Manuscript accepted on :22/08/2019
Published online on: 12-09-2019
Plagiarism Check: Yes
Reviewed by: N.H. Aksoy
Second Review by: Dr Geeta
Final Approval by: Dr. Ian James Martin
Munesh Kumar1, Rajesh Thakur1* and Sandeep Kumar2
1Department of Bio and Nano Technology, Guru Jambheshwar University of Science and Technology, Hisar.
2Department of Veterinary Physiology and Biochemistry, LUVAS, Hisar.
*Corresponding Author E-mail: rthakur99@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1761
Abstract
Syzygium cumini is well known for its medicinal values in the indigenous Indian system of medicine. This study was designed to evaluate the protective effect of methanolic (SCM), ethanolic (SCE) and aqueous (SCA) extracts of Syzygium cumini seeds on arsenic-induced blood cell genotoxicity and hepatotoxicity in Wistar albino rats. Rats were divided into five groups: (1) control, (2) arsenic, (3) SCM, (4) SCE and (5) SCA. After completion of 60 days treatment period, comet assays were performed on isolated blood lymphocytes and serum marker assays indicative of hepatic toxicity were carried out. Arsenic exposed rats expressed significantly higher DNA damage in their lymphocytes than the unexposed rats. Increased activities of serum alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT), and decreased levels of total proteins were observed in arsenic exposed rats. Simultaneous administration of Syzygium cumini seed extracts significantly decreased the arsenic-induced DNA damage and hepatotoxicity. The amelioration of arsenic toxicity was more pronounced with methanolic extract compared to ethanolic and aqueous extracts of Syzygium cumini seeds.
Keywords
Antioxidants; Arsenic; Genotoxicity; Reactive Oxygen Species; Syzygium Cumini
Download this article as:| Copy the following to cite this article: Kumar M, Thakur R, Kumar S. Comparative Efficacy of Syzygium Cumini Seed Extracts in Alleviating Arsenic-Induced Hepatotoxicity and Blood Cell Genotoxicity in Wistar Albino Rats. Biomed Pharmacol J 2019;12(3). |
| Copy the following to cite this URL: Kumar M, Thakur R, Kumar S. Comparative Efficacy of Syzygium Cumini Seed Extracts in Alleviating Arsenic-Induced Hepatotoxicity and Blood Cell Genotoxicity in Wistar Albino Rats. Biomed Pharmacol J 2019;12(3). Available from: http://biomedpharmajournal.org/?p=28400< |
Introduction
Syzygium cumini (L.) Skeels (Myrtaceae) is a tropical plant widely distributed in different countries of the world such as India, Sri Lanka, Australia and Malaysia1. It has been attributed in the Indian folklore system of medicine to possess various medicinal properties2. Different parts of this plant, such as fruits, seeds and leaves are reported to have antidiarrheal3,4, hypoglycemic, antipyretic, anti-inflammatory5,6, and antibacterial7 properties. It has been valued in Ayurveda for possessing astringent, digestive, acrid and wound healing properties2. The leaves are used to strengthen gums and teeth, to treat stomachalgia, leucorrhoea, fever, strangury, dermopathy, constipation and gastropathy. The seeds and fruits are also used to treat pharyngitis, urethrorrhea, spleenopathy and ringworm infections2,8. Different parts of this plant are known to possess various bioactive compounds having free radical scavenging and antioxidant activities1,51.
Arsenic is a widespread pollutant in several parts of the world. Arsenic contaminated water is reported in more than 30 countries worldwide. However, the major affected areas are in the basins of rivers Brahmaputra, Ganga, and Meghna in India and Bangladesh and some parts of China9,10. In Taiwan, Argentina, Mexico, and the Indo-Bangladesh regions, drinking water concentrations of arsenic have been reported to be much above the standard (10 μg/l) adopted by the US Environmental Protection Agency (USEPA) in 200111. Arsenic exists in both organic and inorganic forms in the environment. Inorganic arsenic compounds include trivalent (arsenite or As III) and pentavalent (arsenate or As V) species which are highly toxic for humans and animals, and are considered as class I carcinogens12. Chronic human exposure to inorganic arsenicals is associated with various toxic effects including liver injury, peripheral neuropathy and increased incidences of skin, lung, liver, and bladder cancers9,13.
