Manuscript accepted on :29-11-2019
Published online on: 28-12-2019
Plagiarism Check: Yes
Reviewed by: Amr Ahmed Elhakem
Second Review by: Vijay Rekulapally
Final Approval by: Dr. Ian James Martin
Antonio Ruggiero2 , Giovanna Trombatore2, Silvia Triarico2, Michele Antonio Capozza2, Paola Coccia1, Giorgio Attina2, Stefano Mastrangelo2 and Palma Maurizi2
, Giovanna Trombatore2, Silvia Triarico2, Michele Antonio Capozza2, Paola Coccia1, Giorgio Attina2, Stefano Mastrangelo2 and Palma Maurizi2
1Pediatric Hemato-oncology Unit, Ospedale Salesi, Azienda Ospedali Riuniti Ancona, Ancona, Italy
2Pediatric Oncology Unit, Fondazione Policlinico Universitario A .Gemelli IRCCS, Universita’ Cattolica Sacro Cuore, Roma, Italy
Corresponding Author E-mail: antonio.ruggiero@unicatt.it
DOI : https://dx.doi.org/10.13005/bpj/1791
Abstract
Platinum’ derivates are antineoplastic agents widely adopted for their efficacy for the treatment of many pediatric cancers. The use of cisplatin has positively influenced the results of the cure of different childhood malignancies. However, cisplatin-based treatments are limited by the risk of severe and progressive toxicities, such as oto- or nephrotoxicity, that can be more serious in very young children expecially when high cumulative doses and/or radiotherapy is administered. A correct knowledge of the cisplatin’ pharmacological features might be of interest for clinicians in order to manage its potential toxicities. Based on the positive trend in the cure of children with cancer, it is crucial that all children receiving cisplatin-based chemotherapy have and appropriate and long-term follow-up to improve their quality of life.
Keywords
Cisplatin; Children; Nephrotoxicity; Neurotoxicity; Myelotoxicity; Ototoxicity
Download this article as:| Copy the following to cite this article: Ruggiero A, Trombatore G, Triarico S, Capozza M. A, Coccia P, Attina G, Mastrangelo S, Maurizi P. Cisplatin Toxicity in Children with Malignancy. Biomed Pharmacol J 2019;12(4). |
| Copy the following to cite this URL: Ruggiero A, Trombatore G, Triarico S, Capozza M. A, Coccia P, Attina G, Mastrangelo S, Maurizi P. Cisplatin Toxicity in Children with Malignancy. Biomed Pharmacol J 2019;12(4). Available from: https://bit.ly/2Q2Kkx9 |
Introduction
The use of the cisplatin in the chemo-treatment of pediatric malignancies has contributed to positively impact on the chances of cure of children affected by tumor [1]. Regrettably, the cisplatin use can be limited by different toxicities that can include otological, neurological, nephrological, hematological toxicity, and emesis [2,3]. These potential toxicities can negatively impact on the adherence to the treatment and on the health status of children cured for their cancer.
The purpose of the present manuscript is to describe the main pharmacological and clinical features of cisplatin with a focus on its pharmacokinetic profile and toxic effects in order to promote the development of appropriate strategies for preventing them.
Pharmacokinetics
In the early 70s, cisplatin was among the first platinum compounds available for the clinical practice and since that time it continues to play a fundamental role in the current chemotherapy [4,5].
Cisplatin (cis-diamminedichloroplatinum II) structure contains a platinum atom surrounded by two ammonia groups and two chloride leaving groups in the cis-position (Figure 1). It is intracellulary activated by a replacement reaction in which the cisplatin’ chloride ligands are replaced by water molecules with consequent platinum cations that can covalently bind with the purine DNA bases obtaining intra-strand and inter-strand cross-links [6].
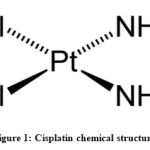 |
Figure 1: Cisplatin chemical structure |
The cisplatin’ antineoplastic effects is mediated by the generation of cisplatin-DNA adducts (N-7 adducts at d(GpC) and d(ApG)) able to block the DNA, RNA and protein synthesis, on the basis of the proliferative index of cancer cells [5,7].
