Manuscript accepted on :17 Sep 2019
Published online on: 30-09-2019
Plagiarism Check: Yes
Reviewed by: Amruta Dashputra
Second Review by: Yilkal Bezie
Final Approval by: Dr. Kishore Kumar Jella
Department of Zoology, S.S. and L.S. Patkar College of Arts and Science and V.P. Varde College of Commerce and Economics, S.V. Road, Goregaon (West), Mumbai- 400 062, India
DOI : https://dx.doi.org/10.13005/bpj/1784
Abstract
Fresh seeds of Piper nigrum were procured from the botanical garden of KokanKrushiVidyapeeth, Dapoli, Ratnagiri. The ethanolic extract of the seeds was carried out by soxhlate extraction method. Sixty four (64) Sprague- Dawley rats (average weight 150 - 240 g) of each sex were used for the experiment. The drugs ETH and PAS drug and Piper nigrum were given to respective groups daily for 28 days. At the end of study various biochemical parameters were analyzed from serum such as, ACP, ALP, SGOT (AST), SGPT (ALT), Bilirubin, Total Cholesterol, HDL Cholesterol, and TGL. Liver tissues were analyzed for Histopathology. The data was statistically analyzed by one way analysis of variance (ANOVA). The value p< 0.05 considered as significant. Administration ETH and PAS in Sprague-Dawley rats showed hepatotoxicity in test groups which was confirmed by biochemical parameters and histoarchitecture examination of liver. Ethanolic extract of Piper nigrum (Linn.) seeds were administered to the test groups have ameliorated the toxic effect of the drugs. It is concluded that the Piper nigrum (Linn.) acts as hepato-protective agent hepato-toxicity caused by Ethionamide and Para amino salicylic acid in Sprague-Dawley rats .
Keywords
Histoarchitecture; Liver; PAS; Piper Nigrumeth; Rats
Download this article as:| Copy the following to cite this article: ZodapeG. V, Gaikwad V. S. Effect of Piper Nigrum (L.) on Hepatotoxicity Induced by Ethionamide and Para Amino Salicylic Acid in Sprague- Dawley Rats. Biomed Pharmacol J 2019;12(3). |
| Copy the following to cite this URL: Zodape G. V, Gaikwad V. S. Effect of Piper Nigrum (L.) on Hepatotoxicity Induced by Ethionamide and Para Amino Salicylic Acid in Sprague- Dawley Rats. Biomed Pharmacol J 2019;12(3). Available from: http://biomedpharmajournal.org/?p=28724 |
Introduction
The liver is a vital organ of paramount importance involved in the maintenance of metabolic functions and detoxification of the exogenous and endogenous challenges like xenobiotics, drugs, viral infections and chronic alcoholism1. Liver disease is still a worldwide health problem. Drug-induced hepatotoxicity is one of the major concerns which limit the therapy and drug use. About 2% of all causes of jaundice in hospitalized patients are drug induced. Approximately quarter of cases of fulminant hepatic failure are thought to be drug related. More than 900 drugs have been implicated in causing liver injury and it is the most common reason for a drug to be withdrawn from the market2.
Tuberculosis (TB) is transmittable diseases caused by Mycobacterium tuberculosis. Despite medical advancement tuberculosis is very lethal and is the main cause of the human deaths in many countries. Every second someone in the world is infected with tuberculosis, with an estimated 9.6 million new cases each year. Approximately a third of the world’s population is currently infected with tuberculosis and up to 10% of these will go to develop active TB, leading to 1.6 million deaths per year 3. It is studied that the development of MDR-TB is due to misuse of proper antibiotic treatment by patients and unfocused physician observations to these patients. Due to very modern issue associated with MDR-TB the Second line Tuberculosis drugs are used.
Ethionamide (eth eye on a mide) is most frequent drug used which shares the similarities with the Isonizid in terms of structure and antimycobacterial function. The daily oral dose of Ethionamide is 250 mg/kg and can be given up to 1 gm if well tolerated by patients. In the some cases of hepatotoxicity induced by ethionamide have been serious and malefic cases are also reported4.
Para-amino salicylic acid (PAS) was the first antibiotic found to be efficient in the treatment of tuberculosis in the 1940s5. PAS treatment is uncommon and a highly drug resistant strain seems to have limited resistance to this drug. Thus, PAS became the principle second line agent for the treatment of MDR-TB6. PAS may cause the hepatitis (Prasad et. al., 2006).Hepatotoxicity is one of the most frequent and serious adverse effects of anti-TB medications and may reduce treatment effectiveness by compromising treatment regimens7,8.
