Manuscript accepted on :21-11-2019
Published online on: 27-12-2019
Plagiarism Check: Yes
Reviewed by: hind shakir
Second Review by: Ankur singh bist
Final Approval by: Dr Ayush Dogra
Ketut Widyani Astuti,  Ni Putu Ayu Dewi Wijayanti
Ni Putu Ayu Dewi Wijayanti , Putu Sanna Yustiantara
, Putu Sanna Yustiantara , Komang Puja Laksana
, Komang Puja Laksana and Putu Surya Anggara Putra
and Putu Surya Anggara Putra
Pharmacy Study Program, Faculty of Mathematics and Natural Sciences, Udayana University Bali, Indonesia
Corresponding Author E-mail: dwijayanti27@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1807
Abstract
Mangosteen (Garcinia mangostana Linn.) rind is known for its anti-inflammatory activity. Inflammation of local tissue can be overcome by topical administration of dosage forms. In an effort to improve the quality of topical drug delivery, nanoparticle technology can be an option.The purpose of this study is to determine the activity of gel and nanoemulgel dosage forms containing fractions of mangosteen rind extract (n-hexane: ethyl acetate). The gel dosage form of mangosteen rind fractions was successfully prepared. Its physical and chemical properties were evaluated, and the results were within the expected range. The spreadibility of the formulations was between 5-7cm and the pH was between 4.5 and 6.5.The 0.0625% and 0.125% mangosteen rind fraction concentrations are the formulas by which nanoemulgel was successfully formed, resulting in non-separating phases, percent transmittance of 96.997 ± 0.137% and 94.253 ± 0.134% respectively, particle size of 17.437 ± 0.427 and 17.240 ± 0.276 nm; potential zeta of 5.183 ± 0.202 and -10.143 ± 0.238. In the inflammatory test of carrageenan induced laboratory mice, nanoemulgel containing 0.0625% and 0.125% mangosteen rind fraction concentrations produced better percent inhibition (p<0.05) compared to gel containing 0.1%, 0.5%, and 1% mangosteen rind fraction concentrationsin the 90th minute, but the difference was not significant in the 120thminute through the end of thetest. The nanoemulgel containing 0.0625% and 0.125% mangosteen rind fraction concentrations have an unsignificant difference in results (p>0.05) when compared to the reference drug (diclofenac sodium) in the 90th minute.
Keywords
mangosteen rind; nanoemulgel; gel; anti-inflammatory activity
Download this article as:| Copy the following to cite this article: Astuti K. W, Wijayanti N. P. A. D, Yustiantara P. S, Laksana K. P, Putra P. S. A. Anti-Inflammatory Activity of Mangosteen (Garcinia Mangostana Linn.) Rind Extract Nanoemulgel and Gel Dosage Forms. Biomed Pharmacol J 2019;12(4). |
| Copy the following to cite this URL: Astuti K. W, Wijayanti N. P. A. D, Yustiantara P. S, Laksana K. P, Putra P. S. A. Anti-Inflammatory Activity of Mangosteen (Garcinia Mangostana Linn.) Rind Extract Nanoemulgel and Gel Dosage Forms. Biomed Pharmacol J 2019;12(4). Available from: https://bit.ly/3659h0A |
Introduction
Inflammation of local tissue, such as osteoarthritis and rheumatoid arthritisis caused by inflammation in the joint area which can impede body movement and affect patients’ daily activities. The WHO data in 2017 reveals that the prevalence of osteoarthritis worldwide is 9.6% in men and 18% in women aged 60 years or more. Meanwhile, the prevalence of rheumatoid arthritis varies between 0.3% -1% of the population aged 20-40 years worldwide withhigher prevalencein women compared to men. Both of these types of arthritis are chronic, and therefore, long-term anti-inflammatory therapy is needed for the symptomatic treatment of the disease (Colmegna et al., 2011).