The drug is characterized by a high binding affinity with plasma proteins: following the intra-venous administration of the cisplatin almost 90% bind with the plasmatic proteins. This binding of the platinum appears to significantly influence its pharmacokinetic profile and activity as only the unbound drug can have antineoplastic activity. The protein-bound platinum can persist in plasma for a long time and detected in urine for many hours or days up to 4 days [8,9]. On the contrary, the unbound drug appears to have a rapid metabolism with a brief half-life of less than 1 hour. In the pediatric population, the total and unbound platinum have very different half-lives ranging from 44 for total drug to 1.3 hours for unbound drug [10]. In addition, about 90% of the platinum is excreted by the kidney via glomerular filtration and tubular secretion and the remaining 10% by biliary excretion
Both the unbound cisplatin and the total platinum, which consists of bound and unbound platinum, can be measurable in serum, although only the ultra-filtrable platinum is related to the antitumor and toxic effects of the drug.
Ototoxicity
Up to 60% of children treated with cisplatin-based chemotherapy can be at risk of developing irreversible bilateral hearing loss [1,11-14]. The otological toxicity may develop when a high dosage of cisplatin is given intraperitoneally or intravenously. However, a single low dose of drug can be nevertheless ototoxic when infused retrograde into the common carotid artery due to the drug first-pass into the vertebral artery that provides blood to the cochlea [14,16].
Sensory cells of the inner ear are protected by a blood labyrinth barrier very similar to the blood-brain barrier. However, the platinum has been found in the cochlea tissues even in presence of a blood labyrinth barrier intact making the passage mechanism unclear [14,17].
This barrier can be evaded with the intrusion of the cisplatin directly into the cochlea or temporarily opening the barrier through the use of loop diuretics [18,19]. Alternatively, the combination of noise and cisplatin injection can favorite the passage of platinum into the cochlea [20]. The blood labyrinth barrier and the stria vascularis can be damaged by the noise exposure; also the cochlea can become more vulnerable due to the induction of the oxidative stress and the concomitant reduction of the antioxidant enzyme levels [18,20].
The damaged cochlear hair cells degenerate first at the base of the cochlea and then gradually towards the apex if the exposure to the drug is repeated over time [14,21]. At the level of the mitochondria of cochlear cells, alkylation of cisplatin is able to determine the mitochondrial production of factors favoring apoptosis and high levels of reactive oxygen species (ROS) with the consequence of activating caspases, thus triggering cell death. Moreover, the high toxic levels of ROS increase and amplify the degeneration following the damage of proteins and lipids and facilitating the exhaustion of the antioxidant agents of the cell [22]. Moreover, some data attribute to p53 a potential role in the initial development of the hair cell death process induced by the cisplatin administration [23,24]. The upregulation of p53 and later the upregulation induction of caspase 8-9, cytochrome-c and Bax can speed up the apoptotic cell death [25].
Myelotoxicity
Cisplatin can induce reversible and dose-related myelotoxicity. Clinically, it is generally of a mild level and can involve all three hematopoietic lines. Data from the literature suggest that cisplatin might determine in mice CFU-S and CFU-C progressive and cumulative toxicity [26]. Previous studies have reported serious leucopenia or thrombocytopenia up to 5% to 6% of patients including episodes secondary to haemolysis and erythropoietic toxicity [ 27-29].
Nephrotoxicity
Over the years, many studies have tried to identify the molecular mechanisms that are the basis of nephrotoxic cisplatin damage, I conclude that the damage is linked to its accumulation in renal tubular cells with the consequence of causing a direct inflammation, stress and damage oxidative, and finally to activate a mechanism of apoptosis with tubular lesion and dysfunction [30,31].
Alterations from cisplatin damage are found mainly in the epithelial cells that are located in the S3 segment of the proximal tubule; this site is particularly damaged for the reason that in this renal site the concentration of cisplatin is about five times greater than the serum concentration. Furthermore, the OCT2 organic cation transporter is also involved in renal cisplatin damage, which is essential for active absorption in renal tubular cells. In fact, by administering cimetidine, which is an OCT2 substrate, the absorption of cisplatin and the consequent nephrotoxicity is reduced. As confirmation of the role of OCT2, Filipski et al. showed that the presence of a non-synonymous single-nucleotide polymorphism (SNP) in the OCT2 gene SLC22A2 (rs316019) is able to reduce its nephrotoxicity in patients receiving cisplatin [32,33].
Other studies on molecular pathogenesis have hypothesized that the nephrotoxicity of cisplatin may be due to the splitting of a cisplatin-glutathione conjugated with gamma-glutamyl transpeptidase (GGT) at the level of the luminal surface of the proximal renal tubules. GGT performs its function by initiating the catalysis of drugs conjugated with glutathione to mercapturic acids, some of which can be severely nephrotoxic [34].