A lot of medicinal plants, traditionally used for thousands of years, are present in group of herbal preparation of the Indian traditional health care system. Today, about 80% of the world population dependent on botanical agents as medicine to meet their health issues9.In developing countries, the traditional plant remedies are widely used to treat various ailments. Many varieties of plants have been used for treating different kinds of diseases including hepatoprotective potentials10. Now days the dietary supplements and herbal remedies have increase the interest of researchers to treat different kind of diseases. In India, over 40 polyherbal commercial formulations reputed to have hepatoprotective action are being used 11 ,12.
Piper nigrum (Linn.) (familyPiperaceae) is one of the most commonly used spices and considered as ’’The King of spices’’ among various spices. Piper nigrum is effective anti-M. Tuberculosis and is active against both drug sensitive and resistant strains of TB13. Piper nigrum along with other phytoconstituents contains major pungent alkaloid Piperine which is known to possess many interesting pharmacological actions. Piperine has been found to enhance the therapeutic efficacy of many drugs, vaccines and nutrients by increasing oral bioavailability by inhibiting various metabolizing enzymes14. It is also known to enhance hepato-protective action15.In view of severe undesirable side effects of synthetic drugs, there is a need to focus on systematic research methodology and to study the scientific basis for the traditional herbal medicines that are claimed to show hepato-protective potential.
Therefore, in view of the above literature survey it was found that ETH and PAS used as antituberculosis drugs but at the same time these drugs are responsible to caused hepatotoxicity in the human beings. Therefore, in the present study, attention has been given to find the effect of Piper nigrum on hepatotoxicity induced by ETH and PAS in the Sprague-Dawley rats.
Materials and Methods
Collection of sample
Fresh seeds of Piper nigrum were procured from the botanical garden of KokanKrushiVidyapeeth, Dapoli, Ratnagiri. The initial identification was done by referring related literature and final identification and confirmation was done at the department of horticulture, KokanKrushiVidyapeeth, Dapoli, Ratnagiri prior to process the sample at the department of Zoology S.S & L.S. Patkar College Goregaon (west), Mumbai India.
Extraction
The ethanolic extract of the seeds was carried out by soxhlate extraction method. The sample was evaporated to dryness and powder was weighed and the yield so obtained was collected in a sterile container and kept at -20 0C till further use. The weight of the powder was calculated based on weight of the seeds.
Purchas of drugs
The drugs ETH (Macleods Pharmaceuticals Ltd) and PAS (Lupin Ltd) were purchased following the Prescription of Physician from B.J. Medical College and Sassoon General Hospital, Pune, Maharashtra.
Experimental Design
Sixty six(64) Sprague- Dawley rats (average weight 150 – 240 g) of each sex were used for the experiment. They were purchased and procured from the National Toxicological Centre, APT Testing & Research Pvt. Ltd. (ATR) Pune. The experimental study was approved by Ethical committee at APT Research Foundation, Pune prior to the experimentation (CPCSEA NO. 40/PO/ReBiRc/S/99/. 11. 03. 2014).The animals were acclimatized, maintained and housed in APT laboratory for a week. The controlled humidity and temperature at 240C; humidity, 12-hlight/12 hrs dark cycle was also maintained by feeding the rats with commercial rat pallets and water available ad libitum.
Biochemical assay
Blood samples of the above groups were taken after 28th day by heart puncture for estimation of liver functional test. Assessment of liver damage were done by biochemical investigations of Serum glutamic-pyruvic transaminase (SGPT) and Serum glutamic–oxaloacetic transaminase (SGOT) by 16Serum bilirubin(Bil) by17 and Serum alkaline phosphatase (ALP) by 18Cholesterol and HDL High density lipid by19Triglysceride by20, Acid Phosphatase by21.
Histopathological analysis
The liver tissue was dissected out and fixed in 10% formalin, dehydrated in gradual ethanol (50–100%), cleared in xylene, and embedded in paraffin. Five micron thick sections were prepared and then stained with hematoxylin and eosin (H–E) dye for photomicroscopic observation, including cell necrosis, fatty degenerative changes, hyaline regeneration, ballooning degeneration as proposed by22, and histological structure of liver tissue were examined under the Biological digital microscope-Motic B1 Series.
Administration of Test Article
The test article at the above concentration was administered to each rat by a single oral gavage. The animals were dosed using a stainless steel intubation needle fitted onto a suitably graduated syringe. The dosage volume administered to individual rat was adjusted according to its most recently recorded body weight. Animal weights were determined weekly along with food consumption.
