HPV) | Double Stranded DNA | Genital warts and skin lesions | Cervical, Anal, Vaginal, Penile, Vulval and Head and Neck Cancers |
| Epstein-Barr Virus (EBV)
|
Double Stranded DNA | Infectious mononucleosis | Predisposes to nasopharyngeal carcinoma and Burkitt’s lymphoma |
| Hepatitis-B Virus (HBV) | Double Stranded DNA | Viral hepatitis | Hepatic Cancer |
| Hepatitis-C Virus (HCV) | RNA | Viral Hepatitis | Hepatic cancer and Non Hodgkins Lymphoma |
| Human Herpes Virus-8 (HHV-8)
|
Double stranded DNA | Herpes | Kaposi’s Sarcoma and Lymphoma |
| Human T-Lymphotrophic Virus-1 (HTLV-1)
|
RNA | __ | Lymphocytic Leukemia and Non Hodgkins Lymphoma |
| Human Immunodeficiency Virus (HIV)
|
RNA | AIDS | Kaposis Sarcoma, Cervical Cancer. Non Hodgkins Lymphoma and Hepatic Cancer |
| Merkel Cell Polyoma Virus (MCPV)
|
Double Stranded DNA | —
|
Skin cancer |
Cancers caused by HPV infections
Human papilloma viruses are a group of double stranded DNA viruses belonging to the family Papovaviridae (15, 16). HPVs are responsible for causing genital warts, non-cancerous lesions and tumors (5, 17); and are implicated in about 98% of cervical cancers and 30 to 50% of Head and Neck cancers (6, 18). Apart from these tumors, about 56 % of vaginal cancers and a small percentage of anal, penile and vulval cancers are also attributed to HPV infections (19-21). However, all HPV infections do not result in cancer (22). But, infection with HPV is necessary to transform precancerous lesions in to cancerous ones. In addition, the virulence due to HPV infection vary from one type to another type. Therefore, based on the virulence, HPVs are classified into “Low Risk” and “High Risk” types (23, 24). To date, although about 202 types of HPVs have been discovered, however, only very few were involved in carcinogenesis (25). The most predominant oncogenic HPVs reported are HPV16 and HPV18 (26, 27). The other high-risk types are 31, 33, 34, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 70 (6). Now, brief summary on the HPV genome structure and organization is provided in the following sections, which helps the reader to better understand the recent developments in the detection methods.
Structure of HPV genome
HPV genome is made up of double stranded circular DNA of about 8 kb (Figure-1A &B). The whole genome is broadly divided into 3 regions: viz., (a) early genes E1 to E7; (b) late genes L1 and L2; and (c) a long control region (LCR) also called as non coding region (NCR) separated by two Poly A regions. The proteins produced from these genes perform variety of functions that help the virus to take-over the control of host cell machinery (Table-2) (28).
Table 2: Functions of proteins coded by HPV genes
| Gene | Function |
| E1 | DNA Helicase activity. On binding to viral origin of replication, uses host cell machinery for replication |
| E2 | Guides E1 to viral origin of replication.
Acts as a transcriptional activator or repressor depending on the region bound by LCR. Helps in DNA replication of virus and packaging |
| E3 | Function(s) not conclusively established |
| E4 | Viral DNA amplification and release |
| E5 | Growth factor signalling of the virus and helps the virus to escape the host immunity |
| E6 | E6 combines with ubiquitin ligase and causes degradation of tumor suppressor protein p53. Acts as an oncoprotein to escape from apoptosis |
| E7 | Acts as an oncoprotein, and activates cellular proteosomal degradation system to decrease pRb tumor suppressor. |
| L1 | Major component of viral capsid protein. On over expression, assembles into virus like particles |
| L2 | Minor component of viral capsid protein, but required for infectious process |
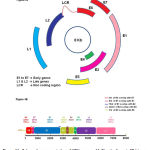 |
Figure 1 A: Schematic representation of HPV genome.1 A-Circular format, 1B-Linear format. |
Mechanism of HPV infection
Human papilloma viruses can replicate only in the stratified epithelial keratinocytes of skin and mucosa(29, 30). Recent studies have shown that HPV can enter into cells containing heparan sulfate, but not the cells lacking heparan sulfate (31). Mechanistically, first, HPV binds to heparan sulfate present on the basal membrane of traumatised epithelial cells with the help of viral capsid protein L1 (32).Next, the binding of L1 to Heparan sulfate results in a change in the conformation of L2, leading to the exposure of N terminal sequences of L2 to cellular Furin (a proteolytic enzyme), causing proteolysis(33, 34). This opens a protected site on L1, which binds to a cell surface receptor on keratinocyte membrane, allowing it to move much closer to the site of wound. After binding to the cell surface receptor, the virus gains cellular entry by endocytosis.The virus removes the protein coat after a period of 12 hours in the late endosome releasing the viral genome L2 complex. This complex enters the nucleus. Next, in the nucleus the viral genome L2 complex copolymerises with Nuclear Domain 10 and initiates RNA transcription.
Among different early-stage proteins, E6 and E7 are carcinogenic (35). E6 contributes to HPV carcinpgenesis by triggering the degradation of p53 (36, 37). Mechanistically, the degradation of p53 by E6 begins when E3 ubiquitin ligase binds to p53 and proapoptotic proteins Bcl2 and Bak thereby marking these proteins for 26-proteosomal degradation (38, 39). In addition, E6 proteins contribute to tumor cell immortalization by increasing the synthesis of h-TERT (40, 41). H-TERT is a catalytic inducer of telomerase activity in cells (42, 43). Telomerase is a reverse transcriptase, which adds new DNA nucleotides to the telomeres. Telomeres are made up of kilobases of TTAGGG nucleotide repeats that are located at the ends of chromosomes. Telomeres and associated protein complex, termed shelterin, protects chromosome ends from end-to-end fusions and degradation by forming special t-loop like structures thereby masking the linear ends of chromosome from being recognized as single and/or double-strand DNA breaks. In normal cells, telomeres are progressively lost during (a) aging; (b) cell divisions; and (c) growth arrest. However, in cancer cells, this loss is much slower due to upregulation of telomerases. While telomerase does not drive the oncogenic process, it is required for the continuous growth of malignant cells (44, 45). Targeted inhibition of telomerase is one of the viable strategies for treating cancers (46). Therefore, studies are required to determine whether targeting E6 proteins reduce cervical cancers. In this regard, recent studies have isolated compounds from natural sources and tested their potential for inhibiting cell lines representing carcinomas of cervix such as HeLa etc (47, 48). Additional studies evaluating these compounds in animals are immediately required.
E7 is another oncogenic protein reported widely in literature. Mechanistically, E7 interacts with p27 family of proteins and inactivate their function (49, 50). Since p27 family proteins negatively control cell cycle progression, and arrest cells in G1S and G2M transition phases, inactivating these proteins promote cell proliferation rates (51). Therefore E7 is considered as another key oncogenic protein to target for effective inhibition of cervical cancers (52, 53). Testing this possibility, a recent study by Jin, B.Y. et al 2018 developed a T cell receptor (TCR) based therapy as a promising cancer treatment modality(54). Experimentally, first, an HPV-16 E7-specific TCR was identified from a uterine cervix biopsy of a woman with cervical intraepithelial neoplasia. Next, human T cells were transduced to express identified TCR to specifically recognize and kill HPV-16+ cervical and oropharyngeal cancer cell lines.
In experimental animal models, these transduced T-cells have been shown to regress established HPV-16+ human cervical cancer tumors (55). Based on this encouraging in vitro and in vivo data a clinical trial is currently testing E7 TCR gene therapy in patients with metastatic HPV+ cancers (NCT02858310). Many other recent studies have also reported the potential of targeting E7 for treating cervical cancers (Sato, N et al 2018; Sen, P et al., 2017)
Another key protein involved in cervical carcinogenesis is E5. E5 contributes to oncogenesis by promoting anchorage independent growth ability of tumor cells (56). In addition, E5 acts in coordination with epidermal growth factor to promote ERK and Akt signalling cascades(56, 57). Elevated ERK and Akt signaling pathways leads to the activation of c-fos and c-jun, thereby promote the expression of vascular endothelial growth factor (VEGF) and cyclo oxygenase 2 (COX-2) (58, 59). VEGF contributes to anchorage independent growth while decreasing the levels p21 and p27(60, 61) . A recent study by Liu, C., et al., 2015, has demonstrated that HPV16 E5 transform normal cervix cells in to cancer cells by down-regulating miR196a(62). However, it is unknown how this downregulation transform normal cells in to cancer cells. More over, recent studies have demonstrated elevated miR196a in cervical cancer patients’ serum as well as in tissues (63). Hence, future studies should explore to address this discrepancy in findings.