As for other heavy metals, chelation therapy is the mainstream treatment for arsenic poisoning also. In chelation therapy, drugs such as British anti-Lewisite (BAL) and dimercaptosuccinic acid (DMSA) are used; however, these compounds are associated with several moderate to severe side effects including nausea, hypertension, itching, abdominal pain and changes in body temperature14-16. Administration of antioxidants from plant sources is reported to be highly effective in reducing arsenic toxicity12,17,18. Some studies reported that combined administration of antioxidants and chelating agents is also beneficial against arsenic poisoning-induced toxicity14,19. The recent trend is to exploit the therapeutic value of medicinal and/or dietary plants with antioxidative potential to alleviate the arsenic toxicity.
Owing to the notable antioxidative properties of Syzygium cumini, the present study was planned to determine the effect of various seed extracts of this plant on arsenic-induced hepatotoxicity and blood cell genotoxicity in Wistar albino rats.
Materials and Methods
Plant Material
Seeds of Syzygium cumini were purchased from the local market and authenticated by Raw Materials Herbarium & Museum, NISCAIR (National Institute of Science Communication and Information Resources), New Delhi.
Seed Extract Preparation
After removing pericarps, seeds were dried at room temperature and then finely powdered. The dried seed powder was extracted with different solvents (methanol, ethanol and water) in a Soxhlet apparatus. The extracts were concentrated in a rotary vacuum evaporator and then freeze dried. The yield of SCM, SCE and SCA were, respectively, 10.8%, 10.2% and 9.8% of the dried powdered seeds. The seed extracts were stored at -20 °C until further use.
Experimental Animals
Wistar albino rats of either sex (100 – 125 g) were obtained from DFSAH (Disease Free Small Animal House), LUVAS, Hisar. Rats were kept under standard laboratory conditions with dark and light cycle (12/12 hr) and fed on a normal balanced rat diet. The studies were approved by the Institutional Animals Ethics Committee (CPCSEA/0436) of Guru Jambheshwar University and all animal experiments were performed in accordance with the guidelines of the same on animal experimentation. Animals were acclimatized for a week prior to the experiment.
Experimental Design
Rats were divided into five groups of 6 animals each and treated as follows.
|
Groups |
Treatment |
|
Control |
Normal drinking water |
| Arsenic | Arsenic in drinking water (100 ppm) ad libitum |
|
SCM |
SCM (400 mg/kg/day) along with arsenic water (100 ppm) ad libitum |
|
SCE |
SCE (400 mg/kg/day) along with arsenic water (100 ppm) ad libitum |
|
SCA |
SCA (400 mg/kg/day) along with arsenic water (100 ppm) ad libitum |
Seed extracts were administered by oral gavage. Body weight of animals, and their food and water intake were monitored throughout the treatment period of 60 days. At the end of treatment period the rats were weighed and blood was collected from the retro-orbital plexus of the eye under ether anaesthesia.
Lymphocyte Isolation
Freshly collected blood samples were diluted (1:1 ratio) with PBS (phosphate buffered saline) and carefully layered on the top of lymphocyte separation medium (LSM 1084) and centrifuged for 30 minutes at 400 x g. The buffy coat interface, which represented the lymphocytes, was aspirated and washed with PBS twice by centrifugation for 10 minutes at 250 x g. The supernatant was discarded and lymphocytes (pellet) were used immediately for the comet assay.
Comet Assay
The comet assay was performed according to Singh et al.20, with slight modifications. 150 µl of 0.5% NMA (normal melting agarose) was layered on to precleaned microscope slides and dried at 65 °C for 10 min. A second layer containing isolated lymphocytes resuspended in 75 µl of 0.5% LMA, was placed on the NMA precoated slides and solidified at 4 °C for 10 min. The slides were covered with 0.5% LMA and stored at 4 °C for 15-20 min. Afterwards the slides were placed in freshly prepared lysing solution (2.5 M NaCl , 100 mM Na2EDTA, 10 mM Tris, 1% Triton X-100, 10% DMSO and pH 10-10.5) at 4 °C for 2 h in the dark. Following lysis, the slides were immersed in an electrophoretic buffer (300 mM NaOH , 1 mM Na2EDTA, pH 13.5) for 25 min at 0 °C and electrophoresed in the same buffer for next 20 min (24 volts, 300 mA). Electrophoresis was conducted under dim light to prevent additional DNA damage. After that, slides were rinsed with 0.4 M Tris (pH 7.5) twice for 5 min, fixed for 3 min in absolute ethanol and stained with 0.4 µg/ml ethidium bromide. Comet images were observed at 400× magnification with a fluorescence
microscope (Olympus CX 41). For each sample, images of randomly selected 50 cells were examined. Open Comet software was used for DNA damage quantification by analysis of the tail percent DNA, tail moments and tail lengths.