Inflammation of local tissue can be overcome by topical administration of formulations. In an effort to improve the quality of topical drug delivery, nanoparticle technology can be an option. Nanoparticles in a topical dosage form can better penetrate the skin layer, thus allowing abetter drug permeability into the skin,and thereby improving the quality of delivery of drug compounds (Martien et al., 2012). Nanoparticles have a large surface area of particles which enables faster penetration of active substances (Williams and Barry, 2004).
A plant material that is widely developed as an anti-inflammatory drug is mangosteen (Garcinia mangostana Linn.) rind. The mangosteen rind contains secondary metabolites, such as xanthones, mangostin, flavonoids, and tannins. The xanthone compounds that have been identified inmangosteen rind include alpha mangostin, beta mangostin, gamma mangostin, gartanine, garcinone E, 8-deoxygartanine and methoxy-B-mangostin (Chavari et al., 2008; Pratiwi, 2010). Khumsupan and Gritsanapanin their study(2013) state that alpha-mangostin has pharmacological activity as an anti-inflammation agent. Other studies show that the mangosteen rind extract has anti-inflammatory activity oncarrageenan inducedpaw edema inlaboratory mice. The mangosteen rind extract with a dosage of 20 mg/100gBW, 40 mg/100gBW, and 80 mg/100gBW can inhibit the increase in edema during the inflammation in mice (Perwitasari, 2015).
Conventionally developed formulations which contain active natural materials has several physical and chemical drawbacks in that they areorganoleptically unstable, easily dissolved, and lack of bioavailability since they havelarge molecules which cannot easilypenetratecell membranes. Tiara (2017) in her studieshas formulated the mangosteen rind extract into nanoemulgel with a carrier in the form of a mixture of oil (Virgin Coconut Oil), cosurfactant (Ethanol 96%) and surfactant (CremoforRH 40) with a fixed ratio of 1:2:7,producing particles of a size of20.6 nm using the SNEDDS (Self-Nanoemulsifying Drug Delivery System) method. The physical properties and anti-inflammatory activity of this optimum formula was tested in carrageenan induced laboratory mice.
Materials and Methods
Mangosteen Fruit Sample Collection and Plant Determination
The selected mangosteen fruit wasblackish purple ripe fruit collected from Luwus Village, Baturiti Sub-regency, Tabanan Regency, Bali. Plant determinationwas carried out at the UPT (Technical Implementation Unit) of the Bedugul Eka Karya Botanical Garden Plant Conservation Center inTabanan, Bali.
Preparation of Mangosteen Rind
The collected mangosteen fruit was washed, and the rind was separated from the flesh of the fruit. The rind of the fruit was thinly sliced and dried. The dry rind was then processed into powder using a blender and sieved using a 20-mesh sieve. The dry powder was then stored in atightly-closeddry container (Fitri, 2016). The water content of the simplicia powder was determined using a moisture analyzer.
Preparation of Mangosteen Rind Extract
The mangosteen rind powder was defatted using n-hexane with a ratio of 1:3 w/v. The defatting process was carried out for 24 hours. The powder that had been defatted wasfiltered to separate it from the solvent and then air-dried. This process was done three (3) times.
The mangosteen rind powder that had been defatted was macerated with methanol solvent with the ratio ofthe powder to the solvent being 1:10 w/v. The powder was soakedfor 3×24 hours. The macerated powderwasmaceratedonce again with the ratio of the powder to the solvent being 1:4 w/v for 24 hours at room temperature.The solvent of the macerated rind was evaporated using a rotary evaporator at 50oC until it became nearly thick, and it was then evaporated again inan oven at 50oC until it became thick (Mardawati et al., 2008).The thick extract was then fractionated using a stationary phase, namely silica powder. The thick extract was then eluted with n-hexane: ethyl acetate with the ratiosbeing (9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 7:3, 8:2, 9:1, 10:0),and prepared as much as 20 mL.The eluatewasplaced in vials each containing 5 mL.It was revealed that the fraction groups number 6,7,8, and 9 positively contained polyphenols and flavonoids. These fractions were collected and then formulated into gel and nanoemulgel dosage forms.