Animals were randomly divided into following groups containing 8 animals (4 males and 4 females) in each group.
| Groups | Specification | Treatment specifications |
| 1 | Normal control | Animals fed with rat pellets and ordinary water |
| 2 | PnS | PnS (500 mg/kg bw) |
| 3 | ETH | ETH (132 mg/kg bw) |
| 4 | PAS | PAS (400 mg/kg bw) |
| 5 | ETH + PAS | ETH (132 mg/kg bw) + PAS (400 mg/kg bw) |
| 6 | ETH + PnS | ETH (132 mg/kg bw) + PnS (500 mg/kg bw) |
| 7 | PAS + PnS | PAS (400 mg/kg bw) + PnS (500 mg/kg bw) |
| 8 | ETH + PAS + Pns | ETH (132 mg/kg bw) + PAS (400 mg/kg bw)+Pns (500 mg/kg bw) |
*ETH=Ethionamide, PAS=Para amino salicylic acid, PnS= Piper nigrum Linn. Seeds ethanol extract
Test drug and inducers were given to respective groups as indicated in the table daily for 28 days. At the end of study various biochemical parameters were analyzed from serum such as, ACP, ALP, SGOT (AST), SGPT (ALT), Bilirubin, Total Cholesterol, HDL Cholesterol, and TGL. Liver tissues were analyzed for Histopathology of Liver by using method as proposed by Davidson; 1979
Statistical analysis
The data was statistically analyzed by one way analysis of variance (ANOVA) . The value p< 0.05 considered as significant
Results and Discussions
Table 1: Showing the mean concentration of Serum Biochemistry in Sparague-Dawley rats
| Group | Wt. of liver/gm |
ACP |
ALP | SGOT
(AST) |
SGPT
(ALT) |
Bilirubin | Total
Cholesterol |
HDL Cholesterol | TGL |
| NC | 3.854 | 0.06 | 287.67 | 146.5 | 44.87 | 0.29 | 89.33 | 24.43 | 144.83 |
| PnS | 4.121 | 0.04 | 331.5 | 154.63 | 52.53 | 0.33 | 94 | 25.63 | 143 |
| ETH | 3.949
|
0.06 | 540.14 | 149.43 | 84.27 | 0.39 | 92.57 | 21.2 | 183.43 |
| PAS | 3.853 | 0.06 | 349.38 | 155.75 | 85.82 | 0.36 | 89 | 11.38 | 157.38 |
| ETH+PAS | 4.075 | 0.06 | 480.75 | 166 | 50.59 | 0.32 | 86.5 | 14.96 | 134.25 |
| ETH+PnS | 3.545 | 0.07 | 286.67 | 160.33 | 52.01 | 0.27 | 66.33 | 16.53 | 134.83 |
| PAS+PnS | 4.129 | 0.05 | 324 | 181.43 | 54.65 | 0.25 | 63.71 | 18.17 | 115 |
| ETH+PAS+PnS | 4.558 | 0.05 | 371.5
|
165.5
|
56.08
|
0.29
|
89.17
|
20.73
|
132.83
|
each value is the mean of 8 determinations.
ACP: Serum Acid Phosphatase IU / L
ALP : Serum Alkaline Phosphatase IU / L
SGOT : Serum Glutamic Oxaloacetic Transaminase IU / L
SGPT : Serum Glutamic Pyruvic Transaminase IU / L
Bilirubin : Serum Bilirubin mg / dl
TC: Total Cholesterol
HDL Cholesterol
TGL: Triglyceride
Table No. 1. The mean concentration of Serum Biochemical investigations of Serum ACP: Serum Acid Phosphatase , ALP : Serum Alkaline Phosphatase, SGOT : Serum Glutamic Oxaloacetic Transaminase, SGPT : Serum Glutamic Pyruvic Transaminase, Bilirubin : Serum Bilirubin , TC: Total Cholesterol, HDL Cholesterol and TGL: Triglyceride
Statistical analysis
ANOVA followed. The p-value is < .00001. The result is significant at p < .05.
There was no mortality in any of the groups. The body weight and relative liver weights of the experimental animals calculated at the end of the study had no statistically significant difference observed when compared to the control animals
Table No. 1. The mean concentration of Serum Biochemical investigations of Serum ACP; Serum Acid Phosphatase, ALP; Serum Alkaline Phosphatase, SGOT; Serum Glutamic Oxaloacetic Transaminase, SGPT; Serum Glutamic Pyruvic Transaminase, Bilirubin; Serum Bilirubin , TC; Total Cholesterol, HDL Cholesterol and TGL; Triglyceride
Body weight was measured weekly during the study period of 28 days wherein, no statistically significant changes were observed in the body weights of Test group animals as compared to Normal Control on respective days. Similarly, food consumption was also measured weekly wherein no statistically significant changes were observed in food consumption of Test group animals as compared to Normal Control.