Once penetrated into the tubular cells, cisplatin acts to damage nuclear DNA and especially mitochondria, leading to activation of both mitochondrial and non-mitochondrial pathways in apoptosis and necrosis processes. Mitochondria are particularly vulnerable to injury, due to the lack of any efficient DNA repair mechanism; their abundant presence in the proximal tubule may be at the basis of the vulnerability of this specific renal site to cisplatin damage [35]. In cisplatin-induced renal cell death processes, both the intrinsic mitochondria pathway and the endoplasmic reticulum stress pathway and extrinsic activation of TNF or Fas receptors are involved, although significant interactions between the two pathways are reported.
Furthermore, cisplatin is able to increase the renal expression of TNF-α, which in turn can induce apoptotic mechanisms, increase ROS production, promote the activation of pro-inflammatory cytokines and chemokines, thus performing a key role in the pathogenesis of cisplatin-induced renal damage.
The production of pro-inflammatory cytokines induces and amplifies an inflammatory response, with the infiltration of macrophages and lymphocytes, with the consequence of inducing severe interstitial fibrosis [36].
The study of the various mechanisms of cisplatin-induced toxicity has led to propose various potentially protective agents. For example, amifostine, vitamin C and E, allopurinol, melatonin, ebselen, erdosteine seem able to act against oxidative stress damage; erythropoietin and amifostine may play a cytoprotective and antiapoptotic role. Salicylates have been shown to reduce renal inflammation in cisplatin toxicity models as fibrates appear to prevent cisplatin nephrotoxicity in animal studies. However, of all these agents only a few have been applied in human studies [37,38].
Cisplatin can cause acute renal failure due to degeneration, necrosis, desquamation of epithelial cells of the proximal, distal tubules and collecting ducts, without evident glomerular morphological changes. Furthermore, tubular necrosis with dilated cystic tubules and interstitial fibrosis is observed in patients receiving long-term cisplatin treatment [38]. The tubular lesion appears before acute renal failure and haemodynamic dysfunction processes. The consequent reduction of mitochondria and ATPase activity and the coexistent reduction of the expression of solute, co-transporter and aquaphorin transporters at the level of the water channel determine a reduction of the tubular reabsorption capacity of sodium and water, thus increasing the water and sodium excreted. Patients may present with polyuria, reduction of urinary osmolarity after 24 and 48 hours from the infusion of cisplatin, although the glomerular filtration rate remains stable (GFR). Subsequently, also the GFR can be reduced and in the patients increases the urinary excretion of electrolytes such as sodium, potassium, magnesium, calcium, glucose and small amounts of proteins, with potential orthostatic hypotension. However, these alterations are often transient and renal function is usually restored 2-4 or more weeks after cisplatin treatment [39,40].
Cisplatin-induced renal damage is mainly acute damage, but may also result in several clinical pictures such as isolated hypomagnesemia (especially in patients receiving prolonged cisplatin treatment), Fanconi-like syndrome, hypocalcemia, renal salt wasting and reduced capacity of renal concentration, hyperuricemia, distal tubular acidosis, thrombotic-based microangiopathy, transient proteinuria, erythropoietin deficiency [41].
In children receiving platinum-based chemotherapy nephrotoxicity, nephron toxicity, GFR reduction, hypocalcemia, hypomagnesaemia, hypopotassemia can occur. However, data on the long-term results of renal cisplatin damage in children are not conclusive, as chronic kidney damage often appears in adulthood [42,43].
Data on children reported that the risk of cisplatin nephrotoxicity is higher in older children (generally older than 10 years) compared to young children as well as the reduction in GFR was less frequent in children when they received a lower dose of cisplatin at 40 mg / m2 / dose [44].
Furthermore, the infusion rate of cisplatin in children appears to significantly influence the severity of renal damage: long-term cisplatin infusions appear to be less nephrotoxic than repetitive and intermittent bolus administrations [43].
Hyper-hydration with isotonic saline was the most frequently adopted strategy for reducing the incidence of renal cisplatin damage. Recent clinical guidelines have also established that the addition of diuretics, such as mannitol and furosemide, does not seem to offer greater nephroprotection than the use of hydration alone [41,45,46]. Hypomagnesemia itself may lead to an increased risk of cisplatin nephrotoxicity; therefore the continuous integration of magnesium (both during the administration of the cisplatin and between one cycle and another) must be part of the routine regimen [47].
In addition, other potentially nephrotoxic agents (such as iphosphamide, antibiotics, intravenous radiographic contrast) can contribute to increased renal damage and therefore should be avoided when patients are receiving cisplatin treatment [48].