Preparation of Gel containing Mangosteen Rind Extract Fractions
The composition in every 100 grams of material is as follows:
| Material | F1 (0.1%) | F2 (0.5%) | F3 (1%) |
| Mangosteen Rind Extract Fractions*
Base Gel* |
0.1
Ad 100 |
0.5
Ad 100 |
1
Ad 100 |
*The materials are measured in grams
Viscolam was dispersed in distilled water using a magnetic stirrer at a speed of 500 rpm. Then, the microcare was dissolved into propylenglycol and glycerin, and then the mangosteen rind fraction (Mixture 1) was added to it. Mixture 1 was added to the viscolam which had been dispersed and stirred at a speed of 500 rpm for 5 minutes. TEA was added to the mixture to obtain a clear and thick base. The mixture was stirred at a speed of 500 rpm for 5 minutes, and then distilled water was added to it to obtain gel with a mass of 100 grams.
Test of Physical and Chemical Properties of Gel ContainingMangosteen Rind Fractions
Organoleptic Test
Organoleptic observation is carried out by direct observation of the texture, color, and smell of the mangosteen rind extract gel made (Department of Health of the Republic of Indonesia, 1979).
Homogeneity Test
The homogeneity test is carried out to produce homogeneous preparations without the presence of coarse particles or fibers. The testis carried out by applying substances to be tested on a glass plate or other suitabletransparent materials (Department of Health of the Republic of Indonesia, 1979).
Adhesion Test
A sample weighing 0.25 grams is placed between 2 glass plates, and then the glass plates are pressed with a force or weight of 1 kg for 5 minutes. Next, the force is removed from the glass plates and the glass plates are put on a testing instrument. The testing instrument is given a force or weight of 80 grams and then the time needed for the gel to detach from the glass plates is recorded (Garg et al., 2002).
Spreadibility Test
As much as 1 gram of gel formulation is carefully placed on a 20 x 20 cm glass plate. Then, it is covered with mica paper and given a weight it until the whole weight reaches 125 grams. The diameter formed is then measured after 1 minute (Garg et al., 2002).
pH Test
The pH of gel formulations is measured using a pH meter. The pH meter electrode is dipped into the solution being tested. The pH meter needle isallowed to move until it indicates a settling position. The pH indicated by the pH meter needle is recorded as suitable (Department of Health of the Republic of Indonesia, 1979).
Viscosity Test
Viscosity tests are carried out by placing samples in the Brookfield viscometer until the spindle is submerged. The spindle and speed used are set. Six speed points areselected, namely 10 rpm, 20 rpm, 30 rpm, 50 rpm, 60 rpm, and 100 rpm (Garg et al., 2002).
Preparation of Nanoemulsions Containing Mangosteen Rind Extract Fractions
The composition of materials in every 12 grams of the preparation is as follows:
| Material | F1 (0.0625%) | F2 (0.125%) | F3 (0.25%) | F4 (0.5%) | F5 (1%) |
| Fraction | 7.5 mg | 15 mg | 30 mg | 60 mg | 120 mg |
| Olive Oil* | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| PEG 400* | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Chremophor RH 40* | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 |
| Distilled water | Ad 12 gram | Ad 12 gram | Ad 12 gram | Ad 12 gram | Ad 12 gram |
*The materials are measured in grams
The mangosteen rind fraction was suspended in olive oil with a magnetic stirrer (at 200rpm for 15 minutes), and then PEG 400 was added, and the mixture wasstirred with a magnetic stirrer (at 200rpm for 15 minutes). The mixture was then added with Chromophore RH 40, and stirred with a magnetic stirrer (at 200rpm for 2 hours). The droplet size was reduced using a sonicator bath for 1 hour, and thendistilled water was added and it was stirred until nanoemulsionswere formed.
Evaluation Test of Mangosteen Rind Extract Nanoemulsions
Physical Stability Test
The physical stability of nanoemulsionswasmeasured by a centrifugation test of nanoemulsions containing mangosteen rindmethanol fractions at a speed of 1,200 rpm for 15 minutes, and then the result was observed. Stable nanoemulsionsare marked byno separation between both oil and water phases (Rachmawati et al., 2014).