The mean weight of liver was found in normal control rats is (3.854/g). With respect to experimental groups the minimum liver weight was recorded in rats treated with ETH and PnS was (3.545/g) whereas maximum liver weight was recorded in rats treated with ETH, PAS and PnS (4.558/g) The body weight and relative liver weights of the experimental animals calculated at the end of the study had no statistically significant difference observed when compared to the control animals.
The mean concentration of Serum Acid Phosphatase was found in normal control rats was (0.06 IU/L). With respect to experimental groups the minimum concentration of Serum Acid Phosphatase found in rats treated with PnS was (0.04 IU / L), whereas maximum concentration of Serum Acid Phosphatase was found (0.07IU / L) in rats treated with ETH+PnS.
The mean concentration of Serum Alkaline Phosphatase was found in normal control rats was (287.67 IU / L). With respect to experimental groups the minimum concentration of Serum Alkaline Phosphatase found in rats treated with ETH and PnS was ( 286.67 IU / L), whereas maximum concentration of Serum Alkaline Phosphatase was found ( 540.14IU / L) in rats treated with ETH drug.
The mean concentration of Serum Glutamic Oxaloacetic Transaminase was found in normal control rats was (146.5 IU / L). With respect to experimental groups the minimum concentration of Serum Glutamic Oxaloacetic Transaminase found in rats treated with PnS was ( 149.43 IU / L), whereas maximum concentration of Serum Glutamic Oxaloacetic Transaminase was found ( 181.43 IU / L) in rats treated with PAS+ PnS.
The mean concentration of Serum Glutamic Pyruvic Transaminase was found in normal control rats was ( 44.87IU / L ). With respect to experimental groups the minimum concentration of Serum Glutamic Pyruvic Transaminase found in rats treated with ETH+PAS was ( 50.59 IU / L), whereas maximum concentration of Serum Glutamic Pyruvic Transaminase was found ( 85.82IU / L ) in rats treated with PAS drug.
The mean concentration of Serum Bilirubin was found in normal control rats was (0.29 mg / dl). With respect to experimental groups the minimum concentration of Serum Bilirubin found in rats treated with PAS + PnS was (0.25 mg / dl ), whereas maximum concentration of Serum Bilirubin was found ( 0.39 mg / dl ) in rats treated with ETH drug.
The mean concentration of Total Cholesterol was found in normal control rats was (89.33mg / dl). With respect to experimental groups the minimum concentration of Total Cholesterol found in rats treated with ETH+ PAS was ( 63.71 mg / dl ), whereas maximum concentration of Total Cholesterol was found ( 94.00 mg / dl ) in rats treated with PnS.
The mean concentration of HDL Cholesterol was found in normal control rats was ( 24.43 mg / dl). With respect to experimental groups the minimum concentration of HDL Cholesterol found in rats treated with PAS was ( 11.38 mg / dl ), whereas maximum concentration of HDL Cholesterol was found (25.63 ) in rats treated withPnS.
The mean concentration of Triglyceridewas found in normal control rats was (144.83 g / dl). With respect to experimental groups the minimum concentration of Triglyceridemg / dl found in rats treated with PAS and PnS was (115.00 g /dl), whereas maximum concentration of Triglyceride was found (183.43) in rats treated with ETH drug.
In our study, in case of ACP there was no statistically significant change observed in all groups when compared to normal control group. The levels of ALP were found to be significantly increased in ETH group (p<0.001) when compared to normal control animals. These levels were found to be significantly decreased in ETH+PnS group (p<0.001) as well as ETH+PAS+PnS group (p<0.01). In PAS group, although the ALP levels were increased as compared to normal control animals the difference was not statistically significant. The ALP levels of ETH+PAS group were also found to be increased significantly (p<0.01) in comparison with normal control animals. Although these levels were decreased in ETH+PAS+PnS group, the difference was not statistically significant. In case of AST (SGOT) there was no statistically significant change observed in all groups when compared to normal control group. In case of ALT (SGPT) levels were significantly increased in ETH and PAS groups (p<0.05) as compared to normal animals. Although the ALT (SGPT) levels were found to be decreased in PnS treated groups, but the difference was not statistically significant.The billirubin levels were found to be increased in PnS, ETH, PAS and ETH+PAS groups in comparison with normal control animals but the difference was not statistically significant. The levels were found to be significantly decreased in ETH+PnS group (p<0.05) when compared to ETH. Along with this, PAS + PnS group has also shown the significant decrease in billirubin levels in comparison with PAS group (p<0.05).The cholesterol levels were found to be significantly decreased in PAS+PnS group in comparison with PAS group (p<0.05). The total Cholesterol levels were found to be significantly reduced in PAS group animals (p<0.001), ETH+PAS group (p<0.001) and in ETH + PnS (p<0.05) comparison with normal control. PAS has also significantly reduced HDL Cholesterol levels as compared to ETH group (p<0.001). In comparison with PAS, the HDL cholesterol levels were increased significantly in PAS + PnS (p<0.05) as well as ETH + PAS + PnS group (p<0.01), there was no statistically significant change observed in all groups when compared to normal control group.
