Manuscript accepted on :03-May-2019
Published online on: 19-07-2019
Plagiarism Check: Yes
Reviewed by: Hind Shakir
Second Review by: Arjun Baidya
Final Approval by: Dr Ayush Dogra
Mina Wassef Girgiss1*, Wassef Girgiss Nicola2, Aly Mohamed Ezz El-Arab3, Dawoud Fakhry Habib4, Nadia Mohamed Ahmed5 and Eman Refaat Youness6
1Lecturer of Internal Medicine, Endocrinology, Diabetology and Metabolism-Medical Department, Medical Division, National Research Centre (NRC), Cairo, Egypt.
2Professor of Internal Medicine, endocrinology and metabolism-Department of Internal Medicine, Medical Division, National Research Centre (NRC), Cairo, Egypt.
3Professor of Nutrition-Department of Nutrition and Food Science, National Research Centre (NRC), Cairo, Egypt.
4Professor of Biochemistry-Medical Biochemistry Department, Medical Division, National Research Centre (NRC), Cairo, Egypt.
5Professor of Biochemistry-Medical Biochemistry Department, Medical division, National Research Centre (NRC), Cairo-Egypt.
6Assisstant Professor of Biochemistry-Medical Biochemistry Department, Medical division, National Research Centre (NRC), Cairo-Egypt.
Corresponding Author E-mail: mwgnicola@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/1732
Abstract
Glucagon hormone being catabolic and hyperglycemic, it acts in an opposite manner to insulin and adds to insulin resistance. The oligosaccharide inulin fructans is indigestible in the small bowel. When it reaches the large intestine, it encourages beneficial microbacteria strains. These latter produce certain peptides which when absorbed they reach the endlocrine L-cells of the small gut. These peptides stimulate L-cells to release glucagon like peptide 1 (GLP-1) which suppresses glucagon and stimulates insulin secretion in a glucose dependant manner. Our aim is to find how inulin suppresses glucagon and to what extent this improves insulin resistance. Fasting serum glucagon and homeostasis model assessment for insulin resistance (HOMA-IR) were estimated in 28 type 2 diabetic female patients before and after twenty one days of daily inulin intake. Fasting serum glucagon and HOMA-IR decreased significantly after the inulin intake period. In conclusion inulin stimulates the release of GLP-1. This acts in a glucose dependant manner thus simulating the novel incretin based drugs in reducing insulin resistance. However, owing to inulin other actions on insulin resistance, it might exceed these novel drugs.
Keywords
Glucagon; Inulin; Incretin Based Drugs, Type 2 Diabetes Mellitus
Download this article as:| Copy the following to cite this article: Girgiss M. W, Nicola W. G, El-Arab A. M. E, Habib D. F, Ahmed N. M, Youness E. R. Inulin Might Exceed Incretin Based Drugs in the Treatment of Type 2 Diabetes Mellitus. Biomed Pharmacol J 2019;12(3). |
| Copy the following to cite this URL: Girgiss M. W, Nicola W. G, El-Arab A. M. E, Habib D. F, Ahmed N. M, Youness E. R. Inulin Might Exceed Incretin Based Drugs in the Treatment of Type 2 Diabetes Mellitus. Biomed Pharmacol J 2019;12(3). Available from: https://bit.ly/30HjNIc |
Introduction
The pancreatic endocrine portion (islets of Langerhans) is strongly involved in the regulation of energy metabolism. These islets comprise four kinds of hormone secreting cells; the α cells secrete glucagon, the β cells secrete insulin and amylin. The δ cells secrete somatostatin while the fourth type of cells secrete the pancreatic polypeptide.1
Insulin and glucagon are the two main hormones which are largely responsible for blood glucose and energy homeostasis.2
Insulin is a hypoglycemic and anabolic hormone. It acts on the liver, muscles and adipose tissue. It enhances glucose uptake by insulin sensitive cells and its conversion into glycogen (in liver & muscles) and to fat (in adipose tissue), besides it has an anabolic effect on protein.3
Glucagon, on the other hand acts in an opposite manner to insulin. It is catabolic and hyperglycemic. It activates glycogenolysis and gluconeogenesis mainly in the liver, also it inhibits glycogenesis and glycolysis in addition to its lipolytic action on the adipose tissue.3 The glycemic effect of glucagon is essential to regain and maintain the blood glucose level in hypoglycemic states. This is very important to vital organs as the brain which is dependant on blood glucose as its main fuel.4
In normal glycemic states, both insulin and glucagon hormones cooperate in maintaining the blood glucose level in the normal range.2,5 In diabetes mellitus, there is a disturbance in glucagon/insulin secretion. There is an abnormal increase in blood glucagon level relative to insulin level.3
Inulin a naturally occuring oligosaccharide of plant origin, has been known to have hepatic lipotropic and blood glucose lowering effect.6,7 It passes undigested in the small intestine to the colon, where it is acted upon by certain strains of the colonic microbiome. These secrete peptides which stimulate the L-endocrine cells of the small bowel to produce glucagon like peptide-1 (GLP-1).8,9 This incretin hormone suppresses glucagon and stimulates insulin secretion.10,11
In this study, we aim to find out, how inulin ingestion affects blood glucagon level and how inulin can participate in the regulation of blood glucose level and glycemic contol.