Clarity Test
The clarity of the formed nanoemulsions can be determined using UV-Vis spectrophotometer measurementsusing the% transmittance parameter at a wavelength of 650 nm with distilled water as blank. Good nanoemulsionsare clear with a percemttransmittanceof 90-100% (Costa et al., 2012).
Measurement of Particle Size
The nanoemulsion droplet size and polydispersity index are determined using Photon Correlation Spectroscopy. As much as 1 gram of nanoemulsion gel containingmangosteen rindmethanol extract is dispersed in 5 mL of distilled water, and the particle size was measured. The droplet size ismeasured to see whether the droplet sizes of the nanoemulsion formulations produced havemet the nanoemulsion droplet size criteria, namely <100 nm (Pratiwi et al., 2016).
Zeta Potential Measurement
Zeta potential is measured using electrophoretic light scattering. As much as 1 gram of mangosteen rind methanol fraction nanoemulsionsis dispersed in 5 mL of distilled water, and the Zeta potential was measured. Zeta potential values that are acceptablefornanoemulsions are from -30 mV to +30 mV (Kale and Deore, 2017).
Test of Physical and Chemical Properties
The physical properties of the nanoemulgel are tested using the same procedure as the Test of Physical and Chemical Properties of Gel Containing Mangosteen Rind Fraction
In vivo Antiinflammatory Activity Test of Mangosteen Rind Extract Nanoemulgel and Gel Formulations
Forty male laboratory mice used in the study were divided into 8 groups, namely the normal group, the negative control group, the positive control group, and the treatment groups number 1, 2, 3, 4, and 5 that were treated with gel and nanoemulgel formulations. Each treatment group consisted of 5 male laboratory mice. The laboratory mice weregroupedusing a simple random sampling technique. Before being given treatment, the mice were not given any food or drink for 18 hours. The mice were then weighed and marked on their rear leftleg joints using a marker pen (Meisyayati and Dewiwaty, 2015). This mark was used as the limit in dipping the paws in the plethysmometer for the measurement of the volume of the edema. After that, the mice’s left paw was dipped into the plethysmometeruntil they reached the marks that had been made before. Then, the initial volume (Vo) of the mice’spaws was recorded.
Then,the mice’s paws volumeafter being injected with 1% (Vt) carrageenan wasmeasured using a plethysmometer every 30 minutes for 360 minutes, namely in the 30th, 60th, 90th, 120th, 150th, 180th, 240th, 270th, 300th, 330th and 360th minute respectively. The results of the measurements of V0 and Vtwere recorded.
Statistical analysis
Statistical data analysis was performed using the Shapiro-Wilk test to see the normality of the data and the Levene test to see the homogeneity of data. When the data had been distributed normally and homogeneously with p>0.05, the analysis was continued with the parametric analysis usingthe one-way ANOVA method and the SPSS program with a confidence level of 95% (p>0.05). Next, a Post Hoc test wascarried outusing the LSD test to find out which groups have the same effects or differenteffects from one another. If one of the requirements of the ANOVA test is not fulfilled (the data is not normally distributed or the data is not homogeneously distributed) then the Kruskall-Wallis test to determine the differences and the Mann-Whitney test to see the differences between each treatment group are carried out (Besral, 2010).
Results and Discussions
Physical Test Results of Formulations ContainingMangosteen Rind Extract Fractions
| Formula | Organoleptic properties | Spreadability | pH | Viscosity |
| F1 (0.1%) | Light brown, transparent, odorless | 6.14 cm | 7.42 | 3509 cPs |
| F2 (0.5%) | Light brown, transparent, odorless | 6.10 cm | 7.62 | 3503cPs |
| F3 (1%) | Light brown, transparent, odorless | 5.75 cm | 7.42 | 3667 cPs |
F1 = gel with 0.1% mangosteen fraction concentration
F2 = gel with 0.5% mangosteen fraction concentration
F3 = gel with 1% mangosteen fraction concentration.