Neurotoxicity
The neuropathy related to cisplatin administration is cumulative and dose-dependent. Up to approximately 50% of patients treated with cumulative doses of cisplatin greater than 300 or 600 mg / m2 may be present [49].
Clinical manifestations can be moderate to severe, with signs and symptoms of peripheral lesions, such as numbness, loss of vibration and sense of position, painful tingling and paresthesia, loss of taste, tremor, ataxia. Symptoms generally improve gradually after cisplatin withdrawal and very rarely neuropathy can become permanent [50].
Some researchers attribute to thiol compounds (such as amifostine, glutathione and melanocortin) a potential protective role against the development of cisplatin neurotoxicity [6]. Glutathione, although further studies are needed on its safety, appears to reduce the incidence of cisplatin neurotoxicity without interfering with the antineoplastic effect of the chemotherapy drug [51].
The concentration of cisplatin in the DRG reduces the reserves of vitamin E, thus reducing resistance to oxidative stress; Pace et al. has shown that supplementing with vitamin E given before and after treatment with cisplatin may have a neuroprotective effect [52].
A deleterious interaction was described when cisplatin treatment was combined with cranial irradiation with the appearance of acute neurological symptoms of different severity such as coma, paralysis of multiple cranial nerves, quadriparesis, convulsions. Furthermore, the hyperhydration used to administer cisplatin can accentuate neurological disorders; therefore, caution should be exercised when treatment with cisplatin is planned in children with brain tumors. This treatment with cisplatin should be carefully considered, given the risk of significant hearing loss and acute neurological deterioration later [53].
Nausea and vomiting
Cisplatin frequently has nausea and vomiting as side effects. Vomiting caused by cisplatin may be early (≤ 24 hours after treatment) or delayed (> 24 hours after treatment). The use of 5-hydroxytryptamine (5-HT3) receptor antagonists has allowed to control and significantly reduce acute cisplatin-related emesis [34].
Risk factors
Studies on cisplatin toxicity have allowed us to identify various risk factors for children. Among the main reports were the cumulative dose of cisplatin, concomitant therapies with nephrotoxic or ototoxic drugs, a young age, suffering from tumors of the central nervous system (CNS) and receiving CNS radiation [54-58].
Age. Young children are at greater risk of developing cisplatin ototoxicity. Children under the age of 5 have a risk up to 21 times greater than developing a moderate / severe high frequency hearing loss compared to patients aged 15 to 20 [59]. This increased risk appears to be related to immaturity of cochlea cells or to a different pharmacokinetic of cisplatin in young children.
Radiation. The severity of hearing loss due to platinum compounds can be increased by previous craniospinal or concomitant radiotherapy treatments. This hearing loss can also occur when patients receive low cumulative platinum doses [60-62].
Cumulative dose. Increasing the cumulative dose of cisplatin increases the risk of ototoxicity. When the cumulative dose> 400 mg / m2 is exceeded, there is a significant risk of developing moderate to severe ototoxicity [59].
Nephro- and ototoxic drugs
Concomitant administration of aminoglycosides, bleomycin and loop-inhibitor diuretics may increase the risk of cisplatin-related ototoxicity [63].
Interpatient variability. The ability of renal excretion and protein binding by cisplatin appear to be determinant factors of interpatient variability. This variability, unlike adults, is very wide especially in very young children where the immaturity of the physiological processes responsible for the metabolism of the drug determines a slower elimination of platinum with a large volume of distribution [64-66]. Among the factors that can increase platinum ototoxicity, polymorphisms of the megalin gene have also been reported [67]. Megalin is a multiligand endocytotic receptor involved in the transport of cisplatin or cisplatin adducts. This receptor is found highly expressed in proximal renal tubular cells and in marginal cells of the vascular stria of the inner ear. Furthermore, the cumulative risk of developing ototoxicity appears to increase if other factors such as renal failure or intravenous bolus administration are present.
Conclusions
Cisplatin, since its discovery, is an important drug in the treatment of children with cancer. However, its widespread use is limited by several toxic effects that can negatively affect patients’ quality of life due to the risk of toxicity of the auditory and nervous system, as well as kidney function and bone marrow.
In order to prevent toxicity and improve the quality of care of patients with pediatric cancer, an adequate knowledge of the pharmacokinetic and toxicological characteristics of platinum is recommended.