fig. 1a and b: NC-Male & Female: the histological architecture of liver sections of healthy rats showed normal cellular architecture with distinct hepatic cells and sinusoidal space.
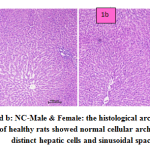 |
Figure 1a and b: NC-Male & Female: the histological architecture of liver sections of healthy rats showed normal cellular architecture with distinct hepatic cells and sinusoidal space. |
fig. 2a and b: PNS-Male & Female: the structural changes of the liver are normal and hepatocytes show no symptoms of necrosis and degeneration.
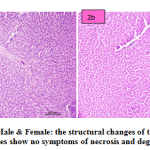 |
Figure 2a and b: PNS-Male & Female: the structural changes of the liver are normal and hepatocytes show no symptoms of necrosis and degeneration. |
fig. 3a and b: ETH-Male & Female: the animals treated with ETH drug shows that mild degenerative effect of liver. In males congested vasculature in hepatic parenchyma. Focal cellular swelling and vacuolar changes of hepatocytes with degenerative changes were occasionally occurring. In females congested vasculature and focal minimal degenerative changes in hepatic parenchyma. Multiple foci of cellular swelling and vacuolar changes in hepatocytes were seen with granular cytoplasm changes. Focal sinusoidal hemorrhages and focal MNC infiltration were occasionally seen.
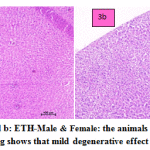 |
Figure 3a and b: ETH-Male & Female: the animals treated with ETH drug shows that mild degenerative effect of liver. |
fig. 4a and b: PAS-Male & Female: the animals treated with PAS drug showed mild degenerative effect of liver. In males congested vasculature in hepatic parenchyma. Focal cellular swelling and vacuolar changes of hepatocytes with degenerative changes of hepatocytes occasionally observed. In females normal hepatic parenchyma with normal histomorphology of hepatocytes and portal vascular tissue were observed. The hepatocytes arranged in cord like manner around central vein with presence of normal sized nucleus and intact cellular borders. Normal bile ducts and portal areas were seen.
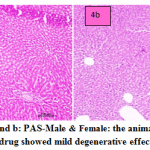 |
Figure 4a and b: PAS-Male & Female: the animals treated with PAS drug showed mild degenerative effect of liver. |
fig. 5a and b: ETH+PAS- Male & Female: in the liver section of the rats intoxicated with ETH+PAS drug, there was disarrangement and degeneration of normal hepatic cells with intense centrilobular necrosis. Congested vasculature and focal minimal degenerative changes were occurring in hepatic parenchyma with cellular swelling and vacuolar changes of hepatocytes. Fatty infiltration in hepatocytes occurred. In female normal hepatic parenchyma with normal histomorphology of hepatocytes and portal vascular tissue were seen. The hepatocytes arranged in cord like manner around central vein with presence of normal sized nucleus and intact cellular borders. Normal bile ducts, and portal areas was clearly seen.
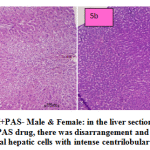 |
Figure 5a and b: ETH+PAS- Male & Female: in the liver section of the rats intoxicated with ETH+PAS drug, there was disarrangement and degeneration of normal hepatic cells with intense centrilobular necrosis. |
fig. 6a and b: ETH+PNS- Male & Female: in the liver section of the rats intoxicated with ETH drug in combination with PNS , there was rearrangement and regeneration of normal hepatic cells with normal hepatic parenchyma with normal histomorphology of hepatocytes and portal vascular tissue. The hepatocytes arranged in cord like manner around central vein with presence of normal sized nucleus and intact cellular borders.