As much as 100 grams of gelscontaining mangosteen rind fractions with concentrations of 0.1%, 0.5%, and 1% were prepared. The physical and chemical tests on the gel formulations containing mangosteen rind fractions was then carried out. The results of the organoleptic property, spreadability, pH, and viscosity tests of the 3 types of gel formulas were not much different,and they were within the expected range. The greater the concentration of the extract used, the greater the viscosity, which also affects the spreadability. Viscosity plays an important role in the stability of the formulations and the efficiency of the release of active substances (Pranita et al., 2016). Increased viscosity illustrates a decrease in surface tension in the water and oil phases which provides better phase stability and slower release of active substances providing a longer chance for absorption in the skin. The spreadability of the formulations has been in the range of 5-7cm which is the optimum value of the formulations. The recommended pH of the formulations is from 4.5 to 6.5. In this study, the pH of the formulationsisstill higher than the recommended range; therefore, materials that can reduce pH are needed.
Results of the Physical Test of the Nanoemulgel Formulations containing Mangosteen Rind Extract Fractions
| Formula | Physical stability | Clarity | Particle Size
(nm) |
Zeta Potential (mV) |
| F1 | Separating | 90.780 ± 0.210% | – | – |
| F2 | Separating | 85.180 ± 0.301% |
– |
– |
| F3 | Separating | 77.630 ± 1.790% |
– |
– |
| F4 | Not separating | 96.997± 0.137% | 17.437 ± 0.427 | -5.183 ± 0.202 |
| F5 | Not separating | 94.253± 0.134% |
17.240 ± 0.276 |
-10.143 ± 0.238 |
F1 = nanoemulgels with 0.1 % mangosteen rind fraction concentration
F2 = nanoemulgels with 0.5 % mangosteen rind fraction concentration
F3 = nanoemulgels with 1 % mangosteen rind fraction concentration
F4 = nanoemulgels with 0.0625 % mangosteen rind fractionconcentration
F5 = nanoemulgels with 0.0125 % mangosteen rind fraction concentration
The mangosteen rind extract fraction which had been made into gel was then made into nanoemulgel, but at the concentrations of 0.1%, 0.5%, and 1% there was phase separation which marked adrawbackin nanoemulsion formulation.
Clarity is a sign of the successful formation of nanoemulsion. The expected clarity is 90%-100%. Nanoemulgelswith 0.0625% and 0.125% mangosteen rind fraction concentrationshave percent transmittance values of 96.997 ± 0.137% and 94.253 ± 0.134% respectively indicating the success of particle size reduction. The greater the percent transmittance, the smaller the particle size will be.
The particle sizes produced by nanoemulgelswith 0.0625% and 0.125% mangosteen rind fractionconcentrations were 17.437 ± 0.427 and 17.240 ± 0.276 nm respectively. These particle sizes havebeen within a range of particle sizes that can be used for Self-Nanoemulsifying Drug Delivery System (SNEDDS) which arefrom 5 to 200 nm. The smaller the particle size that is produced, the higher the penetration rate of the active substance. This will give the opportunity for more active substances to reach the inflammatory area (Devarajan and Ravichandran, 2011).
The zeta potential of nanoemulsionscontaining mangosteen rind fractions was tested using Electrophoretic Light Scathering. It was found that the nanoemulsions with 0.0625% and 0.125%mangosteen rind fraction concentrationshad a value of -5.183 ± 0.202 and -10.143 ± 0.238 respectively. The expected zeta potential of the nanoemulsions was from -30mV to + 30mV. Greater zeta potential values (negative or positive) will provide better stability in the nanoemulgel formulation phase (Maharani, 2018).
| Formula | Organoleptic properties | Spreadability | pH | Viscosity |
| F4 | Light yellow, transparent, odorless | 5.6 cm | 7.62 | 3256 cPs |
| F5 | Light yellow, transparent, odorless | 5.9 cm | 7.44 | 3520 cPs |
F4 = nanoemulgels with 0.0625 % mangosteen rind fractionconcentration
F5 = nanoemulgels with 0.125%mangosteen rind fractionconcentration
The physical properties of nanoemulgelscontaining 0.0625% and 0.125% mangosteen rind fractions were tested. The organoleptic properties, spreadibility, pH and viscosity had met the required values. The pH value was alsohigher than the required ones in the nanoemulgel formula. There was a need to add substances that can reduce pH.