In particular, children, due to the physiological immaturity of metabolic systems, are more at risk of developing serious negative effects such as ototoxicity that can have a negative impact on learning, on language acquisition and academic performance. Therefore, particular attention must be paid to the nefrologic and otological follow-up of these young patients to protect them from the disabling effects of potentially curative treatments.
Acknowledgments
This work was supported by Fondazione per l’ Oncologia Pediatrica.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding support
None
References
- Knight KR,Kraemer DF,Neuwelt EA, 2005. Ototoxicity in children receiving platinum chemotherapy: Underestimating a commonly occurring toxicity that may influence academic and social development. Journal of Clinical Oncology, 23(34): 8588-96
CrossRef - O’Dwyer PJ, Stevenson JP, Johnson SW, 2000. Clinical pharmacokinetics and administration of established platinum drugs. Drugs, 59 (4 Suppl.): 19-27
CrossRef - Ruggiero A, Trombatore G, Triarico S, Arena R, Ferrara P, Scalzone M, Pierri F, Riccardi R, 2013. Platinum compounds in children with cancer: toxicity and clinical management. Anticancer Drugs, 24(10):1007-1019
CrossRef - Lokich J, Anderson N, 1998. Carboplatin versus cisplatin in solid tumors: An analysis of the literature. Annals of Oncology; 9: 13-21
CrossRef - Gietema JA, Meinardi MT, Messerschmidt J, Gelevert T, Alt F, Uges DR, Sleijfer DT, 2000. Circulating plasma platinum more than 10 years after cisplatin treatment for testicular cancer. Lancet, 355(9209): 1075-1076
CrossRef - Sprauten M, Darrah T, Petersonm D, Campbell ME, Hannigan R, Cvancarova M, 2012. Impact of long-term serum platinum concentrations on neuro- and ototoxicity in cisplatin-treated survivors of testicular cancer. Journal of Clinical Oncology, 30(3):300-307, doi: 0.1200/JCO.2011.37.4025
CrossRef - Boulikas T, Pantos A, Bellis E, Petros C, 2007. Designing platinum compounds in cancer: structures and mechanisms. Cancer Therapy; 5: 537-583
- Gormley PE, Bull JM, LeRoy AF, et a, 1979. Kinetics of cis-dichlorodiammineplatinum. Clinical Pharmacology and Therapeutics, 25(3): 351-357.
CrossRef - DeConti RC,Toftness BR,Lange RC, Creasey WA, 1973. Clinical and pharmacological studies with cis-diamminedichloroplatinum (II). Cancer Research, 33(6): 1310-1315.
CrossRef - Pratt CB,Hayes A,Green AA, Evans WE, Senzer N, Howarth CB, Ransom JL, Crom W, 1981. Pharmacokinetic evaluation of cisplatin in children with malignant solid tumours: a phase II study. Cancer Treatment Reports, 65(11-12): 1021-1026.Knight KR, Kraemer DF, Winter C, Neuwelt EA, 2007. Early changes in auditory function as a result of platinum chemotherapy: Use of extended high frequency audiometry and evoked distortion product otoacoustic emissions. Journal of Clinical Oncology, 25(10): 1190-1195
CrossRef - Blakely BW, Myers SF, 1993. Patterns of hearing loss resulting from cisplatinum therapy. Otolaryngology Head and Neck Surgery, 109(3 Pt 1): 385-391
CrossRef - Skinner R, Pearson AD, Amineddine HA, Mathias DB, Craft AW, 1990. Ototoxicity of cisplatinum in children and adolescents. British Journal of Cancer, 61(6): 927-931
CrossRef - Brock PR,Knight KR,Freyer DR, Campbell KC, Steyger PS, Blakley BW, Rassekh SR, Chang KW, Fligor BJ, Rajput K, Sullivan M, Neuwelt EA, 2012. Platinum-induced ototoxicity in children: A consensus review on mechanisms, predisposition, and protection, including a new international society of pediatric oncology Boston ototoxicity scale. Journal of Clinical Oncology, 30(19): 2408-2417
CrossRef - Fetoni AR, Ruggiero A, Lucidi D, De Corso E, Sergi B, Conti G, Paludetti G. 2016. Audiological Monitoring in Children Treated with Platinum Chemotherapy. Audiology. Neurootology, 21(4):203-211
CrossRef - Dickey DT, Wu YJ, Muldoon LL, Neuwelt EA, 2004. Protection against cisplatin-induced ototoxicity by N-acetylcysteine in a rat model. Hearing Research, 193(1-2): 25-30
CrossRef - van Ruijven MW, de Groot JC, Hendriksen F, Smoorenburg GF, 2005. Immunohistochemical detection of platinated DNA in the cochlea of cisplatin-treated guinea pigs. Hearing Research, 203(1-2): 112-121
CrossRef - Ding D, Allman BL, Salvi R, 2012. Ototoxic characteristics of platinum antitumor drugs. Anatomical record, 295(11): 1851-1867, doi: 10.1002/ar.22577