Activities of Serum Markers
Commercially available diagnostic kits were used for assaying the activities of serum ALT, ALP, AST and total proteins.
Statistical Analysis
Data analysis was performed using One-way ANOVA followed by post-hoc Tukey’s test. The differences were considered statistically significant at P < 0.05.
Results
The effects of arsenic on body weight gain in control and experimental rats are depicted in Fig. 1. In arsenic-only treated rats, body weight gain percent was lower than control, SCM, SCE and SCA groups. Among experimental groups, body weight gain percent was highest in SCM group.
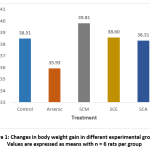 |
Figure 1: Changes in body weight gain in different experimental groups; Values are expressed as means with n = 6 rats per group. |
Activities of serum ALP, AST and ALT were significantly higher in arsenic-only group than in controls, while the same activities were all less in groups treated with Syzygium cumini seed extracts along with arsenic. Activities of ALP and AST were significantly lower in SCM, SCE and SCA groups compared to arsenic-only group. Serum total protein was significantly lower in the arsenic-only group than in control group and significantly greater in SCM, SCE and SCA groups than in arsenic-only group (Fig. 2).
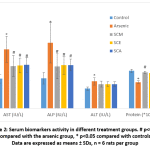 |
Figure 2: Serum biomarkers activity in different treatment groups. # p<0.05 compared with the arsenic group, * p<0.05 compared with controls; Data are expressed as means ± SDs, n = 6 rats per group. |
DNA damage in individual blood lymphocytes was assessed by measuring various comet assay parameters viz.; tail percent DNA, tail moments and tail lengths. Fig. 3 depicts the value of percent tail DNA in different treatment groups. Tail percent DNA was significantly greater in arsenic group than in control. Co-administration of S. cumini seed extracts (SCM, SCE, and SCA) significantly reduced the tail percent DNA. A significant increase in tail moment was observed in arsenic intoxicated rats as compared to control rats. However, tail moment values were significantly reduced in SCM, SCE and SCA group rats (Fig. 4). Similarly, the mean comet tail length was significantly higher in the arsenic-treated rats than in untreated rats, while the same values were significantly lower in SCM, SCE, and SCA rats than in arsenic-only exposed rats (Fig. 5). Among experimental groups treated with both arsenic and S. cumini seed extract, the mean values of tail percent DNA, tail moments and tail lengths were lowest in SCM, followed by SCE and SCA groups. Fig. 6 illustrates the representative comet assay images obtained by fluorescent microscopy.
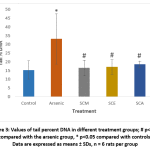 |
Figure 3: Values of tail percent DNA in different treatment groups; # p<0.05 compared with the arsenic group, * p<0.05 compared with controls; Data are expressed as means ± SDs, n = 6 rats per group |
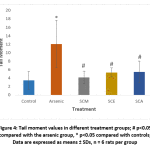 |
Figure 4: Tail moment values in different treatment groups; # p<0.05 compared with the arsenic group, * p<0.05 compared with controls; Data are expressed as means ± SDs, n = 6 rats per group |
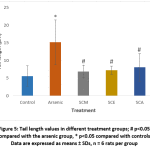 |
Figure 5: Tail length values in different treatment groups; # p<0.05 compared with the arsenic group, * p<0.05 compared with controls; Data are expressed as means ± SDs, n = 6 rats per group |
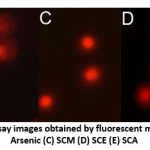 |
Figure 6: Representative comet assay images obtained by fluorescent microscopy; Groups: (A) Control (B) Arsenic (C) SCM (D) SCE (E) SCA |
Discussion
The present study reveals substantial amelioration of arsenic-induced blood cell genotoxicity and hepatotoxicity by various seed extracts of Syzygium cumini. Our results are corroborated by previous studies that have reported protective activity of antioxidant-rich plants such as Emblica officinalis, Camellia sinensis and Trichosanthes dioica against arsenic toxicity21-23. The present findings also support the modulatory effects of Syzygium cumini on genomic damage and oxidative stress induced by various agents such as radiations24, DMBA (7,12-dimethyl benz(a)anthracene) and urethane25, among few others.