Antiinflammatory test
Inflammatory test was carried out by measuring the volume of edema incarrageenan induced laboratory mice. Measurements weremadeusing a plestismometer every 30 minutes for 360 minutes. Theedema volume was inversely proportional to the percent inhibition produced in each formula compared to the control group.
Induction using 1% carrageenan has the effect of releasing inflammatory mediators such as histamine, serotonin, bradykinin, and prostaglandin, causing acute edema for up to 6 hours (Winter et al., 1962). In the first 90 minutes after induction, histamine andseretonin begin to be released.From the 90thto 150th minute bradykinin begins to be released, and from the 150th to 300th minute prostaglandins begin to be released. Maximum inhibition (100%) occurs from the 210th minute to the end of the test (the 360th minute) for all types of formulas compared to reference drugs.
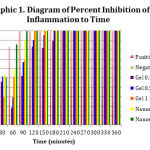 |
Graph 1: Diagram of Percent Inhibition of Inflammation to Time |
Differences in inflammatory inhibition occurred in each formula, seen inthe 30th, 60th, 90th, 120th, 150th, and 180thminute.The average percent inflammation data in 7 test groups had a normal distribution but was not homogeneous (p>0.05), which marked unfulfillment ofan ANOVA test requirement. Theferore,a Kruskal-Wallis test was carried out to determine the differences and a Mann-Whitney test was conducted to see the differences between each treatment group (Besral, 2010).The Kruskall-Wallis test results showed no significant difference (p>0.05) between the formulas in the 30th and 60th minute of the testing time and there was significant difference (p<0.05) in the 90th, 120th, 150th, and 180thminute of the testing time. The inhibitory activity which is not significantly different may be caused by the release of inflammatory mediators that have yet to cause mice paw edema.
The results in the 90th, 120th, 150th, and 180thminute were tested statistically usingthe Mann-Whitney test to see the differences between groups at each test time. The negative control group shows a significant difference when compared with all groups of formulas at all time periods of measurement.This shows that induction of any of all formulas provides the potential to inhibit the occurrence of inflammation. Based on the table below and the Mann-Whitney test, nanoemulgel with0.0625% and 0.125% mangosteen rind extract produced better percent inhibition (p<0.05) compared to the gel with0.1%, 0.5%, and 1% mangosteen rind extract inthe 90th minute. However, the percentinhibition did not differ significantly inthe 120th, 150th, and 180th minute.
This might show a possibility that the nanoemulgel formulation provides a better onset of action compared to conventional gel formulas. Nanoparticles in topical use provides an advantage, namely theyare able to better penetrate the skin layer thereby allowing betterpermeability of the drug into the skin,and thus increasing the quality of delivery of drug compounds (Martien et al., 2012).Nanoparticles have a large surface area of active materials which makes the penetration of active substances faster (Williem and Barry, 2004). The testin the 90th minute also showinsignificantly different result (p>0.05) between the control group and nanoemulgel containing 0.0625% and 0.125% mangosteen rind extract concentrations. This shows that the two nanoemulgel formulas have activities comparable to the reference drug namely diclofenac sodium.