CrossRef - He J, Yin S, Wang J, Ding D, Jiang H, 2009. Effectiveness of different approaches for establishing cisplatin-induced cochlear lesions in mice. Acta Oto-laryngologica, 129(12): 1359-1367, doi: 10.3109/ 00016480902856604
CrossRef - Gratton MA, Salvi RJ, Kamen BA, Saunders SS, 1990. Interaction of cisplatin and noise on the peripheral auditory system. Hearing Research, 50(1-2): 211- 223
CrossRef - Laurell G, Bagger-Sjoback D, 1991. Dose-dependent inner ear changes after i.v. administration of cisplatin. The Journal of Otolaryngology, 20(3):158-167
- Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V, 2007. Mechanisms of cisplatin- induced ototoxicity and prevention. Hearing Research, 226(1-2): 157-167
CrossRef - Boulikas T, Vougiouka M, 2003. Cisplatin and platinum drugs at the molecular level. Oncology Reports, 10(6): 1663-1682
CrossRef - Park CM,Park MJ,Kwak HJ, Moon SI, Yoo DH, Lee HC, Park IC, Rhee CH, Hong SI, 2006. Induction of p53-mediated apoptosis and recovery of chemosensitivity through p53 transduction in human glioblastoma cells by cisplatin. International Journal of Oncology, 28(1): 119-125
CrossRef - Devarajan P,Savoca M,Castaneda MP, Park MS, Esteban-Cruciani N, Kalinec G, Kalinec F, 2002. Cisplatin-induced apoptosis in auditory cells: role of death receptor and mitochondrial pathways. Hearing Research, 174(1-2): 45–54
CrossRef - Egorin MJ, Van Echo DA, Olman EA, Whitacre MY, Forrest A, Aisner J, 1985. Prospective of validation of a pharmacologically-based dosing scheme for the cis-diamminedichloroplatin (II) analogue cis-diammine- cyclobutanedicarboxylato platinum (II). Cancer Research, 45(12 Pt 1): 6502-6506
CrossRef - Go RS, Adjei AA, 1999. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. Journal of Clinical Oncology, 17(1): 409-422
CrossRef - Rothmann SA, Weick JK, 1981. Cisplatin toxicity for erythroid precursors. New England Journal of Medicine, 304(6): 360
CrossRef - Fouladi M,Blaney SM,Poussaint TY, Freeman BB 3rd, McLendon R, Fuller C, Adesina AM, Hancock ML,Danks MK, Stewart C, Boyett JM, Gajjar A, 2006. Phase II Study of oxaliplatin in children with recurrent or refractory medulloblastoma, supratentorial primitive neuroectodermal tumors, and atypical teratoid rhabdoid tumors. Cancer, 107(9): 2291-2297
CrossRef - Yao X, Panichpisal K, Kurtzman N, Nugent K, 2007. Cisplatin nephrotoxicity: a review. The American Journal of Medical Sciences, 334(2): 115-124
CrossRef - Ruggiero A, Ferrara P, Attinà G, Rizzo D, Riccardi R, 2017. Renal toxicity and chemotherapy in children with cancer. British Journal Clinical Pharmacology, 83(12):2605-2614
CrossRef - Ciarimboli G,Deuster D,Knief A, Sperling M, Holtkamp M, Edemir B, Pavenstädt H, Lanvers-Kaminsky C,am Zehnhoff-Dinnesen A, Schinkel AH, Koepsell H, Jürgens H, Schlatter E, 2010. Organic cation transporter 2 mediates cisplatin-induced oto-and nephrotoxicity and is a target for protective interventions. The America Journal of Pathology, 176(3): 1169-1180, doi: 10.2353/ajpath.2010.090610
CrossRef - Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A, 2009. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clinical pharmacology and therapeutics, 86(4): 396-402
CrossRef - Hanigan MH, Gallagher BC, Taylor PT Jr, Large MK, 1994. Inhibition of gamma-glutamyl transpeptidase activity by acivicin in vivo protects the kidney from cisplatin-induced toxicity. Cancer Research, 54(22): 5925-5929
CrossRef - Qian W,Nishikawa M,Haque AM, Hirose M, Mashimo M, Sato E, Inoue M, 2005. Mitochondrial density determines the cellular sensitivity to cisplatin-induced cell death. American Journal of Physiology. Cell Physiology, 289(6): 1466-1475
CrossRef - Yamate J,Machida Y,Ide M, Kuwamura M, Kotani T, Sawamoto O, LaMarre J, 2005. Cisplatin-induced renal interstitial fibrosis in neonatal rats, developing as solitary nephron unit lesions. Toxicologic Pathology, 33(2): 207-217
CrossRef - Miller RP, Tadagavadi RK, Ramesh G, Reeves WB, 2010. Mechanisms of Cisplatin nephrotoxicity. Toxins (Basel), 2(11): 2490-2518
CrossRef - Ali BH, Al Moundhri MS, 2006. Agents ameliorating or augmenting the nephrotoxicity of cisplatin and other platinum compounds: a review of some recent research. Food and Chemical Toxicology, 44(8): 1173-1183.