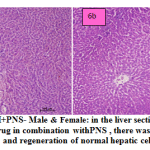 |
Figure 6a and b: ETH+PNS- Male & Female: in the liver section of the rats intoxicated with ETH drug in combination withPNS , there was rearrangement and regeneration of normal hepatic cells. |
fig. 7a and b: PAS+PNS- Male & Female: in the liver section of the rats intoxicated with PAS drug in combination with PNS, there was rearrangement and regeneration of normal hepatic cells with normal hepatic parenchyma with normal histomorphology of hepatocytes and portal vascular tissue. The hepatocytes arranged in cord like manner around central vein with presence of normal sized nucleus and intact cellular borders.
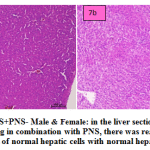 |
Figure 7a and b: PAS+PNS- Male & Female: in the liver section of the rats intoxicated with PAS drug in combination with PNS, there was rearrangement and regeneration of normal hepatic cells with normal hepatic parenchyma |
fig. 8a and b: ETH+PAS+PNS- Male & Female: in the liver section of the rats intoxicated with ETH+PAS drug in combination with PNS, there was rearrangement and generation of normal hepatic cells with normal hepatic parenchyma. histomorphology of hepatocytes and portal vascular tissue showed normal arrangement. The hepatocytes arranged in cord like manner around central vein with presence of normal sized nucleus and intact cellular borders.
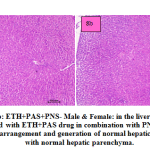 |
Figure 8a and b: ETH+PAS+PNS- Male & Female: in the liver section of the rats intoxicated with ETH+PAS drug in combination with PNS, there was rearrangement and generation of normal hepatic cells with normal hepatic parenchyma. |
Hepatotoxicity is one of the most frequent and serious adverse effects of anti-TB medications and may reduce treatment effectiveness by compromising treatment regimens 23, 24. Different experimental studies on animals suggest that administration of antitubercular drugs results in the rise of ALT, AST and ALP in serum, affecting hepatocellular membrane integrity and its organelles25, 26. Increased activity of hepatocytes leads to hyperbillirubinaemia which helps to determine integrity of liver27. It has been reported that sub acute or chronic treatment with isoniazid induced hepatotoxicity in man28, rat29, and guinea pigs30resulting in the rise of serum transaminases and phosphatase activities. Isoniazid-induced hepatitis is associated with ballooning degeneration, focal hepatocyte necrosis with minimal cholestasis31.
In our study, it was found that piper nigrum at the higher dose levels prevented an increased in ALT, AST, ALP, ACP, Bilirubin, levels.
The increased levels of AST and ALT are indicative of cellular damage and loss of functional integrity of the cell membrane in the liver32. The increase in ALP in liver disease is the result of increased synthesis of the enzyme by cells lining the canaliculi, usually either intra- or extra hepatic, which reflects the pathological alteration in biliary flow33. An abnormal increase in the levels of bilirubin in serum indicates hepatobiliary disease and severe disturbance of hepatocellular function34. The study carried out by 35 found that piperine inhibited the increased level of serum GPT and GOT in dose-dependent manner in a hepato-toxicity model of mice caused by D-galactosamine. The hepatoprotective activity of methanolic extract of Piper nigrum fruits was evaluated in ethanol- CCl4 induced hepatic damage in Wistar rats. In their study they showed significant liver protection as evidenced from the triglycerides levels, Alanine transaminase, Aspartate transaminase, alkaline phosphatase, bilirubin and superoxide dismutase, Catalase, Glutathione reductase and Lipid peroxidation levels to assess the liver functions. The study carried out by36 showed administration of Ethanol-CCl4 exhibited significant boost in triglycerides, Alanine transaminase, Aspartate transaminase, alkaline phosphatase, and bilirubin levels while there was significant decrease in the superoxide dismutase, catalase, and glutathione reductase levels which were restored to normal level after pre-treatment of methanolic extract of Piper nigrum and Piperine. The Morphological and histopathological studies of liver were also supportive of the biochemical parameters. Thus it is concluded that Piper nigrum possesses potential hepato-protective activity due to the presence of piperine alkaloids and have great therapeutic potential in treatment of liver ailments37. Piperine is one of the constituent of Piper nigrum is an active ingredient against acetaminophen induced hepatotoxicity studied by 38in mice. It has hepatoprotective potential and known to be used as thearapeutic medicine for various ailments39.The hepatoprotective effect of aqueous extract of Piper longum and piperine against first line antituberculous drugs having antioxidant property and hence proves its hepatoprotective potential40. Our study beholds the similar view. In our study we have found the intracellular hepatotoxic markers have increased in drugs treated animals and were controlled when treated with Piper nigrum in single or in combination of both the drugs.