In summary, protein products of viral genes play key roles in transforming the infected cells in to tumor cells. Hence, approaches targeting these proteins using pharmacological agents, therapeutic T-cells and antibodies to inhibit infected cells growth are required.
Screening Methods for Cervical Cancer – Where We rre Now?
Cervical cancer is a preventable disease, if detected early and abnormal cells treated timely (64, 65). The detection of abnormal cervical cells, including precancerous cervical lesions, as well as early cervical cancers can be achieved by screening tests that include (a) cytology-based screening (known as the Pap test or Pap smear); and (b) molecular biology based HPV DNA/RNA/Protein identification methods. In general HPVs are detected by hybrid capture-2 (HC-2) or PCR based methods(66). However due to various limitations such as inability to detect all HPV subtypes and poor sensitivity, use of these methods in clinical diagnosis begins to fade (67). Addressing some of the drawbacks associated with existing methods, recent technological developments have produced (a) a plat form to sequence the whole genome using next generation sequencing approach; and (b) high sensitivity biosensors to detect HPV DNA. However, further advancements are urgently warranted to (a) develop devices that can detect different high-risk HPVs using very small sample volume; (b) bring self-testing devices so that infected individuals can easily test samples; (c) prepare low-cost easy to use detection devices, which can be used in various field studies. Therefore, in this book chapter, an attempt was made to provide key information about various HPV detection methods developed and the technological advancements warranted to improve existing devices for more efficient, and specific detection of all high-risk HPVs.
Tests Based on Visual Inspection
Pap test (Papanicolaou test)
This test was invented by Dr. George Papanicolaou in 1928. It is the earliest method developed for cervical cancer screening. This involves the use of a brush or spatula,to collect cervical cells, which will be observed under a microscope for abnormal cytology. An individual is considered Pap test positive if any of the following cell types are present (a) Atypical squamous cells of undetermined significance (ASCUS); (b) Squamous intra epithelial lesions; (c) Atypical glandular cells or (d) Squamous cell cancer or adenocarcinoma cells. Screening with the Pap test not only detects abnormal cells (that may develop into cancer later) but also identify cancer cells. In addition to identifying abnormal and cancer cells, the Pap test can assist in the diagnosis of infections and inflammatory reactions. Hence, Pap test is considered as “Gold Standard”. “National Cervical Cancer Coalition”, a program of the American Sexual Health Association, recommends Pap test along with HPV test for women aged 30 and over. In general the Pap test is performed once in 5 years when it is combined with HPV testing. However, more frequent test is required if an individual is (a) having precancerous cells; (b) HIV infections; (c) weakened immune system and (d) exposed to diethystilbestrol (DES) before birth. DES is a synthetic organic compound similar to female hormone estrogen. Studies have demonstrated that prenatal exposure to DES can induce clear cell carcinoma in women (68-70).
Visual inspection with acetic acid
This is an easy-to-execute method to detect cervical cabcer lesions. This method is developed based on the fact that application of 3% to 5% acetic acid promotes reversible coagulation of proteins in tissues with high amount of DNA (ie., such as in cancerous lesions), which turn these tissues in to white (acetowhite) compared to pink colored surrounding epithelium. This method uses a scope for the observation of cervix treated wth 3 to 5% acetic acid, first, to identify whether the individual is “VIA-positive” or “VIA-negative”. Next, the VIA-positive individuals will be categorized in to “Precancerous” or “Cancerous” by (a) identifying the anatomy of cervix; (b) localizing the abnormal area – in contact or not in contact with transformation zone; (c) excluding a benign lesion. Even today VIA is widely used to detect cervical cancers. However, this procedure requires a well-trained medical staff member, and a laboratory set-up to conduct screening program. Hence, VIA is difficult to execute in community screening programs.
Visual inspection with Lugol’s iodine (VILI)
Lugol’s iodine (5% iodine and 10% potassium iodide) is a glycophilic solution, which stains tissues dark brown if they contain high glycogen content. Since mature cervico-vaginal squamous (CVS) epithelium contain glycogen compared to normal columnar epithelium, a dark brown color is observed upon staining with Lugol’s iodine only in CVS epithelium. Tissues having low glycogen such as precancerous lesions appear yellow in color. Although VILI is simple and easy to execute, there are many drawbacks with this test. For example, moderate specificity, less accuracy especially in post-menopausal women, and require well-trained person and a separate clean room setup to execute this test.
Colposcopy
Colposcopy involves the use of a scope to examine abnormal areas of cervix with illumination. It is usually conducted when the Pap test results are abnormal and need further confirmation. Procedurally, a doctor or nurse will insert a speculum to open the vagina, followed by which application of venegar like solution using a swab to wash the cervix. Then the doctor/nurse will examine using a colposcope with brigh light to identify any abnormalities in the tissues. Colposcopy is easy to execute. However, it still require a doctor/nurse and an examination room, limiting this test to effectively use in community based screening programs.
Advancements in the Detection of Hpv – Integrating Molecular Biology Methods with Electromagnetic Detection Tools
Inability of culturing HPV is the major limiting factor, which made detection of HPV in clinical specimens a challenging task. The conventional methods for screening women with risk of cervical cancer are not fully effective enough to identify all women with cervical cancer (64). More over, although the presence of HPV can be identified using serological, clinical and morphological examination methods, accurate detection and type confirmation can be achieved only through molecular biology based assays. Hence, the advent of molecular techniques such as Southern-, Northern- and Western blotting, Multiplex Polymerase reactions to detect all 13 high risk HPVs in a single reaction and hybrid capture technology have made the detection of HPV feasible even with a very low quantity of sample. To date, there are three tests developed to detect and identify the type of HPV viz., (a) nucleic acid hybridization; (b) signal amplification; (c) target (nucleic acid) amplification procedure. Therefore, in this section, use and limitations of these techniques are discussed.
Nucleic acid hybridization methods
These methods include (1) Southern blotting; (2) In situ hybridization; and (3) Dot blot hybridization. These methods are not widely used due to either protocol complexicity or the technical difficulties and cost (71, 72).
The signal amplification methods
Hybrid capture-2 (HC2) and Cervista HPV HR tests are the two known signal amplification procedures widely used in detecting cervical cancers.
HC2 is one of the FDA approved tests to detect high-risk HPV in clinical samples(73). The kit developed by Digene (Catalogue number 5101-1296) uses hybrid capture technology to detect 13 high-risk (16,18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68) and 5 low-risk HPV types (74, 75). Experimentally, the procedure involves the hybridization of HPV DNA to a RNA probe that has complementary base pairs for 13 high risk and 5 low risk HPV DNA (76). Once the hybrid is formed, the antibodies that recognize specific RNA-DNA hybrids (coated on to a micro titre plate) immobilize the products in the well. The immobilized products will be reacted with alkaline phosphatase conjugated antibodies that specifically recognize the RNA-DNA complexes. Next, luminescent substrate will be added, and the emitted light (luminescence) detected using a luminometer. Since many alkaline phosphatase molecules are conjugated to each antibody and multiple conjugated antibodies bind to each captured RNA-DNA hybrid, the signal gets amplified substatially and allow the detection of very low quantities of viral load (77, 78)(Figure-2).
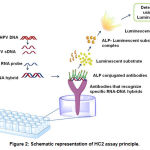 |
Figure 2: Schematic representation of HC2 assay principle.
|
Cervista HPV HR along with Genfind DNA extraction kit is another FDA approved test, which relies on signal amplification principle(79). This test uses the Invader Call Reporter™ software to identify HPV DNA from 14 high-risk genital HPV types. Cervista HPV-HR uses sequence-specific probe and invader oligonucleotides that cycle rapidly on and off the 14 HR-HPV target DNA sequences, creating substrate for the proprietary Cleavase enzyme (Hologic, Inc.). The action of the enzyme results in production of cleaved 5′ oligonucleotide flaps (80). These flaps bind to a universal hairpin fluorescence resonance energy transfer oligonucleotide, creating a second substrate for the Cleavase enzyme. Cleavage of the FRET oligonucleotide results in production of a fluorescent signal. Oligonucleotides specific for the human histone 2 gene act as an internal control in this test (79).