Arsenic is an established carcinogen present ubiquitously in the environment. In addition to its carcinogenic effects, long-term arsenic exposure is associated with hyperpigmentation, hyperkeratosis, type II diabetes mellitus, neurological damage, and black foot and cardiovascular diseases26-30. Chronic arsenic exposure leads to accumulation of arsenic in various body organs, primarily the kidneys, liver, lungs and skin, which adversely affect these organs31-35. Reduction in body weight is also observed in arsenic exposed animals which is supposed to be caused by oxidative damage to body cells and tissues23,36. Our results, too, revealed a decline in the body weight of arsenic challenged rats compared with
controls. However, the combined treatment of arsenic and Syzygium cumini seed extracts resulted in body weight recovery towards control levels. Body weight gain was maximum in SCM, followed by SCE and SCA groups which indicates that methanolic seed extract was most effective in maintaining general body weight and thereby reducing arsenic-induced toxicity in rats with maximum potential among all extracts.
Liver is a potential target organ of arsenic toxicity13,22. Arsenic-mediated increase in activities of serum ALT, AST and ALP indicates hepatic toxicity and these results agree with previous findings6,22. Administration of Syzygium cumini seed extracts in arsenic exposed rats significantly restored the activities of these biochemical variables. Serum total protein levels were significantly less in arsenic exposed rats than the controls and this difference might be due to damaging effect of arsenic on hepatic cells or alterations in protein synthesis and/or metabolism6. Treatment with Syzygium cumini seed extracts significantly increased serum total protein levels towards normal in arsenic treated rats. Methanolic seed extract was most effective in decreasing arsenic-induced hepatic toxicity followed by ethanolic and aqueous extracts.
The comet assay is a simple, fast and reliable method for detection of DNA strand breaks in individual cells20,37. The present results from comet assay show that arsenic exposed rats exhibited significant lymphocyte DNA damage when compared to the controls. Our results are in line with earlier reports demonstrating considerable DNA damage in arsenic intoxicated animals and humans. Balakumar et al. observed increased DNA strand breaks in liver, blood, bone marrow and kidney cells of rats challenged by sodium arsenite38. Elevated levels of DNA damage were detected by DNA fragmentation assay in hepatic cells of experimental rats subchronicaly exposed to arsenic39. In another study, significant DNA damage in peripheral blood lymphocytes was reported in a population exposed to chronic arsenic through contaminated drinking water in West Bengal, India40. The increased lymphocytic DNA damage in arsenic intoxicated rats, as observed in our experiment, was markedly decreased in rats treated with Syzygium cumini seed extracts along with arsenic. Methanolic seed extract provided the maximum protection followed by ethanolic and aqueous seed extracts against the arsenic-induced DNA damage.
The exact mechanism of arsenic toxicity is not yet fully understood; however, the evidences suggest that oxidative stress is involved in arsenic-induced DNA damage and toxicity of various organs. Arsenic can disturb the natural oxidation/reduction balance through several mechanisms involved in intricate redox reactions with endogenous oxidants and antioxidant systems of cells41. Arsenic metabolism generates free radicals and reactive oxygen species (ROS) which induce cell signaling and transcription factor activation eventually leading to gene mutations, DNA strand breakage, sister chromatid exchange, generation of micronuclei and chromosomal aberrations12,42. It is suggested that superoxide anion (O2•⁻) is the primary ROS induced by arsenic in various cellular systems; which triggers formation of other ROS such as hydrogen peroxide (H2O2) and hydroxyl radical (•OH). H2O2 is converted to highly reactive •OH radicals via Fenton reaction. •OH radicals formed in vicinity of DNA may react with deoxyribose backbone of DNA or with DNA bases causing DNA strand breaks or producing damaged bases42,43. Enhanced nitric oxide (NO) production induced by arsenic also plays an important role in oxidative damage to DNA44,45. 8-hydroxy-2′-deoxyguanosine (8-OHdG) is a sensitive biomarker of ROS induced oxidative DNA damage and its elevated levels have been reported in various biological systems exposed to arsenic42,46. Arsenic inhibits DNA repair processes which potentiate the genotoxicity of other DNA damaging agents such as UV radiation, X-rays and benzo[a]pyrene47-49. Arsenic-induced oxidative stress may lead to altered DNA methylation and genomic instability resulting in a higher risk of carcinogenesis36,50.