Conclusion
Administration of Ethionamide and Para amino salicylic acid in Sprague-Dawley rats for 28 days showed hepatotoxicity in test groups. Hepatotoxicity was confirmed by biochemical parameters with the support of histoarchitecture examination of liver. Ethanolic extract of Piper nigrum (Linn.) seeds were administered to the test groups have ameliorated the toxic effect of the drugs. Based on the above results it is concluded that the Piper nigrum (Linn.) acts as hepato-protective agent hepato-toxicity caused by Ethionamide and Para amino salicylic acid in Sprague-Dawley rats and in combination of both. Piper nigrum (Linn) showed the hepatoprotective activity against the drugs however the molecular studies are necessary to elucidate mechanism of action of hepatoprotective activity of Piper nigrum (Linn.) against Ethionamide and Para amino salicylic acid.
Acknowledgement
Authors would like to thank to, Dr. Kishori G Apte, Dr.Bhagyshree ENagarkar, Dr.GayatriDeshmukh,DrR.G.Khandekar for their valuable support during the experimentation.
Conflict of Interest
There is no conflict of interest in the present work.
References
- Dienstag JL, Isselbacher KJ, Toxic and drug‐induced hepatitis, 15th edn. Chapter 296, In: Harrison’s Principles of Internal Medicine. Braunwald E, et al, The McGrawHill Companies, In, 2001; 2:7371742.
- Gordon M.C. and David J.N. Natural Product drug discovery in the next millennium. Pharm. Biol. 2001 39,8-17
- WHO Global Tuberculosis Report, 2015
- Bastian; R Colebunders, Treatment and prevention of multidrug-resistant tuberculosis.Drugs, (1999); 58, 633–661.
- Lehmann, J.Para-Amino salicylic acid in the treatment of tuberculosis. Dis Chest. (1946): 1949 Dec;16(6)
- Mitnick Carole., Jaime Bayona., Eda Palacios., Shin Sonya.,Furin Jennifer., Felix Alcantara. et al. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. Engl.J Med.2003:348:119–128.
- Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D:Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J RespirCrit Care Med 2003; 167: 1472-7.
- Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, DekhuijzenR. :Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J GastroenterolHepatol 2008; 23: 192-202.
- Ogbera, A.O.; Dada, O.; Adeyeye, F. and Jewo, P.I. (2010):Complementary and alternative medicine use in diabetes mellitus. West Afr. J. Med.2010; 29(3): 158-162.
- Ageel MA, Islam MW, Ginawi, OT, AlYahyaO. :Evaluation of the Aphrodisaic activity of Litsea (lauraceace) and Orchis maculate (Orchidaceae) extracts in rats. Phytother Res. 1994; 8:103-105.
- Handa SS, Sharma A ,Chakraborti:Natural products and plants as liver protecting drugs. Fitoterapia 1986; 57:30749.
- Sharma A, Singh RT, Sehgal V, HandaSS. :Antihepatotoxic activity of some plants used in herbal formulations. Fitoterapia, 1991; 62: 131.
- BirdiTannaz, D Souza Desiree,TolaniMonica,Daswani Poonam . Assessment of the Activity of Selected Indian Medicinal Plants against Mycobacterium tuberculosis: A Preliminary Screening Using the Microplate Alamar Blue Assay European Journal of Medicinal Plants 2012; 2(4): 308-323.
- Johnson JJ, Nihal M, Siddiqui IA, Scarlett CO, Bailey HH, et al.: Enhancing the bioavailability of resveratrol by combining it with piperine. See comment in PubMed Commons below MolNutr Food Res 2011; 55: 1169-1176.
- Matsuda H, Ninomiya K, Morikawa T, Yasuda D, Yamaguchi I, et al: Protective effects of amide constituents from the fruit of Piper chaba on D-galactosamine/TNF-alpha-induced cell death in mouse hepatocytes. See comment in PubMed Commons below Bioorg Med Chem Lett (2008) 18: 2038-2042.
- Reitman, S Frankel,S.Am.J. Clin .Path. 1957; 25:56 .
- Jendrassik L. and Grof.P.Biochem. Z.,1938; 297:81.
- Kind, P.R.H., King EJ: J clinpatho. 1954; 7:322.
- Trinder,PAnn.Clin. Biochem. 1969;6:24 .
- Herbert K.in Lipids, In Clinical Chemistry: Theory, Analysis and Correlation,1984; p. 1182, CV Mosby, Toronto.