Target amplification methods
Unlike “Pap test”, HPV DNA testing detects the presence of high-risk HPV types in cervical cells using Polymerase Chain Reaction (PCR) (67, 81). PCR is a chain reaction conducted in the presence of a DNA polymerase to amplify the number of target sequences. It enables the detection of very low quantity of DNA (for example, 50-250ng template DNA is sufficient for carrying out a PCR reaction with genomic DNA; however quantities as low as 1pg to 10ng are sufficient if the sample is a plasmid or viral DNA)(82). Several different HPV tests that detect the DNA or RNA of high-risk HPV are currently available (67). PCR relies on amplification of highly conserved sequences of HPV DNA such as L1 region (83, 84). The amplified PCR products are usually identified using restriction fragment length polymorphisms (RFLP), blotting techniques, or gel electrophoresis.
Although PCR-based methods are more useful in identifying the type of HPV, they suffer from contaminating DNA, which yield false positive test results. Hence, HPV-DNA testing is used in concordance with abnormal Pap smears to conclusively report whether the individual has infection, if so which type. In this section of book chapter, emphasis is given to various PCR based target amplification methods
Detection of HPV DNA using microarray platform
Microarray is a platform developed by spotting a set of DNA sequences (DNA microarray) representing the whole genome of an organism, in general, on to a solid support (usually a slide) (85, 86). DNA microarrays are used to measure the expression of a set of genes in single reaction. In HPV DNA microarray, first, the viral DNA is amplified using PCR (87). The products of PCR are subsequently identified by hybridizing them to a DNA Chip, followed by scanning the chip with a DNA chip scanner. The advantage of this technique over other methods is that it can be automated and thus multiple HPV DNAs can be detected (88). Microarray analysis is more sensitive and specific compared to gel electrophoresis(89). However, this technology require expensive equipment and trained individuals to analyze the data
Using HPV RNA for detection of cervical carcinoma
Detection of HPV RNA is of much better diagnostic value when compared to HPV DNA as it helps in analyzing the transcriptome compared to genome (90, 91). Now, with the advent of transcriptome analysis, RNA sequencing and Bioinformatic softwares, it is feasible to detect the presence of HPV infections with great precision(92). However, RNA is less stable compared to DNA, hence, require much more careful handling and processing (93). In addition, analysis of transcriptome is expensive and require skilled individuals to interpret the data.
Biosensors for the detection of HPV infection
Bio sensors are small probe based instruments that help in detecting HPV DNA(94). Biosensors use oligonucleotide probes that hybridize to HPV DNA (94). The hybridised products are converted to measurable signals. The signals will be amplified by amplifiers and detected by electrochemical or piezo electric detectors (95). The detected signals are processed by analytical software and produce a read out. The advantage of Biosensors over other conventional methods is its’ simplicity, ease of use, decreased preparation and purification time, and high sensitivity. Many types of Biosensors are available in market.
Biosensors using electro chemical detection units
In these biosensors, the detection unit has a biological recognition domain, which is coupled to an electrochemical transducer capable of converting biological recognition into a measurable signal(96, 97). The signals are measured by photometry,voltammetry, conductometry and amperometry. The electrodes used for measuring the signals are single carbon nanotubes, gold nanoparticles, graphene and graphene oxide nanoparticles.
Biosensors using optical systems
The optical biosensors help differentiate positive and negative results making use of colour changes due to differential staining procedures and fluorescent labelling (98). Methods using optical detection systems are fast, simple and can be automated. Flouroscent insitu hybridisation or FISH is one of the techniques utilised in optical biosensors (99). Flouroscence label detects the specific DNA signal while examining the tissues simultaneously. However, optical biosensors or Optrodes are not sensitive enough compared to conventional PCR based methods (100).
Hence, they are combined with spectroscopy, and signals are measured using adsorption / Raman spectroscopy / dispersion spectroscopy (101). Surface plasmon resonance (SPR) is another method of measuring the signals. SPR helps to detect changes in Refractive Index (RI) at the sensor surface, which is coated with gold nanoparticles. The binding of biomolecules at the surface of sensor changes the refractive index. This change is directly proportional to the number of biomolecules bound to the sensor. SPR measures the mass differences to detect HPV strains(102).
Recently, fluoroscently labelled nanoparticles coupled with a specific biological interaction are also being used in the detection of different HPV strains.
Biosensors using piezo electric system
These biosensors convert changes in pressure, temperature or force into electrical charges and measure the levels of different HPV strains (103). For instance, a DNA-based quartz crystal microbalance integrated with isothermal DNA amplification system has been developed recently to detect very low levels (< 100 copies) of HPV-58 DNA(104). Similarly, a DNA piezo electric biosensor was fabricated to detect HR-HPV types 16, 18, 45, 33, 39, 51, 52, 56, 58, 59 and 68. In this method, a Biotinylated detection probe will be attached on gold electrode 9 MHz Quartz crystal microbalance (QCM) using biotin-avidin linking system. Once the target DNA of 11 HR-HPV strains amplified with primers containing overhanging regions with detection probe sequence at their 5’ end added to the developed QCM biosensor, the signals will be detected with great sensitivity. The QCM sensor sensitivity is comparable to the conventional agarose gel electrophoresis.
Magnetic Biosensors
The use of magnetic materials has also been explored in HPV detection. The method involves anchoring of DNA probe on to the transducer, followed by hybridization of biotinylated complementary DNA to the probe, which subsequently detected by streptavidin coated magnetic particles. Variations in the magnetic field strength is measured to detect HPV DNA.
Current Perspectives in the Prevention and Treatment of Hpv Infections
Although several screening programs have been conducted by public and private agencies across the globe, the incidence and mortality rates due to cervical cancers has not shown a huge improvement in the reduction of cases (105). Probable factors contributing to the poor success of these programs include: (a) heavily populated very low and middle income countries; (b) cultural and social stigmas; (c) poor sanitary higene; and (d) low accessibility to various health programs implemented by government (106, 107). Therefore, better approaches and technological advancements are immediately required to educate the public, and promote vaccination of young women. For instance development of an easy to execute, low-cost, self screening test helps to increase the number of women attending screening programs. However, development of such test is challenging and require extensive research. Likewise, developing a treatment agent, which can retard the tumor cells growth is urgently needed. But, to date, no single pharmacological agent showed success in treating malignant cervical tumors.
Therefore, prevention of individuals from getting cervical cancer is the only viable option currently available to reduce the disease incidence and burden. Hence, in this section of book chapter, we have discussed various recent advancements in developing prevention and treatment agents against cervical cancers. In addition, major milestones in the development of anti-cervical cancer agents is also addressed in this section.
Development of vaccines against HPV infections
Vaccine is a biological preparation made upof an inactive or dead version of the organism which causes disease(108-111). HPV vaccines are vaccines that help in preventing infection with HPV strains, especially the high-risk HPV-16 and HPV-18. HPV vaccines are composed of Virus Like Particles (VLP) containing major capsid protein L1(112). These VLPs elicit the same antigenic response as the HPV virus, but fail to cause disease, due to the absence of HPV DNA. The L1 antibodies are natural and 10 times more potent compared to original HPV virus as such (113). Recent methods use recombinant DNA technology to prepare HPV vaccines. Centers for Disease Control and Prevention (CDC), USA, have formulated guidelines for HPV vaccination. According to these guidelines, the Gardasil 9 vaccine should be given if a child is aged 11 or 12 years. The child should get two HPV vaccine shots 6 to 12 months apart. If the two shots are given less than 5 months apart, a third shot will be needed. There could be future changes in recommendations on dosing. Furthermore, HPV vaccine is recommended for young women through age 26, and young men through age 21. Adolescents who get their first dose at age 15 or older need three doses of vaccine given over 6 months. Persons who have completed a valid series with any HPV vaccine do not need any additional doses(114).