CrossRef - Arany I, Safirstein RL, 2003. Cisplatin nephrotoxicity. Seminars in nephrology, 23(5): 460-464
CrossRef - Puma N, Ruggiero A, Scalzone M, Coccia P, Triarico S, Trombatore G, Mastrangelo S, Riccardi R. 2013. Platinum compounds and sodium metabolism in children with diencephalic glioma. Journal Neurooncology, 115(1):113-117
CrossRef - Launay-Vacher V, Rey JB, Isnard-Bagnis C, Deray G, Daouphars M, 2008. Prevention of cisplatin nephrotoxicity: state of the art and recommendations from the European Society of Clinical Pharmacy Special Interest Group on Cancer Care. Cancer Chemotherapy and Pharmacology, 61(6): 903-909, doi: 10.1007/ s00280-008-0711-0
CrossRef - Skinner R,Pearson AD,English MW, Price L, Wyllie RA, Coulthard MG, Craft AW, 1998. Cisplatin dose rate as a risk factor for nephrotoxicity in children. British Journal of Cancer, 77(10): 1677-1682
CrossRef - Skinner R, Parry A, Price L, Cole M, Craft AW, Pearson AD, 2009. Persistent nephrotoxicity during 10-year follow-up after cisplatin or carboplatin treatment in childhood: relevance of age and dose as risk factors. European Journal of Cancer, 45(18): 3213-3219, doi: 10.1016/j.ejca.2009.06.032.
CrossRef - Erdlenbruch B, Pekrum A, Roth C, Grunewald RW, Kern W, Lakomek M, 2001. Cisplatin nephrotoxicity in children after continuous 72-h and 3×1-h infusions. Pediatric Nephrology, 16(7): 586-593
CrossRef - Morgan KP, Buie LW, Savage SW, 2012. The role of mannitol as a nephroprotectant in patients receiving cisplatin therapy. The Annals of Pharmacotherapy, 46(2): 276-281
CrossRef - Ruggiero A, Rizzo D, Trombatore G, Maurizi P, Riccardi R. 2016. The ability of mannitol to decrease cisplatin-induced nephrotoxicity in children: real or not? Cancer Chemotherapy Pharmacology, 77(1):19-26
CrossRef - Willox JC, McAllister EJ, Sangster G, Kaye SB, 1986. Effects of magnesium supplementation in testicular cancer patients receiving cis-platin: a randomised trial. British Journal of Cancer, 54(1): 19-23
CrossRef - Jones DP, Spunt SL, Green D, Springate JE, 2008. Renal late effects in patients treated for cancer in childhood: a report from the Children’s Oncology Group. Pediatric Blood & Cancer, 51(6): 724-731, doi: 10.1002/pbc.21695
CrossRef - van den Bent MJ,van Putten WL,Hilkens PH, de Wit R, van der Burg ME, 2002. Retreatment with dose-dense weekly cisplatin after previous cisplatin chemotherapy is not complicated by significant neuro-toxicity. European Journal of Cancer, 38(3): 387-391
CrossRef - Amptoulach S, Tsavaris N, 2011. Neurotoxicity caused by the treatment with platinum analogues. Chemotherapy Research and Practice, doi: 10.1155/2011/843019
CrossRef - Cavaletti G, Minoia C, Schieppati M, Tredici G, 1994. Protective effects of glutathione on cisplatin neurotoxicity in rats. International Journal of Radiation Oncology, Biology, Physics, 29(4): 771-776
CrossRef - Pace A,Savarese A,Picardo M, Maresca V, Pacetti U, Del Monte G, Biroccio A, Leonetti C, Jandolo B,Cognetti F, Bove L, 2003. Neuroprotective effect of vitamin E supplementation in patients treated with cisplatin chemotherapy. Journal of Clinical Oncology, 21(5): 927-931
CrossRef - Granowetter L, Rosenstock JG, Packer RJ, 1983. Enhanced cis-platinum neurotoxicity in pediatric patients with brain tumors. Journal Neuro-oncology, 1(4): 293-297
CrossRef - Grewal S, Merchant T, Reymond R, McInerney M, Hodge C, Shearer P, 2010. Auditory late effects of childhood cancer therapy: A report from the children’s oncology group. Pediatrics, 125(4): 938-950, doi: 10.1542/peds.2009-1597
CrossRef - Ruggiero A, Rizzo D, Mastrangelo S, Battaglia D, Attinà G, Riccardi R, 2010. Interactions between antiepileptic and chemotherapeutic drugs in children with brain tumors: is it time to change treatment? Pediatric Blood Cancer, 54(2):193-8.