Materials and Method
Subjects
Twenty eight obese, type 2 diabetic women were recruited from the municipal hospitals of Cairo, Egypt. The age range was 40-65 years.
Inclusion criteria: obese (body mass index > 30), type 2 diabetic women, middle aged and above, hypertensive or not and not under antilipidemic drugs.
Exclusion Criteria
Hormonal therapy or contraceptive pills. Endocrine disorders or other metabolic diseases.
Malignancy or organ failure (lung, heart, liver or Kidney). Local or systemic infection including chest, urinary tract, gastro-intestinal tract or skin infection (local or extensive).
Ethical Committee Approval
The present work has been approved by the National Research Centre (NRC) committee of ethics, Cairo, Egypt. (Certificate number 15011).
Consent
All patients signed consent for participation.
Methods
Prebiotic inulin was taken by each of the individuals of the studied group as an add on therapy to their conventional ongoing antidiabetic treatment. Four grams of inulin were given with milk to each patient daily; (half of the dose in the morning and the other half in the evening) for three weeks. This dose was chosen empirically putting in mind that a small amount of inulin can exert a bifidogenic effect.12
Inulin specifications: Inulin A.R (C6H10O5) N ALPHA-CHEMIKA (Mumbai, INDIA). 400002 An ISO: 9001- 2000 Certified company.
Each patient was subjected before and after the period of inulin intake for the following.
A- Full history. B- Thorough clinical examination. C- Laboratory investigations:
Laboratory Investigations
Estimation of Fasting Serum Glucagon
Serum glucagon was estimated by ELISA technique after Bak et al.13. Human GC (Glucagon) ELISA Kit is used from Elabscience. Catalogue number: E-EL-H2237 96T. Elabscience Biotechnology Co., Ltd. Website: www.elabscience.com.
Determination of Insulin Resistance (HOMA-IR)
Insulin resistance (IR) was assessed using the equation of homeostasis model assessment for insulin resistance (HOMA-IR) utilizing fasting serum glucose & fasting serum insulin values, after Mathiews et al.14
The Equation for Insulin Resistance
(HOMA-IR) = fasting glucose value (mg/dl) x fasting insulin value (m I.U./ml)/ 405.
Fasting Serum Insulin Determination
This was quantitatively determined using an enzyme immunoassay method according to the National Committee for Clinical Laboratory Standards.15 Manufacturer of kit used: immunospect corporation- 7018 Owensmouth Ave. Suit 103 Canoga Park- CA, 91303.
Fasting Serum Glucose Assessment
This was assessed by an enzymatic colorimetric method. This is an enzymatic oxidation method for glucose by glucose oxidase enzyme, according to Tietz.16
Kit is from Egyptian Company for biotechnology (S.A.E.) Obour City, Industrial Area. block 20008; piece 19A Cairo. Egypt.
Statistical Methods
Results were coded, tabulated and statistically analyzed. These were done using IBM Statistical Package for Social Sciences (SPSS statistics) software version 22.0; IBM Corp. Chicago- USA- 2013.
Quantitative data were described statistically as minimum and maximum for the range as well as the mean± standard deviation (SD) for quantitative parametric values. The paired t-test was used for inferential analysis of the quantitative variables in cases of two dependent groups having parametric data. Significance level was taken as P value < 0.05.
Results
There was significant decrease in mean fasting serum level of glucagon after inulin ingestion period from 188.3±50.3 to 164.9±46.9 with P value <0.001, table [1] and figure [1].
There was significant decrease in mean fasting serum value of HOMA-IR after inulin intake period from 11.1±8.2 to 7.2±5.0 with P value <0.001, table [1] and figure [2].
Table 1: Shows the estimated values of fasting serum glucagon and HOMA-IR before and after the inulin ingestion period.
| Before | After | ^Change | #P | ||||
| Mean±SD | Range | Mean±SD | Range | Median (IQR) | Range | ||
| Glucagon (pg/mL) | 188.3±50.3 | 115.7–313.0 | 164.9±46.9 | 102.9–278.0 | -9.9 (-40.2–3.1) | -181.5–59.6 | <0.001* |
| HOMA(IR) | 11.1±8.2 | 2.5–32.1 | 7.2±5.0 | 2.1–18.7 | -3.5 (-5.4–-1.6) | -13.4–2.7 | <0.001* |
N=28, ^Negative values= reduction, #P-value for paired t-test, *Significant.