HPV Vaccines in market
Currently there are three vaccines available for preventing further infections with HPV. They are (a) Cervarix; (b) Gardasil and (c) Gardasil 9. Characteristics of these vaccines are as follows.
Cervarix
It is a bivalent vaccine developed by Glaxo SmithKline limited(115). It is effective against highly oncogenic HPV-16 and HPV-18 (115). The vaccine is administered intramuscularly. Cervarix contain recombinant c-terminally truncated L1 proteins from HPV-16 and HPV-18. These recombinant proteins are assembled in to virus-like particles (VLPs). HPV-16 and HPV-18 antigens were prepared by recombinant DNA technology using a Baculovirus expression system in Trichoplusia ni (Hubner) insect cell line. Cervarix uses AS04 adjuvant system, which comprises of Al(OH)3 and 3-O-desacyl-4′ monophosphoryl lipid-A. Since CERVARIX is prepared from VLPs of the major L1 protein of HPV types 16 and 18, and VLPs contain no viral DNA, they cannot infect cells or reproduce. Prior studies using animal models suggested development of humoral immune response and cell-mediated immunity(116). Further studies have demonstrated that transudation of anti-HPV IgG antibodies from the serum to the cervical mucosa is responsible for the protection against persistent oncogenic HPV infection (117).
Gardasil
This vaccine is a quadrivalent vaccine (118). This offers protection against HPV strains 6 and 11, which are responsible for causing genital warts, along with HPV 16 and HPV-18. It uses recombinant particles developed using Saccharomyces cerevisiae. Gardasil uses amorphous aluminum hydroxyphosphate sulphate as adjuvant.
Gardasil
This is a nonavalent HPV vaccine that protects against HPV strains 6,11.16, 18, 31, 33, 45, 52, 58 (119). Like Gardasil, it also uses recombinant particles developed using Saccharomyces cerevisiae, and contain amorphous aluminum hydroxyphosphate sulphate as adjuvant.
 |
Scheme 1
|
Schedule of vaccination, and safety concerns
In general, a dose of 0.5 ml vaccine is administered in the deltoid muscle to individuals at the age of 11 to 12 Years. The vaccination can be given upto the age of 26 years (120). The schedule for Cervarix is 0,1 and 6 months with a minimum gap of 1 month between the first and the second dose and 3 months between second and the third dose (121, 122). The dose for Gardasil is 0, 2 and 6 months. Since the available vaccines do not provide protection against all HPV strains, regular screening is necessary even after vaccination(123). HPV is also responsible for causing anogenital cancers in men, hence, it is advised to vaccinate young boys at the age of 11 and 12 years(124). The vaccine does not cause any serious reactions, however, might cause adverse reactions such as pain and inflammation in the injected area. Vaccines are not advised to girls less than 9 years of age(125). More over, although no serious reactions are observed in animals and clinical trials, vaccination is not advised for pregnant women. Further, it is contraindicated in people with acute illnesses and those who are prone to immediate hypersensitivity reactions (114).
Limitations of the vaccines
The screening should continue in women who are over 26 years of age. More over, the vaccine does not provide protection against all oncogenic HPV viruses. High cost of vaccines is another factor limiting its use in economically challenged countries.
Recent Advances in the Treatment of Cervical Cancer
Advances in Surgery
Use of laproscopic surgery, in contrast to traditional hysterectomy, is one of the advancement made in surgical procedures. Laproscopic surgery is minimally invasive compared to conventional abdominal hysterectomy.
Lapro hysterectomy
It has many advantages over conventional hysterectomy. For example, post operative complicationsl duration of surgery and hospital stay are considerably less in lapro-hysterectomy. In addition, the pain is relatively less and cause a minimal blood loss.
Nerve sparing radical hysterectomy
Conventional hysterectomy results in the removal of the sympathetic and parasympathetic innervations, leading to dysfunction of the bladder, dysfunction of sexual organs, and mobiliyy defects in the anorectal regions. To avoid these complications, a team of surgeons in Japan performed less radical hysterectomy sparing the autonomic innervations to the pelvis but with same surgical oncologic outcomes as that of total radical hysterectomy. This is one of the key advancements in surgical procedures.
Robotic surgery
Robotic radical hysterectomy and robotic radical trachealoctomy are two other methods being used in the treatment of cervical cancer (126). Radical trachelectomy results in the removal of cervix, upper part of the vagina and pelvic lymphnodes. Robotic radical trachelectomy is safe and involves minimal invasive procedures. This is often used in the treatment of early stage cervical cancer. Although radical trachelectomy can be performed through the abdomen or vagina, vaginal trachelectomy is the preferred route.
Pelvic exenteration
(Removal of the entire contents of the pelvis including uterus, cervix, vagina, bladder, and rectum). It is the preferred treatment for recurrent cervical cancers treated earlier by surgery, chemotherapy or radiotherapy (127). PE is performed with the help of Robotics, as it is more advantageous compared to conventional surgery and laproscopy. It uses continous 3D visualisation with minimal invasions and instruments.
Advances in radiation therapy
Radiation therapy, in short radiotherapy, is a procedure of killing cancer cells using high intensity ionizing radiation (128). Mechanistically, radiation damages the cancer cell DNA thereby promotes the destruction of cells. In general, it takes about weeks to months to induce DNA damage in cancer cells using radiation treatment. Conventionally external beam radiation therapy or internal beam therapy have been used in the treatment of cervical cancers. Now, these methods are being combined with newer techniques for better prognosis.
Brachy therapy
This is a technique where a radiation emitting device is implanted in the tissue(129). Devices providing low dose (4-20 Gy/h), medium dose (20-120 Gy/h) and high dose (>120 Gy/h) radiation are currently available in the market (Note: 1Gy = 1 Joule of energy deposited in to 1kg body mass). The radionucleides used in the treatment of gynarcological cancer are Americium 241 (241A) and Californium 225 (225Cf). Low dose remote loading devices are preferred due to low radiation exposure to the hospital personnel.
Intraoperative electron beam radiation therapy
In this technique electron beam is applied to the residual tumor during surgery. This is used to decrease the rate of tumor recurrence.
Intensity modulated radiation therapy and proton therapy
Intensity modulated radiation therapy is used to intensify radiation exposure to the affected tissue and spare the normal tissue (130). This offers better tumor control as it delivers high dosage of radiation to tumor tissues sparing the normal tissues like rectum, bladder and the small intestine.
Proton therapy
Proton therapy uses protons generated with the help of a cyclotron for the treatmet of cervical cancer (131). High speed protons get deeply embedded in the tissue compared to low speed protons. Unlike external beam radiation therapy, the radiation in proton therapy is cconfined to the tumor tissue and does not affect the normal tissue.
Recent advances in chemotherapy for cervical cancer
Conventional chemotherapy for cervical carcinoma involves the use of platinum based drugs such as cisplatin and paclitaxel (132). 5-fluorouracil, gemcitabine are aso used in combination chemotherapy for treating cervical cancers. Concomittant use of radiotherapy and chemotherapy has improved survival rates and reduced the side effects(133).
Angiogenesis inhibitors
Angiogenesis is a process of producing new blood vessels around the tumor. Growth factors such as Vascular Endothelial Growth Factor (VEGF) are involved in the development of new blood vessels. Therefore, targeting VEGF using monoclonal antibody Bevacizumab alone or in combination with chemothrapy has shown promising results in the treatment amd prognosis of cervical cancer(134). Other antiangionesis agents like Sunitinib, pazopanib, and brivanib have been used but with minimum activity. Recenly another angiogenesis inhibitor Cediranib has shown promising results (135). However, further studies are warranted to test the safety and efficacy of this angiogenesis inhibitor as a single agent (136). Recently Cediranib has been tested in combination with carboplatin and paclitaxel for treating cervical cancers(137). Although this combination showed better efficacy in reducing metastatic and recurrent cervical cancers, it yielded severe toxicity (diarrohea, hypertension and febrile neutropenia).