The protective action of Syzygium cumini on arsenic-induced blood cell genotoxicity and hepatotoxicity may be attributed to the presence of various active phytochemicals such as triterpenoids, kaempferol, ellagic acid, myricetin, quercetin and acetyl oleanolic acid in the seeds of this plant1,6. Most of these compounds are reported to exhibit free radical scavenging and antioxidant properties51-54, which might have protected the animals against arsenic toxicity, probably by augmenting endogenous antioxidants12,55, and/or by altering apoptotic pathways12, and/or by directly scavenging DNA-damaging free radicals. The polyphenol ellagic acid is reported to possess antioxidant, antimutagenic and chemopreventive activities52,56. In a previous study, ellagic acid potentially inhibited the lipid peroxidation induced by radiation in the liver of mice57. The flavonoids kaempferol, quercetin and myricetin are potent antioxidants which protect cells by scavenging •OH radicals, nitric oxide and superoxide anion, and by inhibiting lipid peroxidation53,56,58-61. They possess reactive hydroxyl groups and stabilize various ROS by donating hydrogen atom53. Free radical scavenging by flavonoids decreases production of highly damaging peroxynitrite by preventing reaction of nitric oxide with free radicals54. Myricetin has been reported to have even higher antioxidant capacity than Vitamin E (D-α-tocopherol)62. Our results are in consonance with previous reports indicating ameliorative effects of antioxidants such as tetrahydrocurcumin, resveratrol, and vitamins C and E on arsenic-induced toxicity either in vivo36,38 or in vitro63. We also have reviewed the therapeutic potential of various plant-based antioxidants in arsenic genotoxicity, which further supports the results of this study12.
Conclusions
From the observations, we conclude that methanol, ethanol and aqueous seed extracts of Syzygium cumini mitigated arsenic-induced blood cell genotoxicity and hepatotoxicity in Wistar albino rats. Among all, methanol extract was the most effective in alleviating arsenic toxicity. The findings here support the growing evidence that antioxidant-rich plant sources exhibit protective effects against oxidative damage to DNA and other cellular components.
Acknowledgements
We acknowledge the funding provided by the Council of Scientific & Industrial Research (CSIR), India. LUVAS, Hisar for providing experimental animals and NISCAIR, New Delhi, for authenticating Syzygium cumini seeds. We thank Mr. Ravi Kumar, GJUS&T, Hisar, for assisting with animal handling and blood sampling.
Compliance With Ethical Standards
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding Source
Council of Scientific and Industrial Research (CSIR)(09/752 (0043)/2012-EMR-1).
References
- Ayyanar M, Subash-Babu P. Syzygium cumini (L.) Skeels: A review of its phytochemical constituents and traditional uses. Asian Pac. J. Trop. Biomed., 2012; 2: 240-246.
- Warrier P.K, Nambiar V.P, Ramankutty C. Indian Medicinal Plants.; Orient Longman Ltd.: Hyderabad, India. 1996; 5: 225–228.
- Indira G, Mohan R.M. Fruits. National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, India. 1992; 34–37.
- Monteiro F.S, Carvalho A.F.S, Marques E.C, Ribeiro R.M, Borges A.C.R, Borges M.O.R. Antidiarrhoeal and antispasmodic activity of leaves of Syzygium cumini(Myrtaceae) mediated through calcium channel blockage. Afr. J. Pharm. Pharmacol., 2018; 12: 11-18.
- Chaudhuri A.N, Pal S, Gomes A, Bhattacharya S. Antiinflammatory and related actions of Syzygium cumini Phytother. Res., 1990; 4: 5-10.