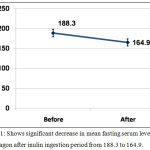 |
Figure 1: Shows significant decrease in mean fasting serum level of glucagon after inulin ingestion period from 188.3 to 164.9.
|
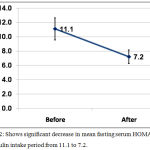 |
Figure 2: Shows significant decrease in mean fasting serum HOMA-IR after inulin intake period from 11.1 to 7.2.
|
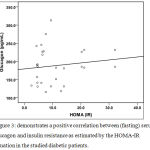 |
Figure 3: Demonstrates a positive correlation between (fasting) serum glucagon and insulin resistance as estimated by the HOMA-IR equation in the studied diabetic patients.
|
Discussion
The glucagon/insulin ratio is a fundamental factor in the control and regulation of hepatic glucose metabolism. In normoglycemic subjects, glucagon secretion by pancreatic α cells is inhibited by blood glucose levels lower than those needed for β cell stimulation and insulin secretion.17,18 In diabetics, on the other hand, there is relative increase in the glucagon/insulin ratio.3 However the disturbed glucagon/insulin ratio is not the only disorder met with. Actual hyperglucagonemia may occur. This increase in glucagon secretion is one of the main factors behind the insulin resistance and the hyperglycemic state in diabetes mellitus.19,20
The present work has shown a positive correlation between the fasting serum glucagon level and the insulin resistance as estimated by HOMA-IR equation in the studied diabetic patients. Also, in the present study, inulin ingestion significantly decreased both fasting serum glucagon level and insulin resistance manifested by lowered HOMA-IR.
In fact, inulin has got no direct action on both α and β cells of the islets of Langerhans in the pancreas. As we mentioned before, it stimulates certain colonic microbacteria. These release particular peptides which when absorbed, reach the endocrine L cells in the small intestine. These L cells release their glucagon like peptide-1 (GLP-1). This incretin suppresses glucagon secretion and enhances insulin release in a glucose dependant manner.10,11 In this respect inulin acts in a similar way like the novel smart incretin based antidiabetics.21 These include the GLP-1 analogues or mimetics and the dipeptidyl peptidase type 4 (DPP4) inhibitors. Being glucose dependant in their action, they protect the diabetic patient against the possible hazardous hypoglycemic side effect of antidiabetic therapy such as insulin or insulin secretagogues.
Moreover, in previous studies by the present authors, inulin reduced insulin resistance by lowering serum triglycerides and improving hepatosteatosis. Fatty liver is the prime and main site for insulin resistance.6,7 Besides, in other previous studies by the present authors, inulin proved to tackle various important facets in the pathogenesis of type 2 diabetes mellitus and its effect on the vascular tree. These studies proved that inulin possesses anti-inflammatory properties22; Also inulin has a protective effect on the microvasculature.23 In addition to its anti-atherogenic effect on the microvasculature.24 Taking into consideration the results of this work together with our preceeding studies,6,7,22-24 inulin might exceed the novel incretin based drugs in the treatment of diabetes mellitus.
Conclusion
In conclusion, in the present work, inulin proved to reduce insulin resistance through lowering glucagon hormone level which has an insulin antagonizing action. This together with its triglycerides lowering effect and hepatic lipotropic effect makes inulin a reasonable antinsulin resistant agent. Moreover, as it works in a glucose dependant manner, it can be added safely to the ongoing treatment of diabetic patients. Also, it can be given alone to prediabetics (patients with impaired fasting glucose or those with impaired glucose tolerance).
Again, we would like to draw the attention for critical cases which deserve meticulous study. Sometimes diabetic patients on insulin therapy whether type 1 or long standing type 2, suffer attacks of hypoglycemia due to failure of responsiveness of their α cells to secrete glucagon25. This abnormal phenomenon has been attributed to multiple failures in α cell regulations including insulin resistance26. The exact underlying causes are still vague. The authors suggest to reduce insulin dose and add inulin as an adjunct therapy to those patients to study such cases.
Acknolwdgements
Thanks for National Research Center, Cairo, Egypt for financing this work. Again, thanks to all patients who volunteered to participate in this study.
Conflict of Interest
There is no conflict of interest.
References
- Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. PNAS.; 103: 2334-2339 (2006).
- Quesada I, Todorova MG, Soria B. Different metabolic responses in a-b-, and d-cells of the islet of Langerhans monitored by redox confocal microscopy. Biophysical Journal.; 90: 2641-2650 (2006a).