Epidermal growth factor receptor inhibitors
EGFR is a 170-kDa trans-membrane glycoprotein receptor encoded by the Her-1 proto-oncogene(138). Functionally active dimeric EGFR activates a tyrosine kinase domain to regulate cell growth, differentiation, gene expression, and development. Expression of EGFR is observed in many normal tissues as well as in a wide variety of solid tumors, including cervical cancer (139). Recent studies have identified the potential utility of targeting EGFR activity using monoclonal antibodies or pharmacological agents in treating cervical cancers. Therefore, several monoclonal antibodies targeting EGFR have been developed and being used in clinical trials (140). For instance erlotinib, lapatinib, cetuximab are some of the EGFR inhibitors tested in clinical trials, however, these inhibitors showed minimal activity {Scheipl, 2016 #309}{Sayar, 2014 #310}. Currently, Nimotuzumab, a monoclonal antibody which targets EGFR is found to be effective in combination with conventional chemotherapeutic agents like cisplatin or gemcitabine for treating cervical cancer (141).
Poly-ADP-ribose polymerase (PARP) inhibitors
Poly(adenosine diphosphate [ADP] ribose) polymerase (PARP) enzymes get activated by DNA damage and serve as a part of base excision repair (BER) pathway of DNA repair (142). In general, the PARP-1 and closely related PARP-2 gets activated by binding to broken DNA to cleave NAD+ and create long homopolymers of ADP ribose, which “flaggs” the damaged point. This causes the release of histones (to loosen the chromatin), and promote the recruitment of additional repair factors to restore intact DNA(143). Therefore, targeting PARP is a viable approach for treating cervical cancers. Several inhibitors have been developed to target PARP (144).
For example, Veliparib, a PARP inhibitor reported to be effective in combination with cisplatin, paclitaxel or topothecan (145). It was found to be 34% effective at all doses, however, at maximal tolerated dose (MTD) an about 60% efficacy has been reported for this combination(145).
Immunotherapy
The major obstacle to cancer therapy is the development of drug resistance(146). Drug resistance is usually reduced by the use of immunotherapty(147).
Adoptive T cell therapy, which involves infusion of cytotoxic T cells (infiltrating T-cells) developed against a specific target into a cancer patient to destruct tumor tissue, helps in killing tumor cells with more efficacy. Recent studies have demonstrated that tumor infiltrating T-cells developed against HPV E6 and E7 proteins showed better prognosis (148).
What We Need – Areas Requiring Research and Development
Despite extensive research and awareness created by public and private sectors, progress in preventing / treating cervical cancers is still lagging. Poor reach of awareness campaigns to rural public, lack of education, and social and family associated stigmas are the major hindering factors, which needs to be addressed immediately. In addition, lack of low cost self-testing devices and appropriate biomarkers for disease monitoring are other factors contributing to the failure of cervical cancer prevention programs. Therefore, research should focus on identifying appropriate biomarkers and developing low cost devices for self testing of cervical cancers. Further, patient education and guidance through mobile phones (m-Health) should be implemented for better reach and monitoring. Research is also required to develop drug-gene alert systems to caution clinicians about the drug resistance and suggest appropriate drug/drug combination(s).
The methods used for screening and prevention of HPV related cancers; particularly cervical cancer has advanced tremendously. The early detection will provide a better prognosis and treatment. Despite this many lacunas exist namely lack of awareness and ignorance among the general public about the use of screening programs and vaccination which must be addressed. This is particularly needed in low and middle income countries as it is both a socioeconomic burden affecting women in their productive years.
In conclusion, many unaddressed areas still exist in cervical cancer prevention and treatment. Hence, future studies should focus on these areas and help in developing better tools for preventing and treating cervical cancers
References
- Yan M, Liu Q. Differentiation therapy: a promising strategy for cancer treatment. Chin J Cancer. 2016;35:3.
- Jogi A, Vaapil M, Johansson M, Pahlman S. Cancer cell differentiation heterogeneity and aggressive behavior in solid tumors. Ups J Med Sci. 2012;117(2):217-24.
- Chen J, Liu T, Gao J, Gao L, Zhou L, Cai M, et al. Variation in Carbohydrates between Cancer and Normal Cell Membranes Revealed by Super-Resolution Fluorescence Imaging. Adv Sci (Weinh). 2016;3(12):1600270.
- Depuydt CE, Beert J, Bosmans E, Salembier G. Human Papillomavirus (HPV) virion induced cancer and subfertility, two sides of the same coin. Facts Views Vis Obgyn. 2016;8(4):211-22.
- Braaten KP, Laufer MR. Human Papillomavirus (HPV), HPV-Related Disease, and the HPV Vaccine. Rev Obstet Gynecol. 2008;1(1):2-10.
- Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16(1):1-17.
- Schiffman M, Wentzensen N. Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(4):553-60.
- Thannesberger J, Hellinger HJ, Klymiuk I, Kastner MT, Rieder FJJ, Schneider M, et al. Viruses comprise an extensive pool of mobile genetic elements in eukaryote cell cultures and human clinical samples. FASEB J. 2017;31(5):1987-2000.
- Hyman P, Abedon ST. Smaller fleas: viruses of microorganisms. Scientifica (Cairo). 2012;2012:734023.
- Boccardo E, Villa LL. Viral origins of human cancer. Curr Med Chem. 2007;14(24):2526-39.
- Lupberger J, Hildt E. Hepatitis B virus-induced oncogenesis. World J Gastroenterol. 2007;13(1):74-81.
- Castello G, Scala S, Palmieri G, Curley SA, Izzo F. HCV-related hepatocellular carcinoma: From chronic inflammation to cancer. Clin Immunol. 2010;134(3):237-50.
- Matsuoka M, Jeang KT. Human T-cell leukemia virus type I at age 25: a progress report. Cancer Res. 2005;65(11):4467-70.
- Moll I, Roessler M, Brandner JM, Eispert AC, Houdek P, Moll R. Human Merkel cells–aspects of cell biology, distribution and functions. Eur J Cell Biol. 2005;84(2-3):259-71.
- Klug A, Finch JT. Structure of Viruses of the Papilloma-Polyoma Type. I. Human Wart Virus. J Mol Biol. 1965;11:403-23.
- Pfister H, Fuchs PG. Anatomy, taxonomy and evolution of papillomaviruses. Intervirology. 1994;37(3-4):143-9.
- Krzowska-Firych J, Lucas G, Lucas C, Lucas N, Pietrzyk L. An overview of Human Papillomavirus (HPV) as an etiological factor of the anal cancer. J Infect Public Health. 2018.
- D’Souza G, Dempsey A. The role of HPV in head and neck cancer and review of the HPV vaccine. Prev Med. 2011;53 Suppl 1:S5-S11.
- Sinno AK, Saraiya M, Thompson TD, Hernandez BY, Goodman MT, Steinau M, et al. Human papillomavirus genotype prevalence in invasive vaginal cancer from a registry-based population. Obstet Gynecol. 2014;123(4):817-21.
- Bansal A, Singh MP, Rai B. Human papillomavirus-associated cancers: A growing global problem. Int J Appl Basic Med Res. 2016;6(2):84-9.
- Giuliano AR, Tortolero-Luna G, Ferrer E, Burchell AN, de Sanjose S, Kjaer SK, et al. Epidemiology of human papillomavirus infection in men, cancers other than cervical and benign conditions. Vaccine. 2008;26 Suppl 10:K17-28.
- Thabet M, Hemida R, Hasan M, Elshamy M, Elfaraash M, Emam M. Human papillomavirus (HPV) is not the main cause of preinvasive and invasive cervical cancer among patients in Delta Region, Egypt. J Exp Ther Oncol. 2014;10(4):247-53.
- Egawa N, Doorbar J. The low-risk papillomaviruses. Virus Res. 2017;231:119-27.
- Brendle SA, Bywaters SM, Christensen ND. Pathogenesis of infection by human papillomavirus. Curr Probl Dermatol. 2014;45:47-57.
- Butel JS. Viral carcinogenesis: revelation of molecular mechanisms and etiology of human disease. Carcinogenesis. 2000;21(3):405-26.
- Faridi R, Zahra A, Khan K, Idrees M. Oncogenic potential of Human Papillomavirus (HPV) and its relation with cervical cancer. Virol J. 2011;8:269.
- Schlecht NF, Burk RD, Palefsky JM, Minkoff H, Xue X, Massad LS, et al. Variants of human papillomaviruses 16 and 18 and their natural history in human immunodeficiency virus-positive women. J Gen Virol. 2005;86(Pt 10):2709-20.