Inflammation caused by 1% carrageenan will peak from the 180th to 240th minute when histamine, serotonin, and bradykinin have all been released,which trigger blood vessel dilation and leukocyte migration (Winter et al., 1962).After 240 minutes of induction, the role of mediators in the inflammatory processbegins to decline. However, there is amigration of leukocyte cells and local production of prostaglandin which marks the presence ofedema in the negative control group through the end of the test (Crunkhorn and Meacock, 1971). The observation after the 180th minute showed an inflammatory inhibition of up to 100% in the control group by any of all test formulas. Inhibition of the formation of inflammatory mediators may be caused by groups of xanthone compounds, such as α- and γ-mangostinswhich have been reported to have anti-inflammatory effects (Chen et al., 2007).
Conclusion
Gel containing 0.1%, 0.5%, and 1% mangosteen rind fraction concentrations and nanoemulgels containing 0.0625% and 0.125% mangosteen rind fraction concentrations were successfully made in this study. In the inflammatory test oflaboratory mice induced with carrageenan, nanoemulgel containing 0.0625% and 0.125% mangosteen rind fraction concentrations produced better percent inhibition (p<0.05) compared to gel with 0.1%, 0.5%, and 1% mangosteen rind fraction concentrations in the 90th
minute, but there was no significant difference in the 120th minute through the end of the testin the 360th minute. In addition, the nanoemulgel containing 0.0625% and 0.125% mangosteen rind fraction concentrationshave results that did not differ significantly (p>0.05) when compared to the reference drug (diclofenac sodium) in the 90th minute.This shows that nanoemulgels with 0.0625% and 0.125% mangosteen rind fraction concentrationshave the potential to be developed as anti-inflammatory topical formulations.
References
- Chen, L. G., Yang, L. L., Wang, C. C. 2008. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food and Chemical Toxicology. Vol 46-2, p: 688-693
- Colmegna I., R.O. Brent, and A.M. Henri. 2011. Current Understanding of Rheumatoid Arthritis Therapy. Journal of Clinical Pharmacology Therapy. 91(4):607–620.
- Crunkhorn, P., and Meacock, C. R. 1971. Mediators of the inflammation induced in the rat paw by carrageenan. Br. J. Pharmac. 42: 392-402.
- Devarajan, V and Ravichandran, V. 2012. Nanoemulsions: as Modified Drug Delivery Tool. International Journal of Comprehensive Pharmacy Vol. 02, Issue 04. p:1-6
- Howes, L.G. 2007. Selective COX-2 Inhibitors, NSAIDs and Cardiovascular Events – is Celecoxib the Safest Choice? Journal Therapeutics and Clinical Management. 3(5):831-845.
- Ko, S.G., C.S. Yin, B. Du, and K.H. Kim. 2014. Herbal Medicines for Inflammatory Diseases. Mediators of Inflammation. 2014:1
- Martien, R., Sa’adah, N., and Saifullah, T. N. S. Formulation and Characterization Insulin Nanoparticle Using Low Molecular Weight Chitosan and Pectin Polymers with Ionic Gelation Method. International Journal of Pharmaceutical and Clinical Research. 8 (5) Suppl: 500-506
- Mycek, M.J., R.A. Harvey, and P.C. Champe. 2001. FarmakologiUlasanBergambar. Jakarta: WidyaMedika.
- Oke, S.L and K.J. Tracey. 2009. The Inflammatory Reflex and the Role of Complementary and Alternative Medical Therapies. The Annals of the New York Academy of Sciences. 1172:172- 180.
- Okin, D. and R. Medzhitov. 2013. Evolution of Inflammatory Diseases. Current Biology. 22(17):733-740.
- Ong, C. K. S, P. Lirk, C. H. Tan, and R.A. Seymour. 2007. An Evidence-Based Update on Nonsteroidal Anti-Inflammatory Drugs. Clinical Medicine & Research. 5(1): 19-34.
- Rainsford, K. D. 2007. Antiinflammatory Drugs in 21st Century. SubcellBiochem. 42:3-27.
- Williams A. C., and Barry, B. W. 2004. Penetration enhancers. Advanced Drug Delivery Reviews. Vol 56-5, p:603-618
- Winter, C. A., Risley, E. A., andNuss, G. W. 1962. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc. Soc. Exp. Biol. Med. 111: 544-7