- Dunning BE, Foley JE, Ahre´n B. Alpha cell function in health and disease: influence of glucagon-like peptide-1. Diabetologia.; 48: 1700-1713 (2005).
- Ahren B. Autonomic regulation of islet hormone secretion – implications for health and disease. Diabetologia.; 43: 393-410 (2000).
- Nadal A, Quesada I, Soria B. Homologous and heterologous asynchronicity between identified alpha-, beta- and delta-cells within intact islets of Langerhans in the mouse. Journal of Physiology.; 517: 85–93 (1999).
- Girgiss MW, Nicola WG, Habib DF, EzzEl-Arab AM, Mohamed NA, Youness ER. Inulin Improves Hepatosteatosis in Humans. Der Pharma Chemica.; 8(23): 35-43 (2016).
- Nicola WG, Ezz El-Arab AM, Girgiss MW, Habib DF, Mohamed NA. Is there a role of inulin in the management of type 2 diabetes mellitus ?! International Journal of PharmTech Research.; 8(10): 01-09 (2015).
- Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 reeceptor. Diabetes.; 55: 1484-1490 (2006).
- Cani PD, Lecourt E, Dewlf EM, Sohet FM, Pachikian BD, Naslain D, et al.. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr.; 90: 1236-1243 (2009).
- Nauck MA, Heimesaat MM, Behle K, Holst JJ, Nauck MS, Ritzel R, et al.. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. Journal of Clinical Endocrinology and Metabolism.; 87: 1239–1246 (2002).
- Guenifi A, Ahren B, Abdel-Halim SM. Differential effects of glucagon like peptide-1 (7–36) amide versus cholecystokinin on arginine-induced islet hormone release in vivo and in vitro. Pancreas.; 22: 58–64 (2001).
- Kelly G. lnulin-type prebiotics — a review part 1. Altern Med Rev.; 13(4): 315-329 (2008).
- Bak MJ, Albrechtsen NW, Pedersen J, Hartmann B, Christensen M, Vilsbøll T, et al. Specificity and sensitivity of commercially available assays for glucagon and oxyntomodulin measurement in humans. Eur J Endocrinol.; 170: 529-538 (2014).
- Mathiews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC, 1985. Homeostasis model assessment: insulin resistance and b–cell function from fasting plasma glucose and insulin concentration in man. Diabetologia.; 7: 412-419 (1985).
- National Committee for Clinical Laboratory Standards. Procedures for the collection of diagnostic blood specimens by venipuncture: approved standards. 4th Ed. NCCLS Document H3-A4, Wayne, PA: (1998).
- Tietz NW, ed. Clinical guide to laboratory tests. 3rd ed. Philadelphia. Wb saunders, : 268-273 (1995).
- MacDonald PE, De Marinis YZ, Ramracheya R, Salehi A, Ma X, Johnson PR, et al. A K ATP channel-dependent pathway within alpha cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biology.; 5: e143 (2007).
- Vieira E, Salehi A, Gylfe E. Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells. Diabetologia.; 50: 370–379 (2007).
- Li XC, Liao TD, Zhuo JL. Long-term hyperglucagonaemia induces early metabolic and renal phenotypes of Type 2 diabetes in mice. Clinical Science.; 114: 591–601 (2008).
- Dunning BE, Gerich JE. The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocrine Reviews.; 28: 253–283 (2007).
- Rosenstock J, Baron MA, Dejager S, Mills D, Schweizer A. Comparison of vildagliptin and rosiglitazone monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabetes Care.; 30: 217-223 (2007).
- Nicola WG, Girgiss MW, Ezz El-Arab AM, Habib DF, Elnemr ME, Mohamed NA, Youness ER. Role of inulin in the protection and management of metabolic inflammation in humans. Biomedical & Pharmacology Journal.; 11: 1083-1090 (2018).
- Nicola WG, Girgiss MW, Ezz El-Arab AM, Habib DF, Elnemr ME, Mohamed NA, Youness ER. Protective effect of inulin and the integrity of the microvasculature in diabetes mellitus. Biomedical & Pharmacology Journal.; 11: 807-813 (2018).
- Nicola WG, Girgiss MW, Ezz El-Arab AM, Habib DF, Mohamed NA. Anti-atherogenic effect of inulin in female type 2 diabetes mellitus patients. Bioscience Research.; 15: 2990-2996 (2018).
- Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of Type I and Type II diabetes. Diabetologia.; 45: 937-948 (2002).
- Zhou H, Zhang T, Harmon JS, Bryan J, Robertson RP. Zinc, not insulin, regulates the rat alpha-cell response to hypoglycemia in vivo. Diabetes.; 56: 1107-1112 (2007a).