- Zheng ZM, Baker CC. Papillomavirus genome structure, expression, and post-transcriptional regulation. Front Biosci. 2006;11:2286-302.
- Stanley MA. Epithelial cell responses to infection with human papillomavirus. Clin Microbiol Rev. 2012;25(2):215-22.
- Kadaja M, Silla T, Ustav E, Ustav M. Papillomavirus DNA replication – from initiation to genomic instability. Virology. 2009;384(2):360-8.
- Giroglou T, Florin L, Schafer F, Streeck RE, Sapp M. Human papillomavirus infection requires cell surface heparan sulfate. J Virol. 2001;75(3):1565-70.
- Shafti-Keramat S, Handisurya A, Kriehuber E, Meneguzzi G, Slupetzky K, Kirnbauer R. Different heparan sulfate proteoglycans serve as cellular receptors for human papillomaviruses. J Virol. 2003;77(24):13125-35.
- Bronnimann MP, Calton CM, Chiquette SF, Li S, Lu M, Chapman JA, et al. Furin Cleavage of L2 during Papillomavirus Infection: Minimal Dependence on Cyclophilins. J Virol. 2016;90(14):6224-34.
- Day PM, Gambhira R, Roden RB, Lowy DR, Schiller JT. Mechanisms of human papillomavirus type 16 neutralization by l2 cross-neutralizing and l1 type-specific antibodies. J Virol. 2008;82(9):4638-46.
- Yim EK, Park JS. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res Treat. 2005;37(6):319-24.
- Lechner MS, Laimins LA. Inhibition of p53 DNA binding by human papillomavirus E6 proteins. J Virol. 1994;68(7):4262-73.
- Yi JW, Jang M, Kim SJ, Kim SS, Rhee JE. Degradation of p53 by natural variants of the E6 protein of human papillomavirus type 16. Oncol Rep. 2013;29(4):1617-22.
- Brooks CL, Gu W. p53 regulation by ubiquitin. FEBS Lett. 2011;585(18):2803-9.
- Zhao F, Huang W, Ousman T, Zhang B, Han Y, Clotaire DZ, et al. Triptolide induces growth inhibition and apoptosis of human laryngocarcinoma cells by enhancing p53 activities and suppressing E6-mediated p53 degradation. PLoS One. 2013;8(11):e80784.
- Bedard KM, Underbrink MP, Howie HL, Galloway DA. The E6 oncoproteins from human betapapillomaviruses differentially activate telomerase through an E6AP-dependent mechanism and prolong the lifespan of primary keratinocytes. J Virol. 2008;82(8):3894-902.
- Liu X, Dakic A, Zhang Y, Dai Y, Chen R, Schlegel R. HPV E6 protein interacts physically and functionally with the cellular telomerase complex. Proc Natl Acad Sci U S A. 2009;106(44):18780-5.
- Misiti S, Nanni S, Fontemaggi G, Cong YS, Wen J, Hirte HW, et al. Induction of hTERT expression and telomerase activity by estrogens in human ovary epithelium cells. Mol Cell Biol. 2000;20(11):3764-71.
- Jin X, Beck S, Sohn YW, Kim JK, Kim SH, Yin J, et al. Human telomerase catalytic subunit (hTERT) suppresses p53-mediated anti-apoptotic response via induction of basic fibroblast growth factor. Exp Mol Med. 2010;42(8):574-82.
- Shay JW, Wright WE. Role of telomeres and telomerase in cancer. Semin Cancer Biol. 2011;21(6):349-53.
- Jafri MA, Ansari SA, Alqahtani MH, Shay JW. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016;8(1):69.
- Cunningham AP, Love WK, Zhang RW, Andrews LG, Tollefsbol TO. Telomerase inhibition in cancer therapeutics: molecular-based approaches. Curr Med Chem. 2006;13(24):2875-88.
- Greenwell M, Rahman PK. Medicinal Plants: Their Use in Anticancer Treatment. Int J Pharm Sci Res. 2015;6(10):4103-12.
- Wang H, Khor TO, Shu L, Su ZY, Fuentes F, Lee JH, et al. Plants vs. cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med Chem. 2012;12(10):1281-305.
- Narisawa-Saito M, Kiyono T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: roles of E6 and E7 proteins. Cancer Sci. 2007;98(10):1505-11.
- Liu X, Clements A, Zhao K, Marmorstein R. Structure of the human Papillomavirus E7 oncoprotein and its mechanism for inactivation of the retinoblastoma tumor suppressor. J Biol Chem. 2006;281(1):578-86.
- Abukhdeir AM, Park BH. P21 and p27: roles in carcinogenesis and drug resistance. Expert Rev Mol Med. 2008;10:e19.
- Zhou J, Li B, Peng C, Wang F, Fu Z, Zhou C, et al. Inhibition of cervical cancer cell growth in vitro and in vivo by lentiviral-vector mediated shRNA targeting the common promoter of HPV16 E6 and E7 oncogenes. Antiviral Res. 2013;98(2):305-13.
- Morrison MA, Morreale RJ, Akunuru S, Kofron M, Zheng Y, Wells SI. Targeting the human papillomavirus E6 and E7 oncogenes through expression of the bovine papillomavirus type 1 E2 protein stimulates cellular motility. J Virol. 2011;85(20):10487-98.
- Jin BY, Campbell TE, Draper LM, Stevanovic S, Weissbrich B, Yu Z, et al. Engineered T cells targeting E7 mediate regression of human papillomavirus cancers in a murine model. JCI Insight. 2018;3(8).
- Draper LM, Kwong ML, Gros A, Stevanovic S, Tran E, Kerkar S, et al. Targeting of HPV-16+ Epithelial Cancer Cells by TCR Gene Engineered T Cells Directed against E6. Clin Cancer Res. 2015;21(19):4431-9.
- DiMaio D, Petti LM. The E5 proteins. Virology. 2013;445(1-2):99-114.
- Mesri EA, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host Microbe. 2014;15(3):266-82.
- Kim HS, Kim T, Kim MK, Suh DH, Chung HH, Song YS. Cyclooxygenase-1 and -2: molecular targets for cervical neoplasia. J Cancer Prev. 2013;18(2):123-34.
- Monje P, Hernandez-Losa J, Lyons RJ, Castellone MD, Gutkind JS. Regulation of the transcriptional activity of c-Fos by ERK. A novel role for the prolyl isomerase PIN1. J Biol Chem. 2005;280(42):35081-4.
- Kanthou C, Dachs GU, Lefley DV, Steele AJ, Coralli-Foxon C, Harris S, et al. Tumour cells expressing single VEGF isoforms display distinct growth, survival and migration characteristics. PLoS One. 2014;9(8):e104015.
- Hua KT, Lee WJ, Yang SF, Chen CK, Hsiao M, Ku CC, et al. Vascular endothelial growth factor-C modulates proliferation and chemoresistance in acute myeloid leukemic cells through an endothelin-1-dependent induction of cyclooxygenase-2. Biochim Biophys Acta. 2014;1843(2):387-97.
- Liu C, Lin J, Li L, Zhang Y, Chen W, Cao Z, et al. HPV16 early gene E5 specifically reduces miRNA-196a in cervical cancer cells. Sci Rep. 2015;5:7653.
- Liu P, Xin F, Ma CF. Clinical significance of serum miR-196a in cervical intraepithelial neoplasia and cervical cancer. Genet Mol Res. 2015;14(4):17995-8002.
- Mishra GA, Pimple SA, Shastri SS. An overview of prevention and early detection of cervical cancers. Indian J Med Paediatr Oncol. 2011;32(3):125-32.
- Safaeian M, Solomon D, Castle PE. Cervical cancer prevention–cervical screening: science in evolution. Obstet Gynecol Clin North Am. 2007;34(4):739-60, ix.
- Kulmala SM, Syrjanen S, Shabalova I, Petrovichev N, Kozachenko V, Podistov J, et al. Human papillomavirus testing with the hybrid capture 2 assay and PCR as screening tools. J Clin Microbiol. 2004;42(6):2470-5.
- Abreu AL, Souza RP, Gimenes F, Consolaro ME. A review of methods for detect human Papillomavirus infection. Virol J. 2012;9:262.