- Kumar M, Thakur R. Syzygium cumini seed extract ameliorates arsenic-induced blood cell genotoxicity and hepatotoxicity in Wistar albino rats. Rep. Biochem. Mol. Biol., 2018; 7: 110-118.
- Jadhav V.M, Kamble S.S, Kadam V.J. Herbal medicine: Syzygium cumini: A review. Pharm. Res., 2009;2: 1212-1219.
- Bhandary M.J, Chandrashekar K.R, Kaveriappa K.M. Medical ethnobotany of the siddis of Uttara Kannada district, Karnataka, India. J. Ethnopharmacol., 1995; 47: 149-158.
- Tchounwou P.B, Centeno J.A, Patlolla A.K. Arsenic toxicity, mutagenesis, and carcinogenesis–a health risk assessment and management approach. Mol. Cell. Biochem., 2004; 255: 47-55.
- Mazumder D.G, Dasgupta U.B. Chronic arsenic toxicity: studies in West Bengal, India. Kaohsiung J. Med. Sci., 2011; 27: 360-370.
- National primary drinking water regulations; arsenic and clarifications to compliance and new source contaminants monitoring. Fed. Regist., 2001; 66: 6975–7066.
- Kumar M, Lalit M, Thakur R. Natural antioxidants against arsenic-induced genotoxicity. Biol. Trace. Elem. Res., 2016; 170: 84-93.
- Chen C.J, Chen C.W, Wu M.M, Kuo T.L. Cancer potential in liver lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br. J. Cancer., 1992; 66: 888–892.
- Kushwaha P, Yadav A, Samim M, Flora S.J. Combinatorial drug delivery strategy employing nano-curcumin and nano-MiADMSA for the treatment of arsenic intoxication in mouse. Chem-Biol. Interact., 2018; 286: 78-87.
- Flora S.J.S, Bhadauria S, Kannan G.M, Singh N. Arsenic induced oxidative stress and the role of antioxidant supplementation during chelation: a review. J. Environ. Biol., 2007; 28: 333–347.
- Inns R.H, Rice P, Bright J.E, Marrs T.C. Evaluation of the efficacy of dimercapto chelating agents for the treatment of systemic organic arsenic poisoning in rabbits. Hum. Exp. Toxicol., 1990; 9: 215–220.
- Gbadegesin M.A, Odunola O.A. Aqueous and ethanolic leaf extracts of Ocimum basilicum (sweet basil) protect against sodium arsenite-induced hepatotoxicity in Wistar rats. Niger. J. Physiol. Sci., 2010; 25: 29-36.
- Adetutu A, Oyewo E.B, Adesokan A.A. Protective effects of Vernonia amygdalina against sodium arsenite-induced genotoxicity in rat. Pharmacognosy, 2013; 5(3):207-211.
- Mishra D, Flora S.J. Quercetin administration during chelation therapy protects arsenic-induced oxidative stress in mice. Biol. Trace. Elem. Res., 2008; 122: 137-147.
- Singh N.P, McCoy M.T, Tice R.R, Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell. Res., 1988; 175: 184-191.
- Acharyya N, Chattopadhyay S, Maiti S. Chemoprevention against arsenic-induced mutagenic DNA breakage and apoptotic liver damage in rat via antioxidant and SOD1 upregulation by green tea (Camellia sinensis) which recovers broken DNA resulted from arsenic- H2O2 related in vitro oxidant stress. J. Environ. Sci. Heal. C, 2014; 32: 338-361.
- Maiti S, Chattopadhyay S, Acharyya N, Deb B, Hati A.K. Emblica officinalis (amla) ameliorates arsenic induced liver damage via DNA protection by antioxidants systems. Mol. Cell. Toxicol., 2014; 10: 75-82.
- Bhattacharya S, Haldar P.K. Trichosanthes dioica fruit ameliorates experimentally induced arsenic toxicity in male albino rats through the alleviation of oxidative stress. Biol. Trace. Elem. Res., 2012; 148: 232-241.
- Jagetia G.C, Baliga M.S. Syzygium cumini (Jamun) reduces the radiation-induced DNA damage in the cultured human peripheral blood lymphocytes: a preliminary study. Toxicol. Lett., 2002; 132: 19-25.
- Arun R, Prakash M.V, Abraham S.K, Premkumar K. Role of Syzygium cumini seed extract in the chemoprevention of in vivo genomic damage and oxidative stress. J. Ethnopharmacol., 2011; 134: 329-333.