- Hillman,G.C.Klin.Biochem, 1971; 9,273-274
- Davidson CS: Guidelines for detection ofhepatotoxicity due to drugs and chemicals.NIHpublication,U.S.Department of Health and Education and Welfare NIH U.S.A.1979.
- Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D: Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J RespirCrit Care Med 2003; 167: 1472-7.
- Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, Dekhuijzen R. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J GastroenterolHepatol 2008; 23: 192-202.
- Parthasarathy, R., Sarma, G. R., Janardhanam, B., Ramachandran, P., Santha, T. and Sivasubramanian, S. Hepatic toxicity in South Indian patients during treatment of tuberculosis with short-course regimens containing isoniazid, rifampicin and pyrazinamide. Tubercle.1986; 67: 99- 108.
- Shakun, N.P. and Tabachuk, O.P. The comparative action of isoniazide, rifampicin and ethambutol on liver function. EKSP. Klin. Farmakol. 1992; 55: 45.
- Singh, .B, Saxena, A.K., Chandan, B.K., Anand, K.K., Suri, O.P., Suri, K.A. and Satti, N.K. :Hepatoprotective activity of verbenalin on experimental liver damage in rodents. Fitoterapia.1998; 69: 135-140.
- Mitchell, J.R., Long, M.W., Thoregeirsson, U.P. and Jollow, D.J. :Acetylation rates and monthly liver function tests during one year of isoniazid preventive therapy. Chest. 1974; 68: 181-190.
- Snodgrass, W., Potter, W. Z., Timbrell, J. A., Jollow, D. J. and Mitchell, J.R: Possible mechanism of isoniazid related hepatic injury. Clin. Res. 1974; 22: 323
- Karthikeyan, S. Hepatotoxicity of isoniazid: A study on the activity of marker enzymes of liver toxicity in serum and liver tissue of rabbits, Indian J.Pharmacol.2004; 36 (4): 247-249.
- Mitchell, J.R., Zimmerman, H.J.,Ishak, K.G.,Thorgeirsson, U.P.,Timbrell, J.A. and Snodgrass, W.R. Isoniazid liver injury: Clinical spectrum, Pathology and probable pathogenesis.Ann. Intern. Med. 1976; 84:181-92.
- Drotman RB, LowhornGT:Serum enzymes as indicators of chemical induced liver damage. Drug ChemToxicol. 1978; 1: 163–171.
- Plaa GL, Hewitt WR: Detection and evaluation of chemically induced liver injury. In: Principles and Methods of Toxicology, 2nd ed. (Wallace Hayes A, ed.), Raven Press, and New York, 1989; 399–428.
- Martin P, Friedman LS:Assessment of liver function and diagnostic studies. In: Handbook of Liver Disease (Friedman LS, Keeffe EB, eds.), Churchill Livingstone, Philadelphia, 1998; 1–14.
- Nirwane A M, Bapat A R: Effect of methanolic extract of Piper nigrum fruits in Ethanol-CCl4 induced hepatotoxicity in Wistarrats. Der Pharmacia Lettre 2012; 4:795-802
- KarunarathnaAmilaUreshani , Chandrika Udumalage, PadumadasaChayanika , SeneviratneBimalka , AbeysekeraAjitaMahendraHepatoprotective activity of link livecaretm in carbon tetrachloride and d-galactosamine induced hepatotoxicity in icr mice j. res. ayurveda pharm. 2017; 8 (suppl 1), 91-96
- Evan Prince SabinaAnnie Deborah Harris SouriyanDeborah JacklineMahaboob Khan Rasool: Piperine, an active ingredient of black pepper attenuates acetaminophen–induced hepatotoxicity in mice Asian pacific journl of medicine. 2010;3(12):971-976
- Mahaboob Khan Rasool; Evan Prince Sabina ; Segu R. Ramya ; PranatharthiharanPreety ; Smita Patel ; Niharika Mandal ; Punya P. Mishra ; Jaisy SamuelHepatoprotective and antioxidant effects of gallic acid in paracetamol-induced liver damage in mice. J Pharm Pharmacol 2010; May;62(5):638-43
- Khursheed A Ansari and Mohd Akram: FilfilSiyah (Piper nigrum Linn) an important Drug of Unani System of Medicine: A Review Journal ofpharmacognosy and phytochemistry.2014; 2(6):219-221.
- Gurumurthy P and Vijaylatha S:Hepatoprotective effect of aqueous extract of Piper longum and Piperine when administered with antituberculosis drugs.2012; 7(4):661-663.