- Tan SY, Tatsumura Y. George Papanicolaou (1883-1962): Discoverer of the Pap smear. Singapore Med J. 2015;56(10):586-7.
- Vilos GA. The history of the Papanicolaou smear and the odyssey of George and Andromache Papanicolaou. Obstet Gynecol. 1998;91(3):479-83.
- Dasanu CA, Herzog TJ. Clear cell adenocarcinoma of the ovary associated with in utero diethylstilbestrol exposure: case report and clinical overview. Medscape J Med. 2009;11(1):6.
- Coutlee F, Mayrand MH, Roger M, Franco EL. Detection and typing of human papillomavirus nucleic acids in biological fluids. Public Health Genomics. 2009;12(5-6):308-18.
- Landry ML. Nucleic acid hybridization in viral diagnosis. Clin Biochem. 1990;23(4):267-77.
- Rabaan AA, Alfaraj SA, Alkhalifah MA. Comparison of the Cepheid Xpert HPV test and the HC2 High-Risk HPV DNA Test for detection of high-risk HPV infection in cervical smear samples in SurePath preservative fluid. J Med Microbiol. 2018.
- Poljak M, Marin IJ, Seme K, Vince A. Hybrid Capture II HPV Test detects at least 15 human papillomavirus genotypes not included in its current high-risk probe cocktail. J Clin Virol. 2002;25 Suppl 3:S89-97.
- Maia LB, Marinho LC, Bocca AL, Cavalcante Neto FF, Velasco LF, Costa PG, et al. Hybrid capture II and PapilloCheck(R) tests for detection of anal high-risk human papillomavirus. Rev Soc Bras Med Trop. 2014;47(2):227-30.
- Tsiodras S, Georgoulakis J, Chranioti A, Voulgaris Z, Psyrri A, Tsivilika A, et al. Hybrid capture vs. PCR screening of cervical human papilloma virus infections. Cytological and histological associations in 1270 women. BMC Cancer. 2010;10:53.
- Li J, Lee JY, Yeung ES. Quantitative screening of single copies of human papilloma viral DNA without amplification. Anal Chem. 2006;78(18):6490-6.
- Vernon SD, Unger ER, Williams D. Comparison of human papillomavirus detection and typing by cycle sequencing, line blotting, and hybrid capture. J Clin Microbiol. 2000;38(2):651-5.
- Boers A, Slagter-Menkema L, van Hemel BM, Belinson JL, Ruitenbeek T, Buikema HJ, et al. Comparing the Cervista HPV HR test and Hybrid Capture 2 assay in a Dutch screening population: improved specificity of the Cervista HPV HR test by changing the cut-off. PLoS One. 2014;9(7):e101930.
- Youens KE, Hosler GA, Washington PJ, Jenevein EP, Murphy KM. Clinical experience with the Cervista HPV HR assay: correlation of cytology and HPV status from 56,501 specimens. J Mol Diagn. 2011;13(2):160-6.
- Tao X, Zheng B, Yin F, Zeng Z, Li Z, Griffith CC, et al. Polymerase Chain Reaction Human Papillomavirus (HPV) Detection and HPV Genotyping in Invasive Cervical Cancers With Prior Negative HC2 Test Results. Am J Clin Pathol. 2017;147(5):477-83.
- Sasagawa T, Mitsuishi T. Novel polymerase chain reaction method for detecting cutaneous human papillomavirus DNA. J Med Virol. 2012;84(1):138-44.
- Lee SH, Vigliotti JS, Vigliotti VS, Jones W. From Human Papillomavirus (HPV) Detection to Cervical Cancer Prevention in Clinical Practice. Cancers (Basel). 2014;6(4):2072-99.
- Lee SH, Vigliotti VS, Vigliotti JS, Pappu S. Validation of human papillomavirus genotyping by signature DNA sequence analysis. BMC Clin Pathol. 2009;9:3.
- Trevino V, Falciani F, Barrera-Saldana HA. DNA microarrays: a powerful genomic tool for biomedical and clinical research. Mol Med. 2007;13(9-10):527-41.
- Bumgarner R. Overview of DNA microarrays: types, applications, and their future. Curr Protoc Mol Biol. 2013;Chapter 22:Unit 22 1.
- Shen-Gunther J, Rebeles J. Genotyping human papillomaviruses: development and evaluation of a comprehensive DNA microarray. Gynecol Oncol. 2013;128(3):433-41.
- Berthet N, Falguieres M, Filippone C, Bertolus C, Bole-Feysot C, Brisse S, et al. Resequencing microarray technology for genotyping human papillomavirus in cervical smears. PLoS One. 2014;9(11):e109301.
- Git A, Dvinge H, Salmon-Divon M, Osborne M, Kutter C, Hadfield J, et al. Systematic comparison of microarray profiling, real-time PCR, and next-generation sequencing technologies for measuring differential microRNA expression. RNA. 2010;16(5):991-1006.
- Fontecha N, Nieto MC, Andia D, Cisterna R, Basaras M. RNA extraction method is crucial for human papillomavirus E6/E7 oncogenes detection. Virol J. 2017;14(1):50.
- Zhang W, Edwards A, Fang Z, Flemington EK, Zhang K. Integrative Genomics and Transcriptomics Analysis Reveals Potential Mechanisms for Favorable Prognosis of Patients with HPV-Positive Head and Neck Carcinomas. Sci Rep. 2016;6:24927.
- Strong MJ, Baddoo M, Nanbo A, Xu M, Puetter A, Lin Z. Comprehensive high-throughput RNA sequencing analysis reveals contamination of multiple nasopharyngeal carcinoma cell lines with HeLa cell genomes. J Virol. 2014;88(18):10696-704.
- Kraeva RI, Krastev DB, Roguev A, Ivanova A, Nedelcheva-Veleva MN, Stoynov SS. Stability of mRNA/DNA and DNA/DNA duplexes affects mRNA transcription. PLoS One. 2007;2(3):e290.
- Mehrotra P. Biosensors and their applications – A review. J Oral Biol Craniofac Res. 2016;6(2):153-9.
- Cagnin S, Caraballo M, Guiducci C, Martini P, Ross M, Santaana M, et al. Overview of electrochemical DNA biosensors: new approaches to detect the expression of life. Sensors (Basel). 2009;9(4):3122-48.
- Grieshaber D, MacKenzie R, Voros J, Reimhult E. Electrochemical Biosensors – Sensor Principles and Architectures. Sensors (Basel). 2008;8(3):1400-58.
- Ronkainen NJ, Halsall HB, Heineman WR. Electrochemical biosensors. Chem Soc Rev. 2010;39(5):1747-63.
- Dey D, Goswami T. Optical biosensors: a revolution towards quantum nanoscale electronics device fabrication. J Biomed Biotechnol. 2011;2011:348218.
- Juskowiak B. Nucleic acid-based fluorescent probes and their analytical potential. Anal Bioanal Chem. 2011;399(9):3157-76.
- Weichert W, Schewe C, Denkert C, Morawietz L, Dietel M, Petersen I. Molecular HPV typing as a diagnostic tool to discriminate primary from metastatic squamous cell carcinoma of the lung. Am J Surg Pathol. 2009;33(4):513-20.
- D’Orazio P. Biosensors in clinical chemistry – 2011 update. Clin Chim Acta. 2011;412(19-20):1749-61.
- Olaru A, Bala C, Jaffrezic-Renault N, Aboul-Enein HY. Surface plasmon resonance (SPR) biosensors in pharmaceutical analysis. Crit Rev Anal Chem. 2015;45(2):97-105.
- Dell’Atti D, Zavaglia M, Tombelli S, Bertacca G, Cavazzana AO, Bevilacqua G, et al. Development of combined DNA-based piezoelectric biosensors for the simultaneous detection and genotyping of high risk Human Papilloma Virus strains. Clin Chim Acta. 2007;383(1-2):140-6.
- Prakrankamanant P, Leelayuwat C, Promptmas C, Limpaiboon T, Wanram S, Prasongdee P, et al. The development of DNA-based quartz crystal microbalance integrated with isothermal DNA amplification system for human papillomavirus type 58 detection. Biosens Bioelectron. 2013;40(1):252-7.
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271-89.