- Chen C.J, Chuang Y.C, Lin T.M, Wu H.Y. Malignant neoplasms among residents of a blackfoot disease-endemic area in Taiwan: high-arsenic artesian well water and cancers. Cancer Res., 1985; 45: 5895–5899.
- Tseng C.H, Chong C.K, Heng L.T, Tseng C.P, Tai T.Y. The incidence of type 2 diabetes mellitus in Taiwan. Diabetes Res. Clin. Pract., 2000; 50: S61–S64.
- Tseng C.H, Chong C.K, Chen C.J, Tai T.Y. Dose-response relationship between peripheral vascular disease and ingested inorganic arsenic among residents in blackfoot disease endemic villages in Taiwan. Atherosclerosis, 1996; 120: 125–133.
- Engel R.R, Hopenhayn-Rich C, Receveur O, Smith A.H. Vascular effects of chronic arsenic exposure: A review. Epidemiol. Rev., 1994; 16: 184–209.
- Goering P.L, Aposhian H.V, Mass M.J, Cebrian M, Beck B.D, Waalkes M.P. The enigma of arsenic carcinogenesis: Role of metabolism. Toxicol. Sci., 1999; 49: 5–14.
- Ratnaike R.N. Acute and chronic arsenic toxicity. Postgrad. Med. J., 2003; 79: 391-396.
- Benramdane L, Accominotti M, Fanton L, Malicier D, Vallon J.J. Arsenic speciation in human organs following fatal arsenic trioxide poisoning—a case report. Clin. Chem., 1999; 45: 301–306.
- Shannon R.L, Strayer D.S. Arsenic-induced skin toxicity. Human. Toxicol., 1989; 8: 99–104.
- Schwartz R.A. Arsenic and the skin. Int. J. Dermatol., 1997; 36: 241–250.
- Hughes M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett., 2002; 133: 1-16.
- Muthumani M. Tetrahydrocurcumin potentially attenuates arsenic induced oxidative hepatic dysfunction in rats. J. Clin. Toxicol., 2013; http://dx.doi.org/10.4172/2161-0495.1000168.
- Fontanetti C.S, Christofoletti C.A, Pinheiro T.G, Souza T.S, Pedro-Escher J. Microscopy as a tool in toxicological evaluations. Microsc. Sci. Technol. Appl. Educ., 2010; 2: 1001-1007.
- Balakumar B.S, Ramanathan K, Kumaresan S, Suresh R. DNA damage by sodium arsenite in experimental rats: ameliorative effects of antioxidant vitamins C and E.
Indian J. Sci. Technol., 2010; 3: 322-327. - Chattopadhyay S, Maiti S, Maji G, Deb B, Pan B, Ghosh D. Protective role of Moringa oleifera (Sajina) seed on arsenic-induced hepatocellular degeneration in female albino rats. Biol. Trace. Elem. Res., 2011; 142: 200-212.
- Basu A, Som A, Ghoshal S, Mondal L, Chaubey R.C, Bhilwade H.N, Rahman M.M, Giri A.K. Assessment of DNA damage in peripheral blood lymphocytes of individuals susceptible to arsenic induced toxicity in West Bengal, India. Toxicol. Lett., 2005; 159: 100-112.
- Miller W.H, Schipper H.M, Lee J.S, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res., 2002; 62: 3893-3903.
- Shi H, Shi X, Liu K.J. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol. Cell. Biochem., 2004; 255: 67-78.
- Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Rhodes C.J, Valko M. Arsenic: toxicity, oxidative stress and human disease. J. Appl. Toxicol., 2011; 31: 95-107.
- Lynn S, Shiung J.N, Gurr J.R, Jan K.Y. Arsenite stimulates poly(ADP-ribosylation) by generation of nitric oxide. Free. Radic. Biol. Med., 1998; 24: 442-449.
- Liu F, Jan K.Y. DNA damage in arsenite- and cadmium-treated bovine aortic endothelial cells. Radic. Biol. Med., 2000; 28: 55-63.