CrossRef - Lazzareschi I, Ruggiero A, Riccardi R, Attinà G, Colosimo C, Lasorella A, 2002. Hypersensitivity reactions to carboplatin in children. Journal Neurooncology, 58(1):33-7
CrossRef - Ruggiero A, Maurizi P, Larocca LM, Arlotta A, Riccardi R, 2006. Childhood CD4+/CD56+ hematodermic neoplasm: case report and review of the literature. Haematologica, 91(12 Suppl):ECR48
- Chiaretti A, Aloe L, Antonelli A, Ruggiero A, Piastra M, Riccardi R, Tamburrini G, Di Rocco C, 2004. Neurotrophic factor expression in childhood low-grade astrocytomas and ependymomas. Childs Nervous System, 20(6):412-419
CrossRef - Li Y, Womer RB, Silber JH, 2004. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. Europena Journal of Cancer, 40(16): 2445-2451.
CrossRef - Kretschmar CS,Warren MP,Lavally BL, Dyer S, Tarbell NJ, 1990. Ototoxicity of pre-radiation cisplatin for children with central nervous system tumors. Journal of Clinical Oncology, 8:1191-1198.
CrossRef - Ruggiero A, Rizzo D, Attinà G, Lazzareschi I, Mastrangelo S, Maurizi P, Migliorati R, Bertolini P, Pastore M, Colosimo C, Riccardi R, 2010. Phase I study of temozolomide combined with oral etoposide in children with recurrent or progressive medulloblastoma. European Journal of Cancer, 46(16):2943-2994
CrossRef - Cefalo G, Massimino M, Ruggiero A, Barone G, Ridola V, Spreafico F, Potepan P, Abate ME, Mascarin M, Garrè ML, Perilongo G, Madon E, Colosimo C, Riccardi R, 2014. Temozolomide is an active agent in children with recurrent medulloblastoma/primitive neuroectodermal tumor: an Italian multi-institutional phase II trial. Neuro Oncology, 16(5):748-753
CrossRef - Lanvers-Kaminsky C,Krefeld B,Dinnesen AG, Deuster D, Seifert E, Würthwein G, Jaehde U, Pieck AC,Boos J, 2006. Continuous or repeated prolonged cisplatin infusions in children: a prospective study on ototoxicity, platinum concentrations, and standard serum parameters. Pediatric Blood & Cancer, 47(2): 183-193.
CrossRef - Gratton MA, Smyth BJ, 2006. Cisplatin. Continuous versus bolus. Journal of Pediatric Hematology/Oncology, 28(2): 60-61.
CrossRef - Murakami T,Inoue S,Sasaki K, Fujimoto T, 1990. Studies on age-dependent plasma platinum pharmacokinetics and ototoxicity of cisplatin. Selective Cancer Therapeutics, 6(3): 145-151.
CrossRef - Stöhr W,Paulides M,Bielack S, Jürgens H, Koscielniak E, Rossi R, Langer T, Beck JD., 2007. Nephrotoxicity of cisplatin and carboplatin in sarcoma patients: a report from the late effects surveillance system. Pediatric Blood & Cancer, 48(2): 140-147
CrossRef - Riedemann L,Lanvers C,Deuster D, Peters U, Boos J, Jürgens H, am Zehnhoff-Dinnesen A, 2008. Megalin genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. The Pharmacogenomics Journal, 8(1): 23-28.
CrossRef