- Franco EL. Cancer causes revisited: human papillomavirus and cervical neoplasia. J Natl Cancer Inst. 1995;87(11):779-80.
- Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87(11):796-802.
- Detmer A, Glenting J. Live bacterial vaccines–a review and identification of potential hazards. Microb Cell Fact. 2006;5:23.
- Smith KA. Louis pasteur, the father of immunology? Front Immunol. 2012;3:68.
- Plotkin S. History of vaccination. Proc Natl Acad Sci U S A. 2014;111(34):12283-7.
- Lee NH, Lee JA, Park SY, Song CS, Choi IS, Lee JB. A review of vaccine development and research for industry animals in Korea. Clin Exp Vaccine Res. 2012;1(1):18-34.
- Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A. 1992;89(24):12180-4.
- Thones N, Herreiner A, Schadlich L, Piuko K, Muller M. A direct comparison of human papillomavirus type 16 L1 particles reveals a lower immunogenicity of capsomeres than viruslike particles with respect to the induced antibody response. J Virol. 2008;82(11):5472-85.
- Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1-30.
- Keam SJ, Harper DM. Human papillomavirus types 16 and 18 vaccine (recombinant, AS04 adjuvanted, adsorbed) [Cervarix]. Drugs. 2008;68(3):359-72.
- Zurek Munk-Madsen M, Toft L, Kube T, Richter R, Ostergaard L, Sogaard OS, et al. Cellular immunogenicity of human papillomavirus vaccines Cervarix and Gardasil in adults with HIV infection. Hum Vaccin Immunother. 2018;14(4):909-16.
- Ahuja A, Anderson SM, Khalil A, Shlomchik MJ. Maintenance of the plasma cell pool is independent of memory B cells. Proc Natl Acad Sci U S A. 2008;105(12):4802-7.
- Villa LL, Ault KA, Giuliano AR, Costa RL, Petta CA, Andrade RP, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine. 2006;24(27-28):5571-83.
- Serrano B, Alemany L, Tous S, Bruni L, Clifford GM, Weiss T, et al. Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infect Agent Cancer. 2012;7(1):38.
- Ahmed F, Temte JL, Campos-Outcalt D, Schunemann HJ, Group AEBRW. Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29(49):9171-6.
- Mishra GA, Pimple SA, Shastri SS. HPV vaccine: One, two, or three doses for cervical cancer prevention? Indian J Med Paediatr Oncol. 2015;36(4):201-6.
- Gilca V, Sauvageau C, Boulianne N, De Serres G, Couillard M, Krajden M, et al. Immunogenicity of quadrivalent HPV and combined hepatitis A and B vaccine when co-administered or administered one month apart to 9-10 year-old girls according to 0-6 month schedule. Hum Vaccin Immunother. 2014;10(8):2438-45.
- Draper E, Bissett SL, Howell-Jones R, Waight P, Soldan K, Jit M, et al. A randomized, observer-blinded immunogenicity trial of Cervarix((R)) and Gardasil((R)) Human Papillomavirus vaccines in 12-15 year old girls. PLoS One. 2013;8(5):e61825.
- Stanley M. HPV vaccination in boys and men. Hum Vaccin Immunother. 2014;10(7):2109-11.
- Bonanni P, Gabutti G, Demarteau N, Boccalini S, La Torre G. Vaccination of boys or catch-up of girls above 11 years of age with the HPV-16/18 AS04-adjuvanted vaccine: where is the greatest benefit for cervical cancer prevention in Italy? BMC Infect Dis. 2015;15:377.
- Park JY, Nam JH. Role of robotic surgery in cervical malignancy. Best Pract Res Clin Obstet Gynaecol. 2017;45:60-73.
- Li L, Ma SQ, Tan XJ, Zhong S, Wu M. Pelvic Exenteration for Recurrent and Persistent Cervical Cancer. Chin Med J (Engl). 2018;131(13):1541-8.
- Bryant AK, Huynh-Le MP, Simpson DR, Mell LK, Gupta S, Murphy JD. Intensity Modulated Radiation Therapy Versus Conventional Radiation for Anal Cancer in the Veterans Affairs System. Int J Radiat Oncol Biol Phys. 2018;102(1):109-15.
- Miglierini P, Malhaire JP, Goasduff G, Miranda O, Pradier O. Cervix cancer brachytherapy: high dose rate. Cancer Radiother. 2014;18(5-6):452-7.
- Mazeron R, Dumas I, El Khouri C, Levy A, Attar M, Haie-Meder C. [Intensity-modulated radiotherapy in cervical cancer: towards a new standard?]. Cancer Radiother. 2014;18(2):154-60; quiz 62, 64.
- Doyen J, Bondiau PY, Benezery K, Chand ME, Thariat J, Leysalle A, et al. [Current situation and perspectives of proton therapy]. Cancer Radiother. 2015;19(3):211-9; quiz 31-2, 35.
- Wong E, Giandomenico CM. Current status of platinum-based antitumor drugs. Chem Rev. 1999;99(9):2451-66.
- Zuliani AC, Esteves SC, Teixeira LC, Teixeira JC, de Souza GA, Sarian LO. Concomitant cisplatin plus radiotherapy and high-dose-rate brachytherapy versus radiotherapy alone for stage IIIB epidermoid cervical cancer: a randomized controlled trial. J Clin Oncol. 2014;32(6):542-7.
- Fisher CM, Schefter TE. Profile of bevacizumab and its potential in the treatment of cervical cancer. Onco Targets Ther. 2015;8:3425-31.
- Bender D, Sill MW, Lankes HA, Reyes HD, Darus CJ, Delmore JE, et al. A phase II evaluation of cediranib in the treatment of recurrent or persistent endometrial cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2015;138(3):507-12.
- Eskens FA. Angiogenesis inhibitors in clinical development; where are we now and where are we going? Br J Cancer. 2004;90(1):1-7.
- Symonds RP, Gourley C, Davidson S, Carty K, McCartney E, Rai D, et al. Cediranib combined with carboplatin and paclitaxel in patients with metastatic or recurrent cervical cancer (CIRCCa): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16(15):1515-24.
- Wells A. EGF receptor. Int J Biochem Cell Biol. 1999;31(6):637-43.
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127-37.
- Bianco R, Daniele G, Ciardiello F, Tortora G. Monoclonal antibodies targeting the epidermal growth factor receptor. Curr Drug Targets. 2005;6(3):275-87.
- Chen YF, Tang WB, Pan XX, Wu CR, Cao Y, Yang W. Safety and efficacy of nimotuzumab combined with chemoradiotherapy in Chinese patients with locally advanced cervical cancer. Onco Targets Ther. 2017;10:4113-9.
- Caldecott KW, Aoufouchi S, Johnson P, Shall S. XRCC1 polypeptide interacts with DNA polymerase beta and possibly poly (ADP-ribose) polymerase, and DNA ligase III is a novel molecular ‘nick-sensor’ in vitro. Nucleic Acids Res. 1996;24(22):4387-94.
- de Murcia JM, Niedergang C, Trucco C, Ricoul M, Dutrillaux B, Mark M, et al. Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc Natl Acad Sci U S A. 1997;94(14):7303-7.
- Kotsopoulos IC, Kucukmetin A, Mukhopadhyay A, Lunec J, Curtin NJ. Poly(ADP-Ribose) Polymerase in Cervical Cancer Pathogenesis: Mechanism and Potential Role for PARP Inhibitors. Int J Gynecol Cancer. 2016;26(4):763-9.
- Thaker PH, Salani R, Brady WE, Lankes HA, Cohn DE, Mutch DG, et al. A phase I trial of paclitaxel, cisplatin, and veliparib in the treatment of persistent or recurrent carcinoma of the cervix: an NRG Oncology Study (NCT#01281852). Ann Oncol. 2017;28(3):505-11.
- Liu FS. Mechanisms of chemotherapeutic drug resistance in cancer therapy–a quick review. Taiwan J Obstet Gynecol. 2009;48(3):239-44.
- Curiel TJ. Immunotherapy: a useful strategy to help combat multidrug resistance. Drug Resist Updat. 2012;15(1-2):106-13.
- Stevanovic S, Draper LM, Langhan MM, Campbell TE, Kwong ML, Wunderlich JR, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol. 2015;33(14):1543-50.