- Yamanaka K, Takabayashi F, Mizoi M, An Y, Hasegawa A, Okada S. Oral exposure of dimethylarsinic acid, a main metabolite of inorganic arsenics, in mice leads to an increase in 8-Oxo-2′-deoxyguanosine level, specifically in the target organs for arsenic carcinogenesis. Biochem. Biophys. Res. Commun., 2001; 287: 66–70.
- Fischer J.M, Robbins S.B, Al-Zoughool M, Kannamkumarath S.S, Stringer S.L, Larson J.S, Caruso J.A, Talaska G, Stambrook P.J, Stringer J.R. Co-mutagenic activity of arsenic and benzo [a] pyrene in mouse skin. Mutat. Res-Gen. Tox. En., 2005; 588: 35-46.
- Qin X.J, Hudson L.G, Liu W, Timmins G.S, Liu K.J. Low concentration of arsenite exacerbates UVR-induced DNA strand breaks by inhibiting PARP-1 activity. Toxicol. Appl. Pharm., 2008; 232: 41-50.
- Jha A.N, Noditi M, Nilsson R, Natarajan A.T. Genotoxic effects of sodium arsenite on human cells. Mutat. Res., 1992; 284: 215-221.
- Bhattacharjee P, Banerjee M, Giri A.K. Role of genomic instability in arsenic-induced carcinogenicity. A review. Environ. Int., 2013; 53: 29-40.
- Ruan Z.P, Zhang L.L, Lin Y.M. Evaluation of the antioxidant activity of Syzygium cuminiMolecules, 2008; 13: 2545-2556.
- Priyadarsini K.I, Khopde S.M, Kumar S.S, Mohan H. Free radical studies of ellagic acid, a natural phenolic antioxidant. J. Agr. Food Chem., 2002; 50: 2200-2206.
- Korkina L.G, Afanas’ Ev I.B. Antioxidant and chelating properties of flavonoids. Adv. Pharmacol., 1996; 38: 151-163.
- Shutenko Z, Henry Y, Pinard E, Seylaz J, Potier P, Berthet F, Girard P, Sercombe R. Influence of the antioxidant quercetin in vivo on the level of nitric oxide determined by electron paramagnetic resonance in rat brain during global ischemia and reperfusion. Biochem. Pharmacol., 1999; 57: 199-208.
- Nijveldt R.J, Van Nood E.L, Van Hoorn D.E, Boelens P.G, Van Norren K, Van Leeuwen P.A. Flavonoids: a review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr., 2001; 74: 418-425.
- Tanaka T. Cancer chemoprevention by natural-products. Oncol. Rep., 1994; 1: 1139-1155.
- Thresiamma K.C, George J, Kuttan R. Protective effect of curcumin, ellagic acid and bixin on radiation induced toxicity. Indian J. Exp. Biol., 1996; 34: 845-847.
- Abalea V, Cillard J, Dubos M.P, Sergent O, Cillard P, Morel I. Repair of iron-induced DNA oxidation by the flavonoid myricetin in primary rat hepatocyte cultures. Radic. Biol. Med., 1999; 26: 1457-1466.
- Maridonneau-Parini I, Braquet P, Garay R.P. Heterogenous effect of flavonoids on K+ loss and lipid peroxidation induced by oxygen-free radicals in human red cells. Pharmacol. Res. Commun., 1986; 18: 61-72.
- Jagetia G.C, Baliga M.S, Venkatesh P. Influence of seed extract of Syzygium cumini (jamun) on mice exposed to different doses of γ-radiation. J. Radiat. Res., 2005; 46: 59-65.
- Vanacker S.A, Tromp M.N, Haenen G.R, Vandervijgh W.J, Bast A. Flavonoids as scavengers of nitric oxide radical. Biochem. Bioph. Res. Co., 1995; 214: 755-759.
- Bennett C.J, Caldwell S.T, McPhail D.B, Morrice P.C, Duthie G.G, Hartley R.C. Potential therapeutic antioxidants that combine the radical scavenging ability of myricetin and the lipophilic chain of vitamin E to effectively inhibit microsomal lipid peroxidation. Bioorg. Med. Chem., 2004; 12: 2079–2098.
- Chen C, Jiang X, Hu Y, Zhang Z. The protective role of resveratrol in the sodium arsenite-induced oxidative damage via modulation of intracellular GSH homeostasis. Biol. Trace. Elem. Res., 2013; 155: 119–131.







